Abstract
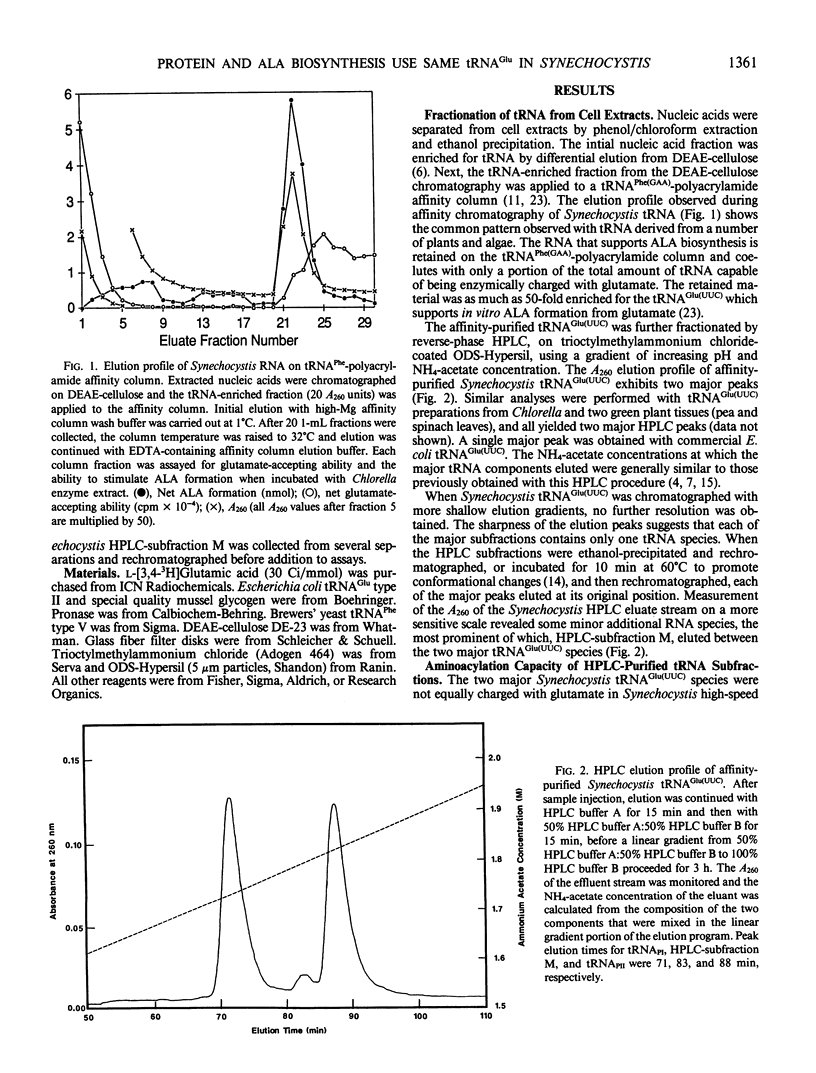
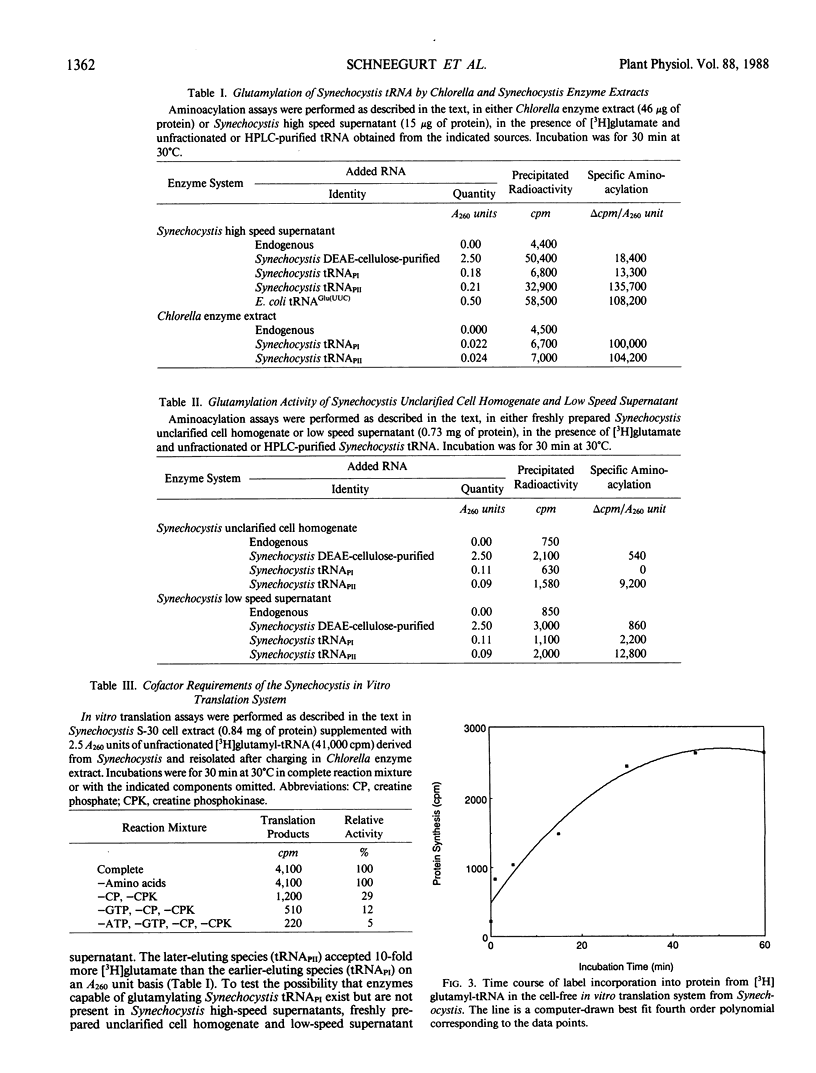
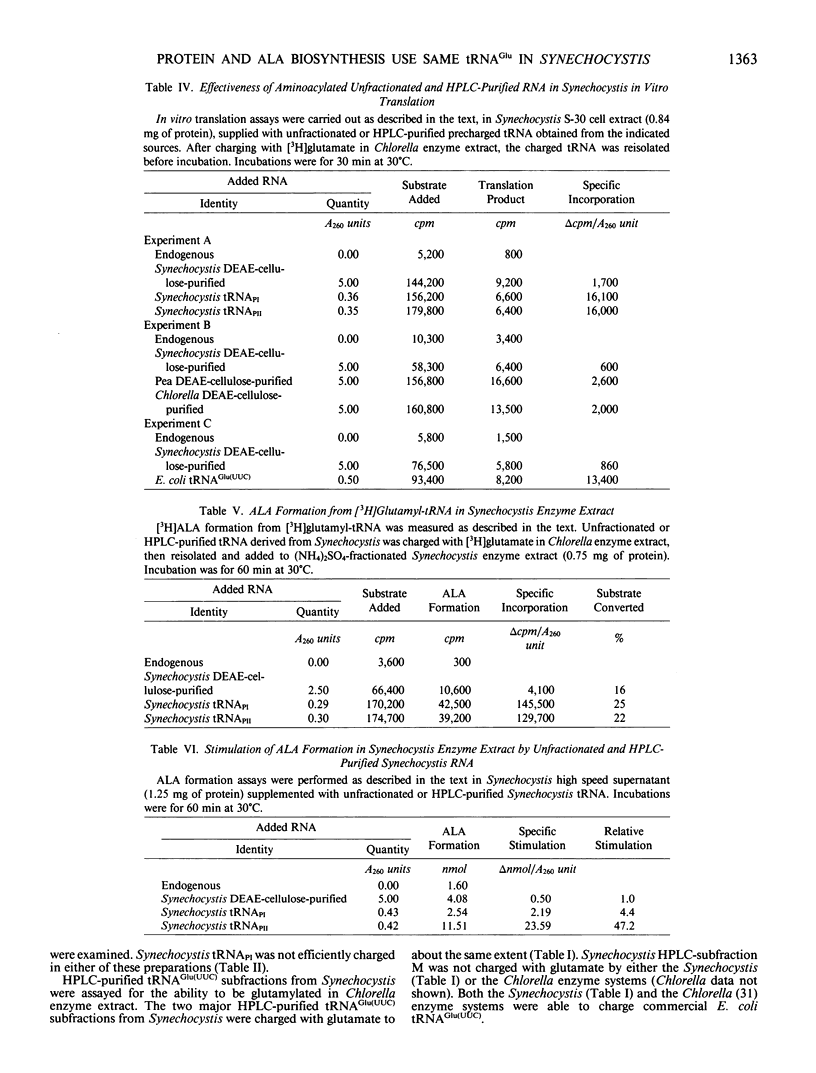
RNA is an essential component for the enzymic conversion of glutamate to δ-aminolevulinic acid (ALA), the universal heme and chlorophyll precursor, as carried out in plants, algae, and some bacteria. The RNA required in this process was reported to bear a close structural resemblance to tRNAGlu(UUC), and it can be isolated by affinity chromatography directed against the UUC anticodon. Affinity-purified tRNAGlu(UUC) from the cyanobacterium Synechocystis sp. PCC 6803 was resolved into two major subfractions by reverse-phase HPLC. Only one of these was effectively charged with glutamate in enzyme extract from Synechocystis, but both were charged in Chlorella vulgaris enzyme extract. When charged with glutamate, the two glutamyl-tRNAGlu(UUC) species produced were equally effective in supporting both ALA formation and protein synthesis in vitro, as measured by label transfer from [3H]glutamyl-tRNA to ALA and protein. These results indicate that one of the two tRNAGlu(UUC) species is used by Synechocystis for both protein biosynthesis and ALA formation. Both of the tRNAGlu(UUC) subfractions from Synechocystis supported ALA formation in Chlorella enzyme extract. Escherichia coli tRNAGlu(UUC) was charged with glutamate, but did not support ALA formation in Synechocystis enzyme extract. Unfractionated tRNA from Chlorella, pea, and E. coli, having been charged with [3H] glutamate by Chlorella enzyme extract and then re-isolated, were all able to transfer label to proteins in the Synechocystis enzyme extract.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bard J. D., Bourque D. P., Zaitlin D. Coupled transcription-translation in chloroplast lysates. Methods Enzymol. 1986;118:270–295. doi: 10.1016/0076-6879(86)18078-5. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Graeser E., McLaughlin L. W. tRNA separation by high-performance liquid chromatography using an aggregate of ODS-Hypersil and trioctylmethylammonium chloride. J Chromatogr. 1983 Mar 4;257(2):305–315. doi: 10.1016/s0021-9673(01)88186-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Galling G. Stimulierung des Aminosäure-Einbaus durch verschiedene Nucleinsäur-Präparationen in zell-- freien Systemen aus Chlorella und Anacystis. Z Naturforsch B. 1967 Jun;22(6):687–688. [PubMed] [Google Scholar]
- Gray J. E., Herson D. S. Functional 70S hybrid ribosomes from blue-green algae and bacteria. Arch Microbiol. 1976 Aug;109(1-2):95–99. doi: 10.1007/BF00425118. [DOI] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Takada C., Petre J. Complex formation between transfer RNAs with complementary anticodons: use of matrix bound tRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):882–893. doi: 10.1016/0006-291x(73)90175-7. [DOI] [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y. Chlorophyll biosynthesis in Chlamydomonas starts with the formation of glutamyl-tRNA. J Biol Chem. 1986 Oct 15;261(29):13451–13455. [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y., Gough S. P., Kannangara C. G. delta-Aminolevulinic acid-synthesizing enzymes need an RNA moiety for activity. Science. 1984 Sep 28;225(4669):1482–1484. doi: 10.1126/science.6206568. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. In vitro protein synthesis and measurement of the stability of messenger RNA in the blue-green alga, anabaena variabilis. J Gen Microbiol. 1974 Mar;81(1):47–58. doi: 10.1099/00221287-81-1-47. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Rieble S., Beale S. I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Synechocystis sp. PCC 6803 and other oxygenic prokaryotes. J Biol Chem. 1988 Jun 25;263(18):8864–8871. [PubMed] [Google Scholar]
- Rodriguez-López M., Muñoz M. L., Vazquez D. The effects of the rifamycin antibiotics on algae. FEBS Lett. 1970 Sep 6;9(3):171–174. doi: 10.1016/0014-5793(70)80346-5. [DOI] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Biosynthesis of protoheme and heme a from glutamate in maize. Plant Physiol. 1986 Aug;81(4):965–971. doi: 10.1104/pp.81.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Characterization of the RNA Required for Biosynthesis of delta-Aminolevulinic Acid from Glutamate : Purification by Anticodon-Based Affinity Chromatography and Determination That the UUC Glutamate Anticodon Is a General Requirement for Function in ALA Biosynthesis. Plant Physiol. 1988 Feb;86(2):497–504. doi: 10.1104/pp.86.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Kannangara C. G., Gough S., Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988 Jan 14;331(6152):187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. RNA is required for enzymatic conversion of glutamate to delta-aminolevulinate by extracts of Chlorella vulgaris. Arch Biochem Biophys. 1985 May 15;239(1):87–93. doi: 10.1016/0003-9861(85)90814-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Formation of delta-Aminolevulinic Acid from Glutamic Acid in Algal Extracts : Separation into an RNA and Three Required Enzyme Components by Serial Affinity Chromatography. Plant Physiol. 1987 Jun;84(2):244–250. doi: 10.1104/pp.84.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Stimulation of delta-Aminolevulinic Acid Formation in Algal Extracts by Heterologous RNA. Plant Physiol. 1986 Dec;82(4):1096–1101. doi: 10.1104/pp.82.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]


