Abstract
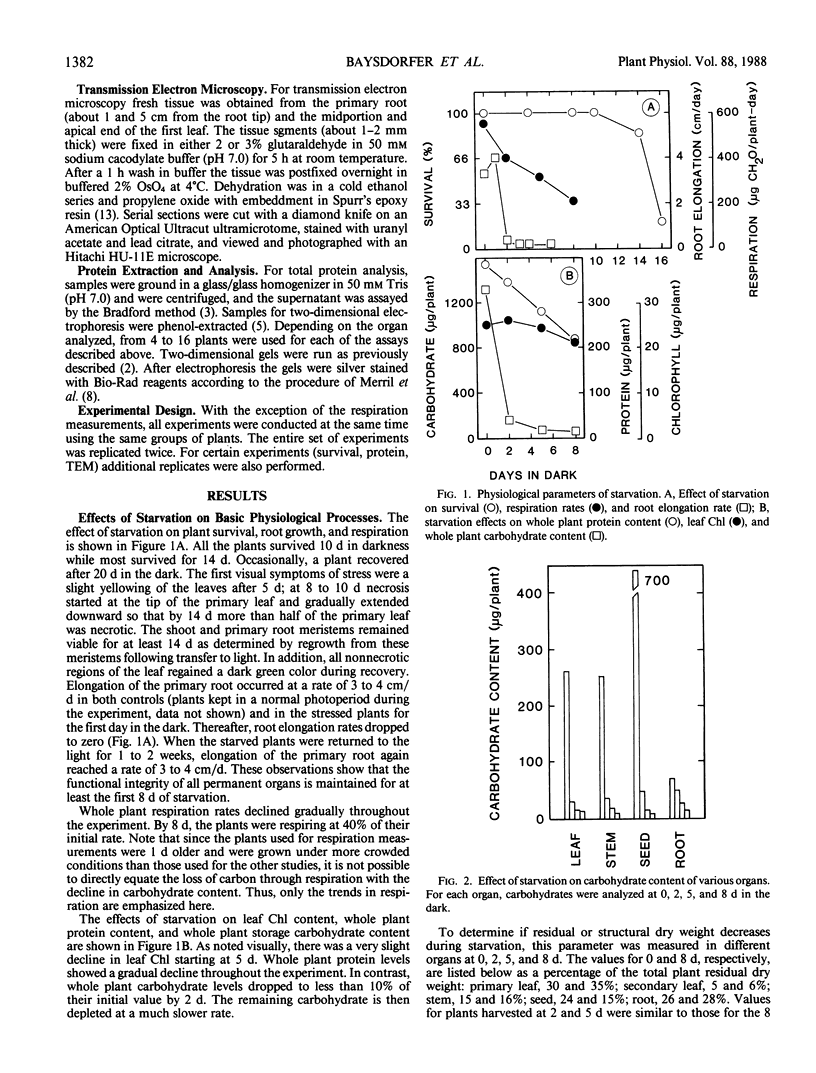
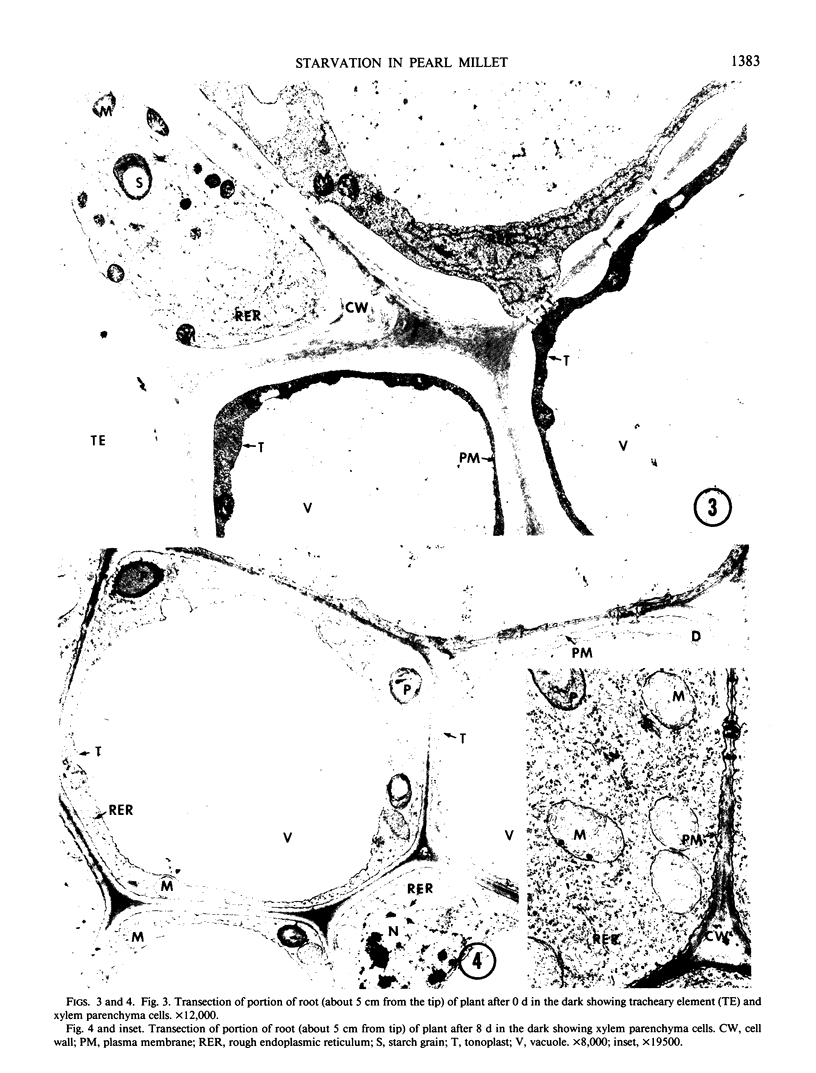
The response of pearl millet (Pennisetum glaucum [L.]) seedlings to prolonged starvation was investigated at the biochemical and ultrastructural level. After 2 days of darkness the bulk of the seedling carbohydrate reserves were depleted. After 8 days in the dark the respiratory rate had declined to less than 50% of its initial value and the plants had lost half of their total protein content. Unlike the situation with carbohydrate depletion, protein loss was restricted to specific organs. The secondary leaf and stem (including the apical meristem) showed little or no protein loss during this period. In the primary leaf, seed, and roots, protein loss was substantial. In spite of the high rate of protein degradation in the primary leaf and roots, these organs showed no ultrastructural changes suggestive of tissue, cellular, or subcellular degradation. In addition, ribulose bisphosphate carboxylase was not preferentially degraded during starvation and only a small decline in chlorophyll content was observed after 8 days in the dark. During the period from 8 to 14 days, cell death started at the tip of the primary leaf and gradually spread downward. Both shoot and root meristems remained alive up to 14 days. Consequently, the eventual death of the plant was due to the loss of the carbohydrate-producing regions rather than the meristems. We suggest that these results provide an explanation for the high degree of starvation tolerance exhibited by pearl millet.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baysdorfer C., Robinson J. M. A Rapid Increase in Spinach Leaf Fructose 2,6-Bisphosphate Occurs during a Light to Dark Transition. Plant Physiol. 1985 Nov;79(3):911–913. doi: 10.1104/pp.79.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysdorfer C., Vanderwoude W. J. Carbohydrate Responsive Proteins in the Roots of Pennisetum americanum. Plant Physiol. 1988 Jul;87(3):566–570. doi: 10.1104/pp.87.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986 Mar 5;261(7):3193–3199. [PubMed] [Google Scholar]
- Kerr P. S., Rufty T. W., Huber S. C. Changes in Nonstructural Carbohydrates in Different Parts of Soybean (Glycine max [L.] Merr.) Plants during a Light/Dark Cycle and in Extended Darkness. Plant Physiol. 1985 Jul;78(3):576–581. doi: 10.1104/pp.78.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Silver staining methods for polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:230–239. doi: 10.1016/s0076-6879(83)96021-4. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Kleinkopf G. E., Huffaker R. C. Evidence for lack of turnover of ribulose 1,5-diphosphate carboxylase in barley leaves. Plant Physiol. 1973 Jun;51(6):1042–1045. doi: 10.1104/pp.51.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C., Martin J. B., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987 Apr 15;262(11):5000–5007. [PubMed] [Google Scholar]
- Rébeillé F., Bligny R., Martin J. B., Douce R. Effect of sucrose starvation on sycamore (Acer pseudoplatanus) cell carbohydrate and Pi status. Biochem J. 1985 Mar 15;226(3):679–684. doi: 10.1042/bj2260679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C. Metabolic adaptation to low intakes of energy and protein. Annu Rev Nutr. 1986;6:495–526. doi: 10.1146/annurev.nu.06.070186.002431. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Induced senescence of intact wheat seedlings and its reversibility. Plant Physiol. 1977 Jun;59(6):1039–1042. doi: 10.1104/pp.59.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A., Lin W., Hebert R. R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982 Jan;69(1):98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]









