Abstract
Species within the genus Neisseria are especially adept at sharing adaptive allelic variation across species’ boundaries, with commensal species repeatedly transferring resistance to their pathogenic relative N. gonorrhoeae. However, resistance in commensal Neisseria is infrequently characterized at both the phenotypic and genotypic levels, limiting our ability to predict novel and potentially transferable resistance mechanisms that ultimately may become important clinically. Unique evolutionary starting places of each Neisseria species will have distinct genomic backgrounds, which may ultimately control the fate of evolving populations in response to selection, as epistatic and additive interactions may coerce lineages along divergent evolutionary trajectories. However alternatively, similar genetic content present across species due to shared ancestry may constrain the adaptive solutions that exist. Thus, identifying the paths to resistance across commensals may aid in characterizing the Neisseria resistome – or the reservoir of alleles within the genus, as well as its depth. Here, we use in vitro evolution of four commensal species to investigate the potential for and repeatability of resistance evolution to two antimicrobials, the macrolide azithromycin and the β-lactam penicillin. After 20 days of selection, commensals evolved elevated minimum inhibitory concentrations (MICs) to penicillin and azithromycin in 11/16 and 12/16 cases respectively. Almost all cases of resistance emergence converged on mutations within ribosomal components or the mtrRCDE efflux pump for azithromycin-based selection, and mtrRCDE or penA for penicillin selection; thus, supporting constrained adaptive solutions despite divergent evolutionary starting points across the genus for these particular drugs. However, continuing to explore the paths to resistance across different experimental conditions and genomic backgrounds, which could shunt evolution down alternative evolutionary trajectories, will ultimately flesh out the full Neisseria resistome.
Keywords: experimental evolution, Neisseria, azithromycin, penicillin, antibiotic resistance, experiential learning
INTRODUCTION
The emergence of antibiotic resistance within bacterial populations is mediated by natural selection, whereby mutations encoding drug-protective mechanisms are produced stochastically, and subsequently increase in frequency as a result of only the cells harboring these mutations surviving exposure events. However, a key question for both understanding evolutionary process and also the enhancement of surveillance efforts is: how repeatable and predictable is resistance evolution at the genotypic level? Two alternate hypotheses can be advanced: (1) adaptive landscapes are constrained to one or few solutions (i.e., genotypic constraint), or (2) a multitude of fitness peaks exist created by many mutations imparting similar phenotypic outcomes. Many prior studies support some level of genotypic constraint on resistance evolution at the strain or species-level1–5, however less frequently has the repeatability of resistance evolution been interrogated across species’ boundaries. Applying selection across different genomic backgrounds at the species-level may lead us to predict a higher likelihood of divergent evolutionary outcomes, with different mutations giving rise to similar phenotypic resistance in different species. We may predict this given that the pre-existing suite of potentially additive and/or epistatically-interacting mutations already present in each species’ genomes will likely be unique as a result of both genetic drift since the time of lineage divergence and also niche-specific adaptation. However, if genotypic convergence is observed across species, this suggests constrained ranges of adaptive solutions between high-level taxonomic groupings (e.g., genera, families, etc.) due to their shared ancestral history and conserved genetic makeup. Here, we begin to interrogate this question: does genotypic constraint or divergence govern the emergence of resistance evolution within the genus Neisseria?
The genus Neisseria is comprised of several Gram-negative, typically diplococcoid, oxidase-positive, and often catalase-positive species, which most frequently colonize the nasopharyngeal or oral niche in humans or animals6. Most human-associated Neisseria are carried harmlessly as commensals in 100% of healthy human adults and children, however N. gonorrhoeae and N. meningitidis are obligate and opportunistic pathogens respectively and are carried in a smaller percentage of the population (between 0.01–10%)7–11. Within the N. gonorrhoeae population, rates of resistance to multiple classes of antimicrobials are rising. For example, according to the latest Gonococcal Isolate Surveillance Project (GISP) report12 ~15% of surveyed isolates were resistance to penicillin, ~20% resistant to tetracycline, 33.2% to ciprofloxacin, 5.8% to azithromycin, and 0.3% to cefixime in the United States; and although resistance (≥ 0.25 μg/ml) was not observed in 2020 to ceftriaxone, isolates with reduced susceptibly have been identified in previous years (2017–2019) as a part of the GISP collection12. Additionally, surveillance studies in other countries have identified higher rates of circulating ceftriaxone resistance (e.g., 4.2% in Taiwan13, 16% in in Guangdong, China14); with recent observations indicating global dissemination (Japan, China, Europe, Australia, North America and Southeast Asia) of high-level ceftriaxone-resistant strains15–20. Though the genetic basis of some resistance phenotypes appears to be exclusively encoded by recurrently acquired mutations (i.e., ciprofloxacin resistance is almost always caused by amino acid substitutions in the DNA gyrase subunit A (GyrA S91F and D95G/D95A21,22)); the complete genetic bases of other resistance phenotypes is currently not fully described and/or is clearly imparted by multiple additive or epistatically-interacting loci (i.e., penicillin23–27 and azithromycin22,28 resistance). Thus, experimentally interrogating the paths to resistance and their repeatability will become an important component of both identifying novel contributing mutations, and understanding their potential prevalence and evolution within populations.
Studies on the paths to resistance within gonococci have previously been explored in vitro (e.g.,29–34). However, gonococci in addition to gaining resistance through de novo mutations, are also superbly adept at acquiring resistance from their close commensal relatives5,28,35–37. This allelic exchange across Neisseria species likely occurs in their shared colonization sites of the naso- and oropharyngeal niches38, with the whole genus often being referred to as a consortium with ‘fuzzy’ borders due to the high frequency of DNA donation through horizontal gene transfer (HGT)39–41. Commensal species thus serve as a bubbling cauldron of new adaptive solutions and reservoir of resistance for gonococci, with each species containing a unique genomic background in which novel resistance genotypes may emerge. Therefore, expanding the investigation on the repeatability of evolution to the entire genus may serve two important goals in the fight against the spread of resistance in gonococci: 1) identifying resistance phenotypes for which a multitude of genotypic paths exist, either within distinct genomic contexts or across several, and 2) determining which drugs and/or drug classes have limited adaptive solutions within the genus. Both of these findings may guide the development of nucleic acid-based resistance tests (i.e. NAAT or WGS) for surveillance programs by defining the scope of mutations which must be surveyed.
Here, we begin to interrogate the paths to resistance to two drugs with as-of-yet not fully identified genotypic bases within the pathogenic Neisseria. We use four different genomic contexts across the Neisseria genus (N. cinerea, N. subflava, N. elongata, and N. canis), and select for increasing minimum inhibitory concentrations (MICs) by passaging each species across selective gradients as previously described5. Though the scope of this initial and a prior study5 have been limited (i.e., limited species and experimental replicates) we imagine that by continuing to ‘roll the evolutionary dice’ we will ultimately coalesce on the possible and quantity of paths to resistance, addressing the repeatability of evolution to different drug classes across the genus. Finally, both this and our previous study5 were conducted as part of exercises within undergraduate classrooms at the Rochester Institute of Technology, highlighting the power of experimental evolution in addressing fundamental questions impacting global public health, while also providing important experiential learning opportunities for students.
RESULTS
Rolling the dice: Evolving Neisseria commensals
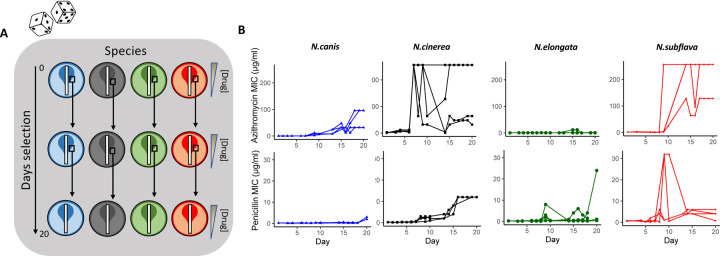
Four Neisseria commensal species were selected as distinct evolutionary starting points for antibiotic selection (N. cinerea (AR-0944), N. subflava (AR-0953 and AR-0957), N. elongata (AR-0945), and N. canis (AR-0948)). All are human-associated commensals except for N. canis, which colonizes the oral cavity of dogs and cats, but has also been isolated from human patients with dog and cat bite wounds42–44. All isolates had been phenotyped for their minimum inhibitory concentrations (MICs) to penicillin and azithromycin (Table 1), and the majority sequenced previously45. One isolate, AR-0944, was sequenced as a part of this study (accession: SAMN37441995; length 2.13 Mbp, 131 contigs, N50= 250 kbp, GC content 50.78%).
Table 1.
Minimum inhibitory concetrations (MICs) for ancestral and average MICs for evolved strains
| Ancestral Strains | Azi MIC (μg/ml) | Average Azi MIC (μg/ml) evolved (n=4) | Pen MIC (μg/ml) | Average Pen MIC (μg/ml) evolved (n=4) |
|---|---|---|---|---|
| AR-0944 (N. cinerea ) | 8 | 152 | 0.38 | 12 |
| AR-0945 (N. elongata ) | 0.5 | 0.69 | 0.25 | 6.72 |
| AR-0948 (N. canis ) | 0.38 | 64 | 0.25 | 5.44 |
| AR-0953 (N. subflava ) | 2 | 224 | 1.5 † | |
| AR-0957 (N. subflava ) | 8 † | 1 | 3.69 |
AR-0953 was only selected with azithromycin and AR-0957 was only selected with penicillin; see disucssion for further details.
For each species and drug combination, four replicate lineages were passaged with selection created by application of Etest strips on standard growth media as previously described5 (Figure 1). Cells were passaged for 20 days, or ~480 generations, by sweeping the entire zone of inhibition (ZOI) and a 1 cm band surrounding the ZOI, and plating any collected cells on new selective growth media. For azithromycin, the average MICs of evolved N. cinerea (MIC=152 ± 120.79 μg/ml), N. canis (64 ± 36.95 μg/ml), and N. subflava (224 ± 64 μg/ml) lineages crossed the breakpoint of reduced susceptibility as defined by the Clinical and Laboratory Standards Institute (CLSI) guidelines for N. gonorrhoeae of ≥ 2 μg/ml46. N. elongata lineages however did not surpass this breakpoint (0.69 ± 0.36 μg/ml). For penicillin, the average MICs for evolved lineages of all species surpassed the CLSI-defined breakpoint concentration of ≥ 2 μg/ml46: N. cinerea (MIC=12 ± 0 μg/ml), N. elongata (6.75 ± 11.53 μg/ml), N. canis (5.44 ± 1.38 μg/ml), and N. subflava (3.69 ± 2.17 μg/ml). Control populations (n=3 per species) with no drug selection showed no significant increase in azithromycin or penicillin MICs compared to the ancestral stocks (Supplementary Table 1).
Figure 1.
Azithromycin and penicillin-mediated selection of four species of commensal Neisseria. (A) Four species with distinct genetic backgrounds were selected as unique starting points for in vitro evolution to two antimicrobials. Each experimental replicate and species/drug combination can be envisioned as an independent “roll of the dice”, in which new derived mutations and evolutionary trajectories may emerge. In brief, 4 experimental replicates were passaged for each species and drug combination for 20 days (~480 generations) on selective gradients created with Etest strips. Cells for each passage were selected by sweeping the entire zone of inhibition (ZOI) and a 1 cm band in the bacterial lawn surrounding the ZOI. (B) Overall, after 20 days, evolved azithromycin minimum inhibitory concentrations (MICs) tended to be higher than of penicillin MICs; with species also differing in their evolutionary trajectories towards elevated MICs within a drug class.
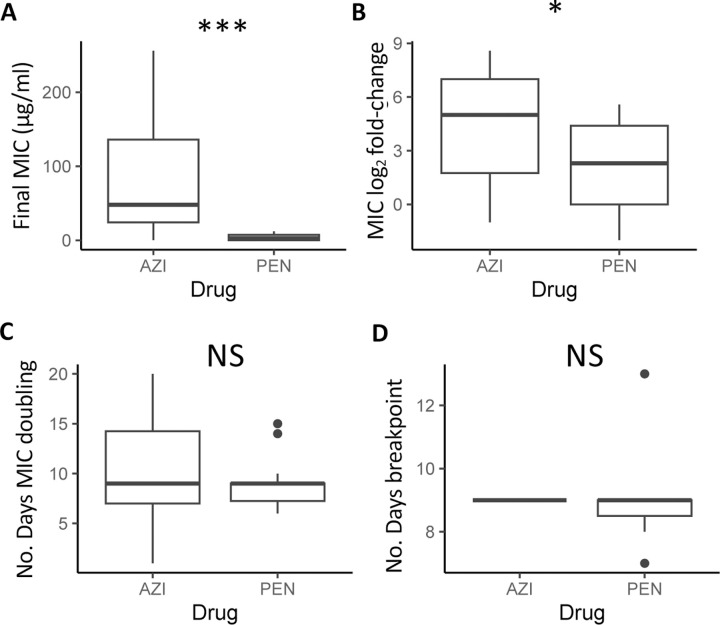
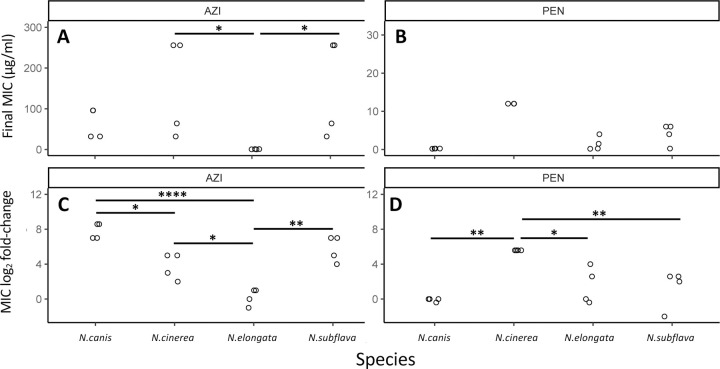
Final recorded MICs for azithromycin (92.17 ± 25.57 μg/ml) were significantly higher across all commensal species compared to the MICs for penicillin (4.45 ± 1.23 μg/ml) (W = 38.5, P = 0.00073; Figure 2A). Azithromycin MIC fold-changes (4.39 ± 0.77) were also significantly higher than that of penicillin MICs (2.08 ± 0.65) across species (W = 74, P = 0.043; Figure 2B). The number of days for MICs to double for azithromycin (10.75 ± 1.34) compared to penicillin (9.07 ± 0.70) were not significantly different (W = 92.5, P = 0.41; Figure 2C); nor was the day the CLSI resistance breakpoint was passed at 9.0 ± 0 and 9.0 ± 0.45 respectively (W = 18, p-value = 0.56; Figure 2D) – with species starting with above breakpoint values at the beginning of the experiment omitted for this last analysis. Between species for azithromycin, N. subflava and N. cinerea had significantly higher evolved MICs compared to N. elongata (Tukey’s HSD: p = 0.036; and p = 0.036 respectively; see also Figure 3A and Supplementary Table 1). There were no significant differences for final MICs between species for penicillin (Figure 3B). However, between species fold-change in MIC was significantly different for four contrasts for azithromycin (Tukey’s HSD: p < 0.05; Figure 3C) and three contrasts for penicillin (Tukey’s HSD: p < 0.01; Figure 3D).
Figure 2.
Across all species, evolved azithromycin MICs were significantly elevated compared to penicillin MICs in both (A) their final values (p < 0.0001), and (B) their fold-increase from ancestral MICs (p < 0.01). The (C) time for MICs to double was not significantly different between drugs (p > 0.05), as was the number of days to surpass the breakpoint value as defined by CLSI guidelines for Neisseria gonorrhoeae (P >0.05).
Figure 3.
Evolved MICs and MIC log-fold change values separated by drug and species. (A) For azithromycin, N. subflava and N. cinerea had significantly higher MICs compared to N. elongata after selection (Tukey’s HSD: p = 0.036; and p = 0.036 respectively). (B) Species were not significantly different between any contrast for penicillin. However, between species fold-change in MIC after evolution was significantly different for (C) four contrasts for azithromycin (Tukey’s HSD: p < 0.05) and (D) three contrasts for penicillin (Tukey’s HSD: p < 0.01).
The frequency and identity of derived mutations
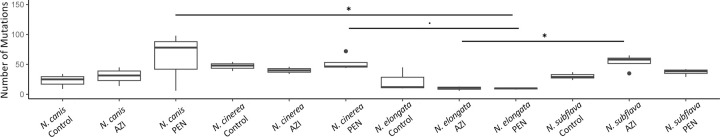
For each evolved lineage, a single colony was picked for further characterization and whole-genome sequencing (Supplementary Table 1). There were no significant differences between the number of derived mutations after the 20-day long experiment between drugs across all species, however each species and interaction between drugs and species (2-way ANOVA: p = 0.0008) had a significant and nearly significant (2-way ANOVA: p = 0.055) impact on the number of derived mutations respectively. N. elongata had significantly fewer derived mutations compared to N. canis (Tukey’s HSD: p = 0.02), N. cinerea (Tukey’s HSD: p = 0.0007), and N. subflava (Tukey’s HSD: p = 0.004). When separated by drug class, for penicillin both N. canis and N. cinerea had significantly more derived mutations compared to N. elongata (Tukey’s HSD: p = 0.02 and p = 0.059 respectively; Figure 4); and for azithromycin N. subflava had significantly more novel mutations compared to N. elongata (Tukey’s HSD: p = 0.045; Figure 4).
Figure 4.
The number of derived mutations after the 20-day long experiment for azithromycin and penicillin selected lines, as well as control lineages. For penicillin both N. canis and N. cinerea had significantly more derived mutations compared to N. elongata (Tukey’s HSD: p = 0.02 and p = 0.059 respectively); and for azithromycin N. subflava had significantly more derived mutations compared to N. elongata (Tukey’s HSD: p = 0.045).
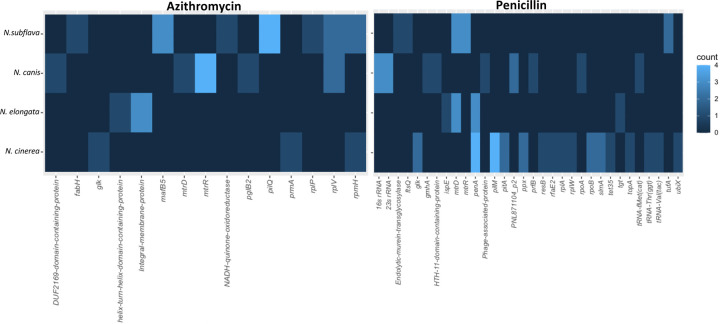
Mutations within coding domain sequences (CDSs) were identified for all evolved lineages, and after correcting for mutations also present in control lineages with no drug exposure, were considered candidates for imparting resistance (Figure 5). For azithromycin, all replicate lineages of N. subflava, N. canis, and N. cinerea evolved resistance, however none of the N. elongata strains did (Figure 1). The most frequent mutation occurring in N. subflava lineages was located within pilM. Additional mutations that emerged included those in fabH, mafB5, nadh, rplP, rplV, and rpmH. For N.canis, the most frequent mutations occurred in mtrR; followed by rplV, duf2169, mtrD, and pglB2. Finally, for N.cinerea, mutations emerged in glk, prmA, and rpmH. For penicillin, all replicate evolved lineages gained resistance except for one N. elongata strain and all N.subflava strains; however each of these lineages developed increased MICs compared to the ancestral strains, and had MICs ≥ 1 μg/ml. Mutations in N. subflava lineages which emerged includes those in mtrD and mtrR, tufA, and a murin transglycosylase. The most frequent mutations in N. canis includes those in the 16s and 23s rRNAs, followed by those in PNL71104_P2, gmhA, an HTH11-domain coding protein, a phage-associated protein, prfB, rpoA, and tRNA-fMet(cat). In N. elongata, derived mutations include those in mtrD, penA, ispE, and tgt. Finally in N. cinerea, mutations included those in penA, pilM, glk, pitA, ppx, rpoB, and slmA; along with some additional singleton mutations (Figure 5).
Figure 5.
Identity of derived mutations in coding domain sequences (CDSs) for drug-selected lineages. The frequency of a mutations within a gene are displayed as a heatmap, with brighter blue coloration indicating more frequent occurrence of a mutation within a CDS in replicate evolved lineages for each species.
DISCUSSION
Commensal Neisseria have repeatedly donated resistance alleles to their pathogenic relative N. gonorrhoeae28,35–37, and beyond doubt serve as a bubbling cauldron of new adaptive solutions to address ‘the antibiotic crisis’ that N. gonorrhoeae faces. However, we do not yet understand the full suite of resistance alleles that commensal Neisseria can carry, if the pool of mechanisms is large or small, and if the pool size varies by antibiotic. Here, we to role the evolutionary dice using antibiotic selection across divergent commensal Neisseria genomic contexts to begin to answer three important questions: 1) what are the identities of resistance mutations that can emerge in commensals, 2) are the paths to resistance evolution constrained or broad, and 3) do the answers to the two prior questions vary by drug class?
Azithromycin is a macrolide antibiotic that inhibits protein synthesis by binding to the 23S rRNA component of the 50S ribosome. Mutations that impact the conformation or block the binding site of the drug have previously been described in N. gonorrhoeae to impart resistance and include: mutations in the 23S rRNA azithromycin binding sites (C2611T and A2059G)47,48, a G70D mutation in the RplD 50S ribosomal protein L449, rplV tandem duplications22, and variants of the rRNA methylase genes ermC and ermB50. Here, we also find a suite of variants that emerged post-selection within the CDSs encoding ribosomal proteins. For example, in both N. subflava and N. canis we uncovered mutations emerging in rplV encoding the 50S ribosomal protein L22; with 2/4 N. subflava lineages and 2/4 N. canis lineages evolving tandem duplications within this gene; previously predicted to block the azithromycin binding site22. In-frame insertions in rpmH, which encodes the 50S ribosomal L34 protein, were also frequent; and found within 2/2 surviving N. cinerea and 2/4 N. subflava strains. N. cinerea strains both evolved distinct rpmH variants (18-bp variant: GATAAGTGCGTTTCATGA; 21-bp variant: GTTGATAAGTGCGTTTCATGA), while N. subflava strains evolved the same variant (24-bp variant: AAACGCACTTATCAACCTTCCGTT). The N. cinerea rpmH variants were nearly identical to those previously described in N. elongata5 and N. gonorrhoeae30, which were found to be casual to high-level azithromycin resistance through transformation in N. elongata5, and thus are the likely mechanisms imparting high-level resistance in N. cinerea strains within this study. Interestingly the N. elongata strains evolved in this study did not evolve reduced azithromycin susceptibility (Figure 1; Table 1); however, in our prior work5, only 44% of replicate N. elongata lineages evolved resistance, and only 43% of these resistant isolates gained resistance through mutations in rpmH. With only 4 replicate N. elongata strains selected in this study we speculate that we did not have sufficient power to uncover these mutations. Finally, we find evidence for a duplication within the rplP gene encoding the 50S ribosomal protein L16 within a single N. subflava strain, however we find no difference in MICs between this strain which also harbors a rplV duplication and a second strain with just a rplV duplication, suggesting that the variant uncovered in rplP may not contribute to the elevated MICs observed. Manoharan-Basil et al. (2021)51 describe multiple recombination events in genes encoding ribosomal proteins across pathogenic and commensal Neisseria, supporting the possibility of transfer of these types of resistance mutations in natural Neisseria populations.
The Multiple transferable resistance efflux pump (Mtr) is a primary mechanism by which N. gonorrhoeae gains resistance to both azithromycin and penicillin. The Mtr efflux pump is comprised of the MtrC-MtrD-MtrE cell envelope proteins, which together export diverse hydrophobic antimicrobial agents such as antibiotics, nonionic detergents, antibacterial peptides, bile salts, and gonadal steroidal hormones from the cell52–55. Overexpression of the pump, through mutations that ablate or decrease the expression of the repressor of the pump (MtrR) have been demonstrated to increase resistance to both azithromycin and penicillin22,26,56,57; and substitutions within the inner membrane component MtrD have been shown to decrease susceptibility to azithromycin28,36. Here, in response to azithromycin-based selection, all four experimental replicates of N. canis evolved mutations in MtrR: two with a G172D substitution, one A37V, and one insertion impacting the reading frame and resulting in a premature stop codon. 3/4 replicates of N. subflava evolved mtrR mutations in response to penicillin exposure which resulted in a T11I substitution in MtrR. MtrD mutations also emerged in response to penicillin-selection in N. subflava (L996I) and N. elongata (with all three strains carrying different mutations: V139G, F604I, or A1009T). Finally, a MtrD mutation also emerged in 1/4 N. canis strains after azithromycin selection E823K. Interestingly, this last E823K MtrD substitution was predicted to be the causal mutation imparting azithromycin resistance in mosaic commensal Neisseria alleles transferred to N. gonorrhoeae28,36.
β-lactams, such as penicillin, target the penicillin binding proteins and inhibit cell wall biosynthesis. Mutations in Penicillin-Binding Protein 2 (PBP2, encoded by penA) in particular have been well documented to impart elevated penicillin MICs in N. gonorrhoeae25,58, and also other β-lactams including the extended spectrum cephalosporin ceftriaxone, through both native gonococcal alleles59 and non-native alleles acquired from commensal Neisseria 22,37,58,60. These mutations act by lowering the affinity of the beta-lactam antibiotics for PBP2 and also by restricting the motions of PBP2 which are important for acylation by beta-lactams61. Therefore, unsurprisingly we observed multiple mutations emerge in penA, though only in two species: N. elongata and N. cinerea. 3/3 surviving N. elongata evolved lines had penA mutations emerge: P399S, V574E, and A581S; and all four experimental N. cinerea replicates evolved penA mutations encoding the amino acid substitutions: F518S, V548E, and A549E.
Additional derived mutations of note that emerged after selection include those in the RNA polymerase and components of the pilus. Here, after penicillin selection a rpoA mutation emerged in N. canis, and rpoB mutations emerged in N. cinerea. In N. gonorrhoeae, both RpoD (E98K and Δ92) and RpoB (R201H) mutations impact ceftriaxone susceptibility, likely through increased expression of PBP1 and reduced expression of D,D-carboxypeptidase62. Here, the rpoA G147A nucleotide substitution in N. canis resulted in a silent change so does not likely contribute to elevated penicillin MICs; however, the evolved rpoB mutations did encode amino acid substitutions (E345A and P591S) in 2/4 N. cinerea replicate lineages. Finally, the pilus-associated mutations in PilM in N. cinerea in response to penicillin selection and PilQ in N. subflava in response to azithromycin likely impact drug diffusion across the outer membrane in some way similar to gonococci63, however are not likely to be evolutionarily maintained in natural Neisseria populations due to the importance of the pilus in host-cell attachment64.
The aforementioned ribosomal, MtrRCDE, and PenA mechanisms seem to be the likely contributors to the emergence of reduced susceptibly in all of the Neisseria commensals investigated in this study for both penicillin and azithromycin-based selection (Figure 6). Therefore, despite 2/2 N. canis replicates evolving low-level penicillin resistance with as-of-yet unexplained genetic bases; with 19/21 cases of Neisseria evolution converging on known resistance mechanisms, we must accept a constrained range of adaptive solutions to antibiotic selection within the genus at this point. Remaining questions do exist however. For example: MICs varied greatly among experimental replicates of the same species, so what other modulating mutations emerged that impact resistance phenotype? Furthermore, here we only investigate coding-domain regions, thus important mutations in intergenic regions were likely missed (i.e., promoter region mutations). We also acknowledge that our small sample of strains and experimental replicates may have limited the pool of potential resistance mechanisms uncovered. For example, some mechanisms may be less frequently observed due to high fitness costs, necessitating the evolution of compensatory mutations. These types of mutations may therefore be missed in small-scale experimental studies. Finally, evolution does not occur in controlled laboratory environments, so what is the role of intergenus gene exchange in Neisseria resistance emergence? Can other genera transfer clinically relevant resistance mechanisms to the Neisseria (see Goytia & Wadsworth (2022)35 for a discussion on this possibility)? In summary, our current results highlight conserved paths to resistance within the Neisseria genus, though continued tosses of the evolutionary dice may ultimately paint a different picture.
Figure 6.
Paths to resistance emergence across members of the Neisseria genus. For azithromycin selection, all species with evolved resistance converged on mutations within ribosome components or the mtrRCDE efflux pump system. For penicillin resistance, N.cinerea, N. elongata, and N. subflava all strains evolving resistance acquired mutations in either the mtrRCDE efflux pump system or penA. N. canis experimental replicates evolving penicillin resistance acquired as-of-yet undescribed resistance mutations.
METHODS
Bacterial strains and culturing
Stocks of Neisseria were obtained from the Centers for Disease Control and Prevention (CDC) and Food and Drug Association’s (FDA) Antibiotic Resistance (AR) Isolate Bank “Neisseria species MALDI-TOF Verification panel”. Evolved strains included: AR-0944 (N. cinerea), AR-0945 (N. elongata), AR-0948 (N. canis), AR-0953 (N. subflava), and AR-0957 (N. subflava). Bacteria were cultivated for all subsequent protocols on GC agar base (Becton Dickinson Co., Franklin Lakes, NJ, USA) media plates containing 1% Kelloggs solution (GCP-K plates) for 18–24 hours at 37°C in a 5% CO2 atmosphere. Bacterial stocks were stored in trypticase soy broth (TSB) containing 50% glycerol at −80°C.
Experimental evolution and MIC testing
Minimum inhibitory concentrations (MICs) were measured by Etest strips (bioMérieux, Durham, NC) on GCB-K plates according to the manufacturer specifications. In brief, cells from overnight plates were suspended in TSB to a 0.5 McFarland standard and inoculated onto GCB-K plates. Etest strips were incubated on these plates for 18–24 hours at 37°C in a 5% CO2 incubator. MICs were subsequently determined by reading the lowest concentration that inhibited growth of bacterial lawns.
For each of the four Neisseria sp. used in the study, four replicates were passaged on GCB-K plates containing a selective gradient of either penicillin or azithromycin. Selective gradients were created using Etest strips as described above and previously5, and MICs were recorded each day. Cells to be passaged were collected from the entire zone of inhibition (ZOI) and a 1 cm region in the bacterial lawn surrounding the ZOI (Figure 1). Cells were suspended in TSB, and spread onto a new GCB-K plate containing a fresh Etest strip. Strains were exposed to azithromycin and penicillin for 20 days, or ~480 generations. Controls for each species were passaged on GCB-K plates as described above, however they did not contain any antibiotic.
Genomic sequencing and comparative genomics
DNA was isolated from cells using the PureLink Genomic DNA Mini kit (Thermo Fisher Corp., Waltham, MA), following lysis in TE buffer (10 mM Tris [pH 8.0], 10 mM EDTA) with 0.5 mg/mL lysozyme and 3 mg/mL proteinase K (Sigma-Aldrich Corp., St. Louis, MO). Resultant genomic DNA was treated with RNase A and prepared for sequencing using the Nextera XT kit (Illumina Corp., San Diego, CA). Libraries were uniquely dual-indexed and pooled, and sequenced on the Illumina MiSeq platform at the Rochester Institute of Technology Genomics Core using V3 600 cycle cartridges (2×300bp). Sequencing quality of each paired-end read library was assessed using FastQC v0.11.965. Trimmomatic v0.3966 was used to trim adapter sequences, and remove bases with phred quality score < 15 over a 4 bp sliding window. Reads < 36 bp long, or those missing a mate, were also removed from subsequent analysis. Draft assemblies had been previously published for all strains45, except for N. cinerea AR-0944. This assembly was constructed using SPAdes v.3.14.167 and all assemblies were annotated with Bakta v.1.8.168. Assembly quality was assessed using QUAST (http://cab.cc.spbu.ru/quast/). Trimmed reads were mapped back to draft assemblies using Bowtie2 v.2.2.469 using the “end-to-end” and “very-sensitive” options and Pilon v.1.1670 was used to call variant sites. Data analysis and visualizations were conducted in R71.
Supplementary Material
Supplementary Figure 1.
Ancestral azithromycin MICs started significantly higher across species compared to penicillin MICs (P < 0.001).
Acknowledgements
This work was produced by the members of the Fall 2022 Genomics course (BIOL340) at the Rochester Institute of Technology (RIT) – a big thank you to every student for their effort in the design and implementation of these experiments. The authors would like to acknowledge the generous support provided by the RIT College of Science and the Thomas H. Gosnell School of Life Science for this work. Work reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R15GM149587. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would also like to thank Girish Kumar at the RIT Genomics Core for providing support and sequencing services.
Footnotes
Data Availability
All scripts and datasets are available on: https://github.com/wadsworthlab. Read libraries for the genomics datasets generated in this study can be accessed on the Sequence Read Archive for evolved strains can be access as a part of the BioProject PRJNA1018855. The assembly for AR-0944 has been deposited to GenBank (accession: SAMN37441995).
Reference
- 1.Zampieri M. et al. Metabolic constraints on the evolution of antibiotic resistance. Mol. Syst. Biol. 13, 917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreich DM, Delaney NF, Depristo MA & Hartl DL. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 7, 111–4 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Vogwill T., Kojadinovic M., Furió V. & MacLean R. C. Testing the role of genetic background in parallel evolution using the comparative experimental evolution of antibiotic resistance. Mol. Biol. Evol. 31, 3314–3323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jørgensen K. M. et al. Sublethal ciprofloxacin treatment leads to rapid development of high-level ciprofloxacin resistance during long-term experimental evolution of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57, 4215–4221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raisman J. C. et al. Evolutionary paths to macrolide resistance in a Neisseria commensal converge on ribosomal genes through short sequence duplications. PLOS ONE 17, e0262370 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G., Tang C. M. & Exley R. M. Non-pathogenic Neisseria: Members of an abundant, multi-habitat, diverse genus. Microbiology 161, 1297–1312 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Dong H. V. et al. Decreased cephalosporin susceptibility of oropharyngeal Neisseria species in antibiotic-using men who have sex with men in Hanoi, Vietnam. Clin. Infect. Dis. 70, 1169–1175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro G., Ibarz-P A. B. & Gabastou J. M. Carriage of Neisseria meningitidis and other Neisseria species among children and young adults in Paraguay. J. Med. Microbiol. [DOI] [PubMed] [Google Scholar]
- 9.Vanbaelen T. et al. Global epidemiology of antimicrobial resistance in commensal Neisseria species: A systematic review. Int. J. Med. Microbiol. 312, 151551 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Diallo K. et al. Pharyngeal carriage of Neisseria species in the African meningitis belt. J. Infect. 72, 667–677 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon C. R. & Schwartz I. S. Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg. Infect. Dis. 24, 1195–1203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Sexually Transmitted Disease Surveillance 2020: Gonococcal Isolate Surveillance Project Profile. (2022).
- 13.Lin H.-H. et al. Emergence of a predominant sequence type ST7363 and the increasing trend of resistance to cefixime and ceftriaxone in Neisseria gonorrhoeae in Southern Taiwan, 2019–2021. J. Microbiol. Immunol. Infect. 56, 833–841 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Liao Y. et al. Dissemination of Neisseria gonorrhoeae with decreased susceptibility to extended-spectrum cephalosporins in Southern China, 2021: A genome-wide surveillance from 20 cities. Ann. Clin. Microbiol. Antimicrob. 22, 39 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Shao-Chun, Han Yan, Yuan Liu-Feng, Zhu Xiao-Yu & Yin Yue-Ping. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg. Infect. Dis. 25, 1427–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X. et al. Dissemination and genome analysis of high-level ceftriaxone-resistant penA 60.001 Neisseria gonorrhoeae strains from the Guangdong Gonococcal antibiotics susceptibility Programme (GD-GASP), 2016–2019. Emerg. Microbes Infect. 11, 344–350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berçot B. et al. Ceftriaxone-resistant, multidrug-resistant Neisseria gonorrhoeae with a novel mosaic penA-237.001 gene, France, June 2022. Eurosurveillance 27, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picker M. A. et al. Notes from the Field: First Case in the United States of Neisseria gonorrhoeae Harboring Emerging Mosaic penA 60 Allele, Conferring Reduced Susceptibility to Cefixime and Ceftriaxone. MMWR Morb. Mortal. Wkly. Rep. 69, 1876–1877 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eyre D. W. et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Eurosurveillance 24, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahra M. M. et al. Cooperative Recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg. Infect. Dis. 24, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortimer T. D. & Grad Y. H. Applications of genomics to slow the spread of multidrug-resistant Neisseria gonorrhoeae. Ann. N. Y. Acad. Sci. 1435, 93–109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grad Y. H. et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J. Infect. Dis. 214, 1579–1587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olesky M., Zhao S., Rosenberg R. L. & Nicholas R. A. Porin-mediated antibiotic resistance in neisseria gonorrhoeae : Ion, solute, and antibiotic permeation through PIB proteins with penB Mutations. J. Bacteriol. 188, 2300–2308 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesky M., Hobbs M. & Nicholas R. A. Identification and analysis of amino acid mutations in Porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46, 2811–2820 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ropp P. A., Hu M., Olesky M. & Nicholas R. A. Mutations in ponA, the gene encoding Penicillin-Binding Protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46, 769–777 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veal W. L., Nicholas R. A. & Shafer W. M. Overexpression of the MtrC-MtrD-MtrE Efflux Pump due to an mtrR Mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J. Bacteriol. 184, 5619–5624 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao S., Tobiason D. M., Hu M., Seifert H. S. & Nicholas R. A. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability: Gonococcal pilQ mutants with increased antibiotic resistance. Mol. Microbiol. 57, 1238–1251 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadsworth C. B., Arnold B. J., Sater M. R. A. & Grad Y. H. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 9, e01419–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foerster S. et al. In vitro antimicrobial combination testing of and evolution of resistance to the first-in-class spiropyrimidinetrione zoliflodacin combined with six therapeutically relevant antimicrobials for Neisseria gonorrhoeae. J. Antimicrob. Chemother. 74, 3521–3529 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Laumen J. G. E. et al. Molecular pathways to high-level azithromycin resistance in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 76, 1752–1758 (2021). [DOI] [PubMed] [Google Scholar]
- 31.González N. et al. Alternative pathways to ciprofloxacin resistance in Neisseria gonorrhoeae: An in vitro study of the WHO-P and WHO-F reference strains. Antibiotics 11, 499 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golparian D., Jacobsson S., Holley C. L., Shafer W. M. & Unemo M. High-level in vitro resistance to gentamicin acquired in a stepwise manner in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 78, 1769–1778 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Z. et al. Novel genes related to ceftriaxone resistance found among ceftriaxone-resistant Neisseria gonorrhoeae strains selected in vitro. Antimicrob. Agents Chemother. 60, 2043–2051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson S. R. et al. In vitro selection of Neisseria gonorrhoeae mutants with elevated mic values and increased resistance to cephalosporins. Antimicrob. Agents Chemother. 58, 6986–6989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goytia M. & Wadsworth C. B. Canary in the coal mine: How resistance surveillance in commensals could help curb the spread of AMR in pathogenic Neisseria. mBio 13, e01991–22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouquette-Loughlin C. E. et al. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 9, e02281–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ameyama S. et al. Mosaic-like structure of penicillin-binding protein 2 gene ( pena ) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46, 3744–3749 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donati C. et al. Uncovering oral Neisseria tropism and persistence using metagenomic sequencing. Nat. Microbiol. 1, 16070 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Smith J. M., Smith N. H., O’Rourke M. & Spratt B. G. How clonal are bacteria? Proc. Natl. Acad. Sci. 90, 4384–4388 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanage W. P., Fraser C. & Spratt B. G. Fuzzy species among recombinogenic bacteria. BMC Biol. 3, 6 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold B. et al. Fine-Scale haplotype structure reveals strong signatures of positive selection in a recombining bacterial pathogen. Mol. Biol. Evol. 37, 417–428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailie W. E., Stowe E. C. & Schmitt A. M. Aerobic Bacterial flora of oral and nasal fluids of canines with reference to bacteria associated with bitest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guibourdenche M., Lambert T. & Riou J. Y. Isolation of Neisseria canis in mixed culture from a patient after a cat bite. J. Clin. Microbiol. 27, 1673–1674 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoke C. & Vedros N. A. Characterization of atypical aerobic gram-negative cocci isolated from humans. J. Clin. Microbiol. 15, 906–914 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiore M. A., Raisman J. C., Wong N. H., Hudson A. O. & Wadsworth C. B. Exploration of the Neisseria resistome reveals resistance mechanisms in commensals that may be acquired by N. gonorrhoeae through horizontal gene transfer. Antibiotics 9, 656 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clinical and Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing. (2020).
- 47.Chisholm S. A., Dave J. & Ison C. A. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob. Agents Chemother. 54, 3812–3816 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng L.-K., Martin I., Liu G. & Bryden L. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46, 3020–3025 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma K. C. et al. Increased power from conditional bacterial genome-wide association identifies macrolide resistance mutations in Neisseria gonorrhoeae. Nat. Commun. 11, 5374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demczuk W. et al. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J. Clin. Microbiol. 54, 1304–1313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manoharan-Basil S. S. et al. Evidence of horizontal gene transfer of 50s ribosomal genes rplB, rplD, and rplY in Neisseria gonorrhoeae. Front. Microbiol. 12, 683901 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagman K. E. & Shafer W. M. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 177, 4162–4165 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagman K. E. & Snyder L. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/noduIation/division protein family constituting part of an efflux system. [DOI] [PubMed]
- 54.Delahay R. M., Robertson B. D., Balthazar J. T., Shafer W. M. & Ison C. A. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology 143, 2127–2133 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Lucas C. E., Balthazar J. T., Hagman K. E. & Shafer W. M. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J. Bacteriol. 179, 4123–4128 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohneck E. A. et al. A Novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2, e00187–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagman K. E. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the rntrRCDE efflux system. [DOI] [PubMed]
- 58.Spratt Brian. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332, 173–176 (1988). [DOI] [PubMed] [Google Scholar]
- 59.Tomberg J., Unemo M., Davies C. & Nicholas R. A. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae : Role of Epistatic Mutations. Biochemistry 49, 8062–8070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohnishi M. et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55, 3538–3545 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh A. et al. Mutations in penicillin-binding protein 2 from cephalosporin-resistant Neisseria gonorrhoeae hinder ceftriaxone acylation by restricting protein dynamics. J. Biol. Chem. 295, 7529–7543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palace S. G. et al. RNA polymerase mutations cause cephalosporin resistance in clinical Neisseria gonorrhoeae isolates. eLife 9, e51407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nandi S., Swanson S., Tomberg J. & Nicholas R. A. Diffusion of antibiotics through the PilQ Secretin in Neisseria gonorrhoeae occurs through the immature, sodium dodecyl sulfate-labile form. J. Bacteriol. 197, 1308–1321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heckels J. E. Structure and function of pili of pathogenic Neisseria species. CLIN MICROBIOL REV 2, (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrews S. FASTQC: A quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 66.Bolger A. M., Lohse M. & Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bankevich A. et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwengers O. et al. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genomics 7, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker B. J. et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9, e112963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.R Core Team. R: A language and environment for statistical computing. R Found. Stat. Comput. Vienna Austria: 2015. Available: https://www.R-Proj.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.