Abstract
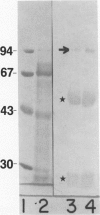
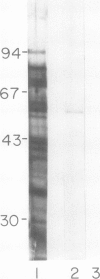
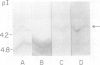
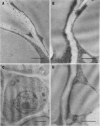
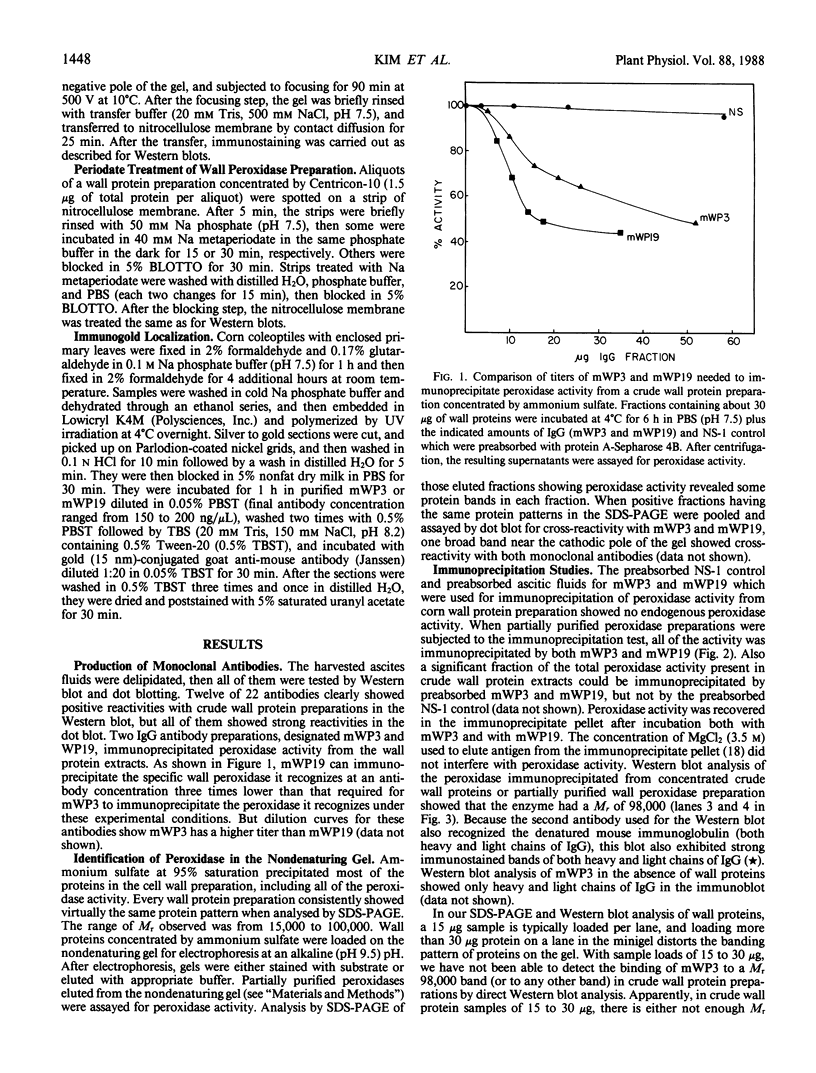
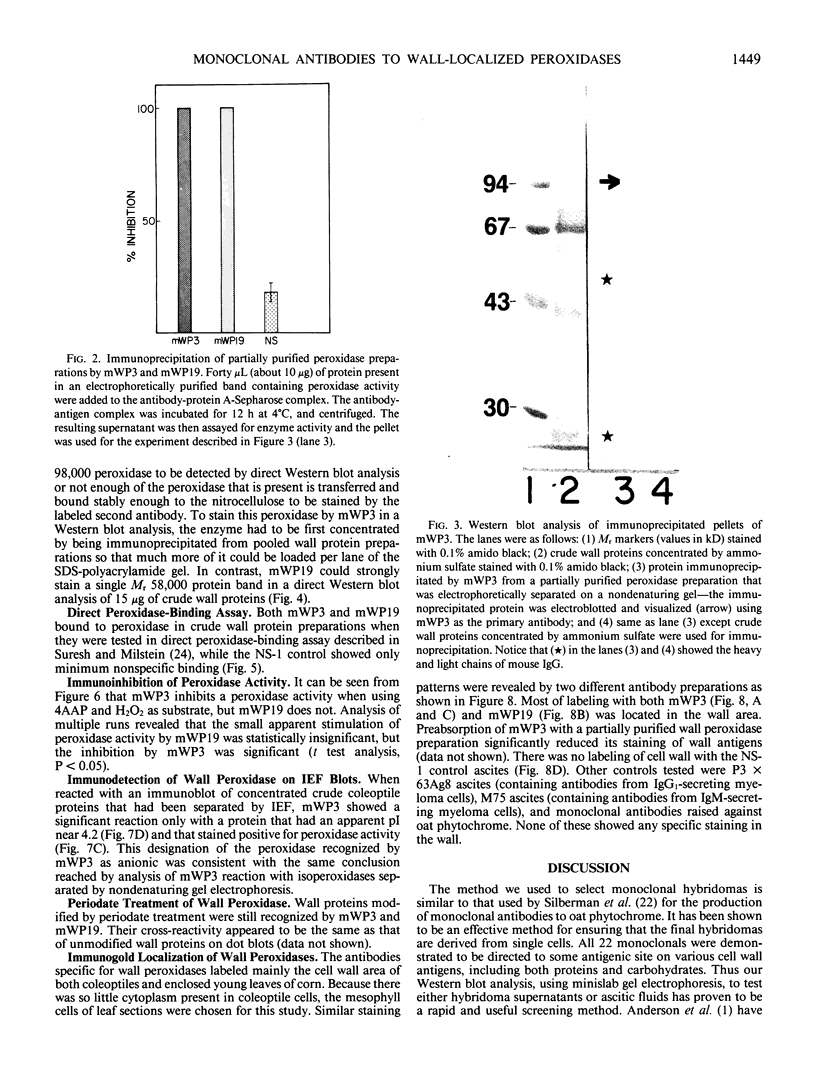
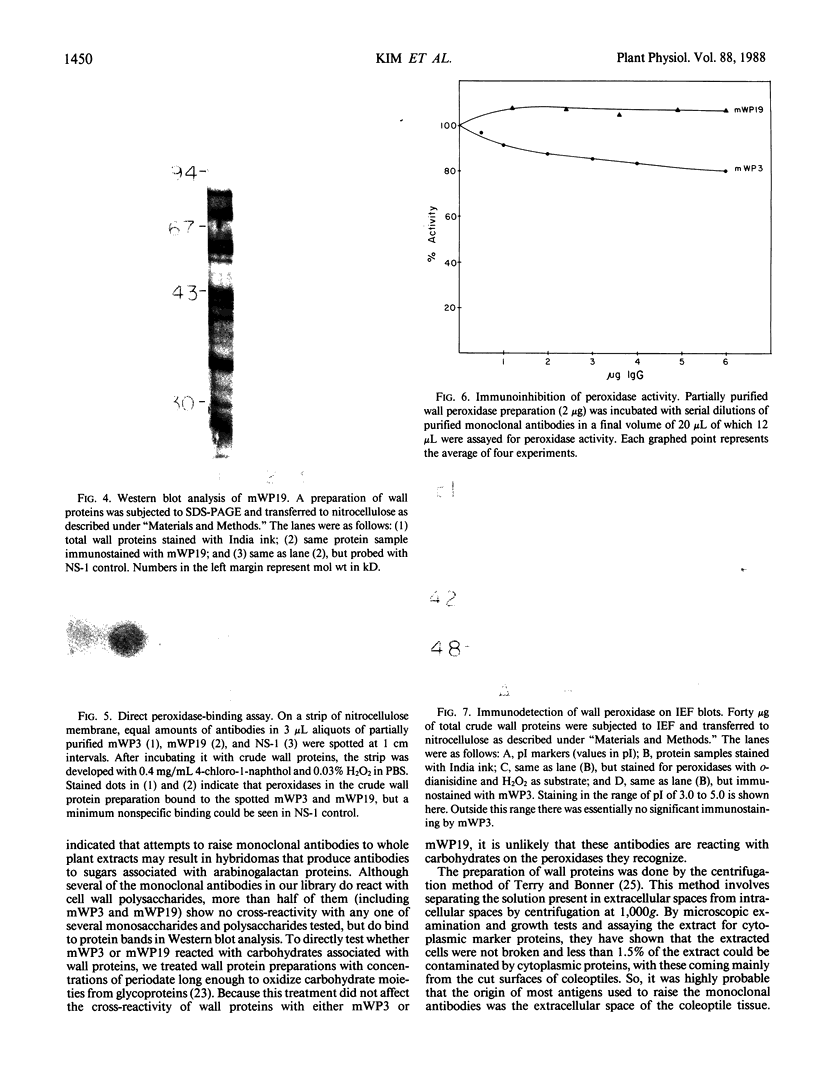
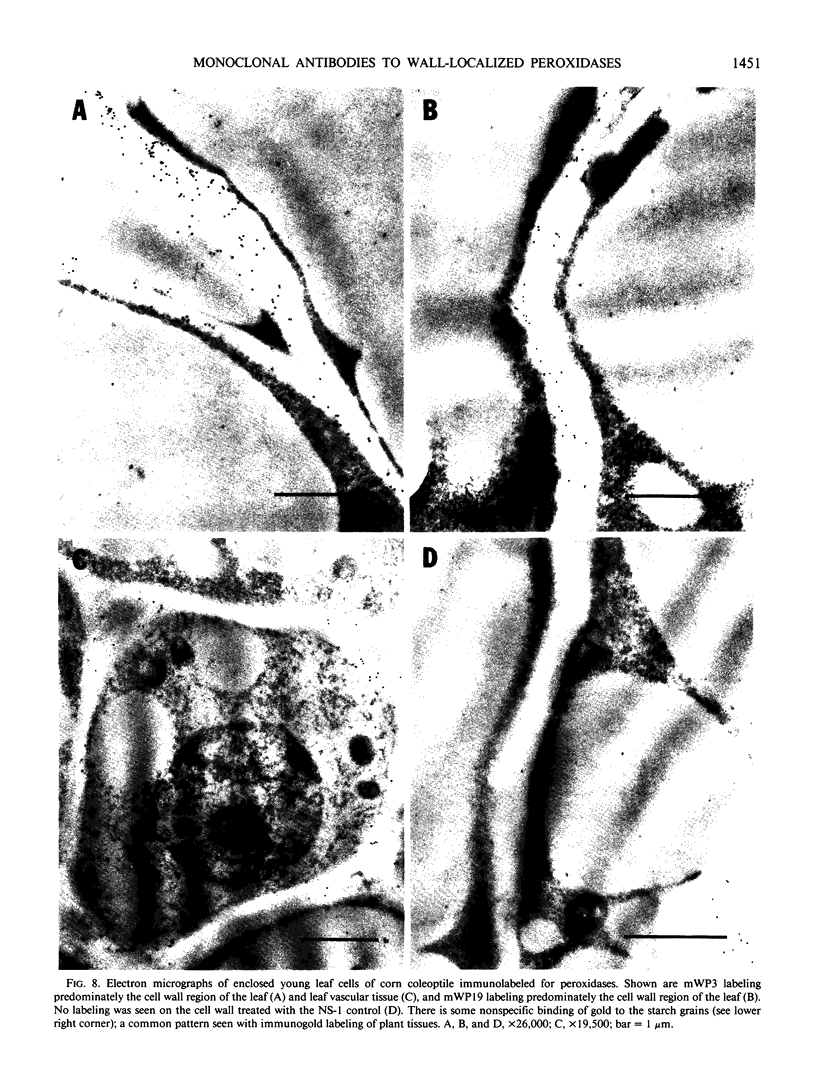
A library of 22 hybridomas, which make antibodies to soluble wall antigens from the coleoptiles and primary leaves of etiolated corn (Zea mays L.) seedlings, was raised and cloned three times by limit dilution to assure monoclonal growth and stability. Two of these hybridomas made immunoglobulin G antibodies, designated mWP3 and mWP19, which both effectively immunoprecipitated peroxidase activity from crude and partially purified preparations of wall peroxidases. Direct peroxidase-binding assays revealed that both antibodies bound enzymes with peroxidase activity. As judged by immunoblot analyses, mWP3 recognized a Mr 98,000 wall peroxidase with an isoelectric point near 4.2, and mWP19 recognized a Mr 58,000 wall peroxidase. Immunogold localization studies showed both peroxidases are predominately in cell walls.
Full text
PDF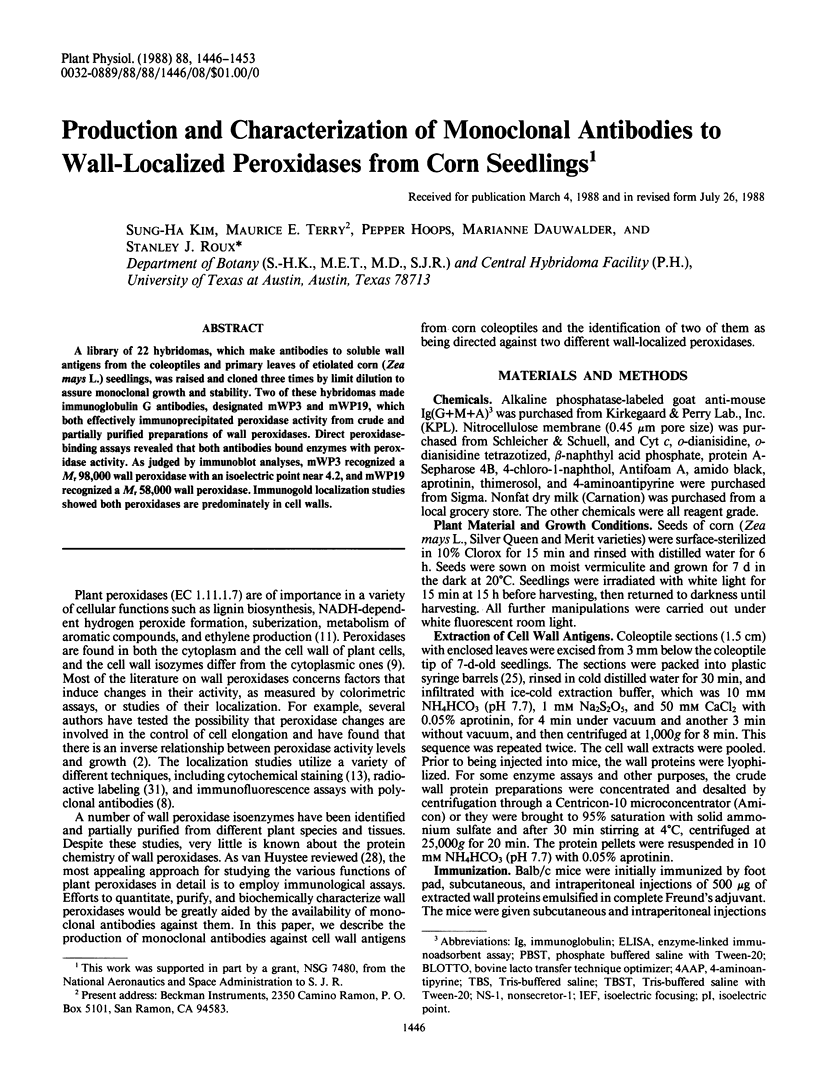



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Sandrin M. S., Clarke A. E. A high proportion of hybridomas raised to a plant extract secrete antibody to arabinose or galactose. Plant Physiol. 1984 Aug;75(4):1013–1016. doi: 10.1104/pp.75.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibbar R. N., van Huystee R. B. Characterization of peroxidase in plant cells. Plant Physiol. 1984 Aug;75(4):956–958. doi: 10.1104/pp.75.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy J. M., Borzelleca D. C., McDonell L. A. Homology of Plant Peroxidases: AN IMMUNOCHEMICAL APPROACH. Plant Physiol. 1982 Jan;69(1):28–31. doi: 10.1104/pp.69.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dao M. L. An improved method of antigen detection on nitrocellulose: in situ staining of alkaline phosphatase conjugated antibody. J Immunol Methods. 1985 Oct 10;82(2):225–231. doi: 10.1016/0022-1759(85)90354-0. [DOI] [PubMed] [Google Scholar]
- Espelie K. E., Franceschi V. R., Kolattukudy P. E. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986 Jun;81(2):487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye L., Mouatassim B., Ghorbel A. Cell Wall and Cytoplasmic Isozymes of Radish beta-Fructosidase Have Different N-Linked Oligosaccharides. Plant Physiol. 1986 Jan;80(1):27–33. doi: 10.1104/pp.80.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee A. P., Borsos T., Boyle M. D. Interaction between components of the human classical complement pathway and immobilized Cibacron Blue F3GA. J Immunol Methods. 1979;30(2):119–126. doi: 10.1016/0022-1759(79)90086-3. [DOI] [PubMed] [Google Scholar]
- Hernandez J., Pérez-Ojeda E., Serrano J. S., Castillo J. R., Serrano M. I. Possible involvement of epinephrine in the cardiovascular effect of naloxone in humans. Clin Ther. 1985;7(4):418–423. [PubMed] [Google Scholar]
- Hu C., Carbonera D., van Huystee R. Production and Preliminary Characterization of Monoclonal Antibodies against Cationic Peanut Peroxidase. Plant Physiol. 1987 Sep;85(1):299–303. doi: 10.1104/pp.85.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacSween J. M., Eastwood S. L. Recovery of antigen from staphylococcal protein A--antibody adsorbents. Methods Enzymol. 1981;73(Pt B):459–471. [PubMed] [Google Scholar]
- Marucci A. A. Inhibition of horse radish peroxidase by specific antisera. Immunochemistry. 1973 Apr;10(4):278–280. doi: 10.1016/0019-2791(73)90207-3. [DOI] [PubMed] [Google Scholar]
- Neoh S. H., Gordon C., Potter A., Zola H. The purification of mouse monoclonal antibodies from ascitic fluid. J Immunol Methods. 1986 Jul 24;91(2):231–235. doi: 10.1016/0022-1759(86)90483-7. [DOI] [PubMed] [Google Scholar]
- Silberman L. G., Datta N., Hoops P., Roux S. J. Characterization of monoclonal antibodies to oat phytochrome by competitive radioimmunoassays and comparative immunoblots of phytochrome peptides. Arch Biochem Biophys. 1985 Jan;236(1):150–158. doi: 10.1016/0003-9861(85)90614-9. [DOI] [PubMed] [Google Scholar]
- Suresh M. R., Milstein C. A direct antigen-binding assay to screen hybridoma supernatants. Anal Biochem. 1985 Nov 15;151(1):192–195. doi: 10.1016/0003-2697(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Terry M. E., Bonner B. A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980 Aug;66(2):321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]