Abstract
Purpose
To estimate the association of psychiatric polygenic scores with healthcare utilization and comorbidity burden.
Methods
Observational cohort study (N = 118,882) of adolescent and adult biobank participants with linked electronic health records (EHRs) from three diverse study sites; (Massachusetts General Brigham, Vanderbilt University Medical Center, Geisinger). Polygenic scores (PGS) were derived from the largest available GWAS of major depressive depression, bipolar disorder, and schizophrenia at the time of analysis. Negative binomial regression models were used to estimate the association between each psychiatric PGS and healthcare utilization and comorbidity burden. Healthcare utilization was measured as frequency of emergency department (ED), inpatient (IP), and outpatient (OP) visits. Comorbidity burden was defined by the Elixhauser Comorbidity Index and the Charlson Comorbidity Index.
Results
Participants had a median follow-up duration of 12 years in the EHR. Individuals in the top decile of polygenic score for major depressive disorder had significantly more ED visits (RR=1.22, 95% CI; 1.17, 1.29) compared to those the lowest decile. Increases were also observed with IP and comorbidity burden. Among those diagnosed with depression and in the highest decile of the PGS, there was an increase in all utilization types (ED: RR=1.56, 95% CI 1.41, 1.72; OP: RR=1.16, 95% CI 1.08, 1.24; IP: RR=1.23, 95% CI 1.12, 1.36) post-diagnosis. No clinically significant results were observed with bipolar and schizophrenia polygenic scores.
Conclusions
Polygenic score for depression is modestly associated with increased healthcare resource utilization and comorbidity burden, in the absence of diagnosis. Following a diagnosis of depression, the PGS was associated with further increases in healthcare utilization. These findings suggest that depression genetic risk is associated with utilization and burden of chronic disease in real-world settings.
INTRODUCTION
It is widely recognized that mental health care is paramount in the management of chronic medical conditions when they co-occur with serious mental illness (SMI) including major depression, bipolar disorder, and schizophrenia. For example, in a Canadian cohort of adults with at least one of chronic medical condition (asthma, congestive heart failure, myocardial infarction, diabetes, epilepsy, hypertension, chronic pulmonary disease, and chronic kidney disease), those with comorbid depression (MDD) or schizophrenia (SCZ) were found to have an increase mean total 3-year cost of care compared to those without any mental health disorder1. Mental health and substance abuse treatment costs among individuals with bipolar (BP) and MDD were 20 and three times, respectively, as high as general medical outpatients2. In the same study, the authors observed similar costs of general medical care between individuals with bipolar or depression and diabetes. Several other published studies have found increased costs and utilization among patients with co-morbid disease and depression3–6.
In parallel, mounting evidence indicates that genetic factors can increase the risk of both SMI and chronic medical conditions7,8. For example, prior work from the PsycheMERGE consortium, a multi-center network of electronic health record- (EHR-) linked biobanks, demonstrated that polygenic scores (PGS) for MDD also increase the risk of cardiovascular disease even in patients without diagnosed depression9. Moreover, depression is a modifiable risk factor for cardiovascular disease10. Other findings from PsycheMERGE have shown that a PGS for SCZ is associated with a range of non-psychiatric medical conditions, including, hepatitis, neurologic disorders, and disorders of the urinary system11. This raises the hypothesis that PGS for SMI may be associated with comorbidity burden or health care utilization, even in the absence of SMI. If so, this could have significant implications for selecting effective models of care that reduce comorbidity and health care costs. For example, the collaborative chronic care model (CCM) takes a team-based patient-centered approach and has demonstrated efficacy in reducing comorbidities for patients with SMI12,13. Recent genetic findings described above, raise the hypothesis that a CCM which integrates psychiatric and mental health care may be a viable preventative model of care for patients with high genetic risk of psychiatric illness, even in the absence of SMI.
Studies exploring the clinical utility use of PGS in psychiatry have been limited to date14. A large body of research has established that psychiatric PGS capture significant risk of psychiatric disorder, largely in cohorts ascertained for research. However, the prospect of implementing PGS as risk indices in real-world health settings has been under-studied. In addition, little is known about whether PGS are associated with disease burden and care utilization. The observation that genomic risk for psychiatric disorders is correlated with a range of medical outcomes suggests that psychiatric PGS may be associated with multimorbidity and consequent healthcare service use in the absence of a PGS’s cognate disorder. In this report, we leverage the PsycheMERGE network to examine whether polygenic scores for major psychiatric disorders (SCZ, BD, MDD) are associated with important clinical outcomes: healthcare utilization and comorbidity burden. We focused on the utilization outcomes related to inpatient, outpatient, and ED usage, and the number of comorbid conditions as index by two widely used measures: the Elixhauser Comorbidity Index (ECI)15 and the Charlson Comorbidity Index (CCI)16.
MATERIALS AND METHODS
Participating Sites:
Participating study sites included two major academic medical centers and a large rural healthcare system in the United States; Mass General Brigham (MGB), Vanderbilt University Medical Center (VUMC), and Geisinger, representing diversity in race/ethnicity and rural/urban status. Each site includes a hospital-based biobank; the MGB Biobank17, the VUMC biobank (BioVU)18, and the Geisinger MyCode Community Health Initiative (MyCode)19. At each of these sites, participants were recruited from the general healthcare system population without systematic recruitment for any particular disease or diagnosis. Biobank procedures and patient recruitment were approved at each health system’s institutional review board. For this study only participants aged 15 or older with at least three encounters over a period of at least five years in the EHR were included. Data were extracted from the beginning of the EHR to April 2021 at each site.
Psychiatric Conditions:
Major Depressive Disorder (MDD) and Schizophrenia (SCZ):
Cases of MDD and SCZ were identified by the presence of Phecodes for these conditions in their medical record. Phecodes were mapped from ICD-9/-10 diagnosis codes using Phecode Map 1.220. A case was defined as positive if the corresponding phecodes were present on at least two outpatient (OP) visits at least 30 days apart, or at least one emergency department (ED) visit, or at least one inpatient (IP) visit, or present on the Problem List (PL), where available.
Bipolar Disorder (BD):
Cases of BD were defined using a rule-based phenotype algorithm developed at MGB as part of the International Cohort Consortium for Bipolar Disorder and referred to as “Bipolar Coded-Broad”21. This rule-based algorithm demonstrated high positive predictive value (0.80) against direct-interview diagnostic assessment based on the SCID-IV. To meet Bipolar Coded-Broad criteria, cases had at least two BD diagnostic (ICD) codes recorded at any time with a minimum of four weeks between each code and a history of two or more medications used to treat BD within one year of index BD diagnosis. To rule out patients with closely related disorders, we required the number of diagnostic codes for MDD, SCZ, Schizoaffective disorder, or Organic Affective Syndrome to total fewer than half the number of BD codes.
Polygenic Scores:
To generate PGS, we used publicly available summary statistics from the Psychiatric Genomics Consortium’s (PGC) genome-wide association studies (GWAS) for MDD22, SCZ23, and BD24, respectively. VUMC used the latest available GWAS for BD by the Psychiatric Genomics Consortium (PGC)25 as these results were available when VUMC ran their analyses. To derive posterior GWAS coefficients adjusted for linkage disequilibrium (LD), we applied PRS-CS (September 10, 2020, software release), applying default settings26. For computational feasibility, PRS-CS is restricted to 1,120,697 quality-controlled HapMap 3 SNPs. PGS were computed for each participant by summing genotypes weighted by the posterior coefficients using PLINK1.9, build 6.1727. In the Supplement, we provide details on the genotyping, imputation, and quality-control procedures.
Outcomes:
Healthcare utilization
The number of ED, IP, and OP visits were enumerated for each participant across the span of their EHR from the age of 15 to date of the data extraction. An ED visit was defined as only those that did not result in an admission. OP visits included ambulatory and telehealth encounters.
Comorbidity burden
Comorbidity burden was measured using the ECI15 and the CCI16 as indicators of how ill the participant was at the end of their EHR span. The ECI consists of 31 conditions and the CCI consists of 17 conditions (see Supplement Table S1). A given condition was coded as positive following the same rules used to define MDD and SCZ. The ECI includes psychosis and depression as two of the conditions which may induce a correlation with the PGS. Results were compared when including and excluding these two conditions and found to be similar. Therefore, results are shown for the full ECI.
Statistical Analysis:
Descriptive statistics are presented as median and inter-quartile range for continuous variables, and frequency and percentages for categorial variables for each participating site. To estimate the effect of psychiatric PGS on healthcare utilization and comorbidity burden the negative binomial (NB) regression model was used. The NB distribution fit better than the Poisson as visually assessed by a rootogram28 and compared using a likelihood ratio test. To account for the varying lengths of EHR follow up time, an offset term, the natural logarithm of the EHR duration in years, was included in the models to allow for the standardized interpretation as the average number of visits per year. The models also controlled for participant age at the end of the observation period, the first ten ancestry principal components derived from GWAS data, and genotyping batch. Analyses were performed separately at each site and results combined using an inverse-variance weighted meta-analysis. PGS were standardized at each site and treated as a continuous predictor as well as categorized into deciles. For the latter, the primary comparison was between the highest and lowest decile. Lastly, in a subgroup analysis, we assessed the association between MDD polygenic score and utilization after the diagnosis of depression. Results are presented as rate ratios (RR) and 95% confidence intervals (CIs). Analyses were preformed using R v4.0.329 with the countreg30 and MASS31 packages.
Participants meeting the definition of MDD, SCZ, or BD had their EHR record truncated at the day prior to the first indication of a diagnosis. That is, the calculation of healthcare utilization and comorbidity burden only used EHR data up until the day before diagnosis while still requiring a minimum of 5 years in the health system. For participants that did not receive a diagnosis of these conditions, all data in the EHR were used to calculate the outcomes.
RESULTS
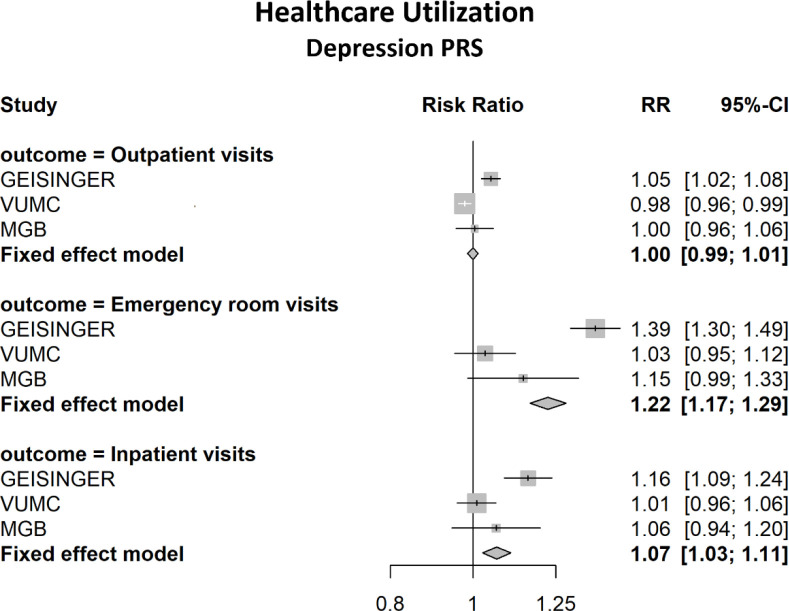
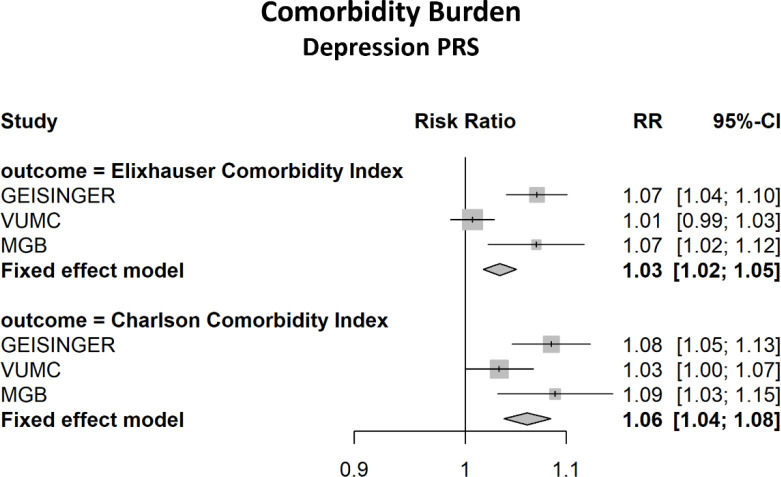
A total of 111,991 biobank participants were included in the MDD PGS analysis, and 118,882 in the SCZ and BD PGS analyses. The majority were female (53%–58%) and median duration in the EHR was at least 12 years in all sites (see Table 1). Additional summary information is presented in Table 1 and Supplement Table S2. Table 2 reports the meta-analysis results for the association between the MDD PGS and healthcare utilization and comorbidity burden. The yearly average number of ED visits was increased by 22.4% among those in the highest decile of MDD PRS compared to the lowest decile (RR=1.22, 95% CI: 1.17, 1.29). There was a 6.6% increase in yearly average number of ED visits for each standard deviation (SD) increase in the MDD PGS. Increases were observed in IP visits, ECI and CCI, however these effects were small and clinically not significant (see Figures 1 and 2). The SCZ and BD PGS were not significantly associated with comorbidity burden, however there was a very modest though significant association with the number of ED visits for both BD (RR = 1.02 (1.01, 1.03) and SCZ (RR = 1.04 (1.02, 1.04) per SD increase in PGS. (see Supplement Tables S3–S4). Additionally, we examined the association between MDD PGS and healthcare utilization following a diagnosis of depression. In this subgroup analysis, there was a total of 53,595 participants with depression across sites. Those in the highest PGS decile had a 55% increase in the average number of ED visits/year compared to the lowest decile (RR=1.56, 95% CI: 1.41, 1.72). Similarly, there was a 13% increase per standard deviation increase in the standardized PGS (RR=1.13, 95% CI: 1.10, 1.15). Statistically significant associations were also found with OP and IP visits post-diagnosis. (see Table 3).
Table 1:
Descriptive Statistics of Major Depressive Disorder Analysis Cohort
| Variable | GHS (N=51,505) | MGB (N=24,826) | VUMC (N=35,660) |
|---|---|---|---|
| Age, median (IQR)a | 58.94 (17.17)a | 61.77 (15.40) | 59.22 (16.59) |
| Female Sex, n (%)b | 30,072 (58.4%) | 9,804 (53.1%) | 20,686 (58.0%) |
| EHR Duration (years), median (IQR) | 14 (10, 19)b | 15 (9, 20) | 12 (8, 16) |
| Emergency Department Visits, median (IQR) | 1 (0, 3) | 0 (0, 2) | 0 (0, 1) |
| Inpatient Visits, median (IQR) | 0 (0, 1) | 5 (0, 16) | 1 (0, 2) |
| Outpatient Visits, median (IQR) | 52 (27, 88) | 160 (79, 286) | 63 (33, 116) |
| ECI, median (IQR) | 4 (2, 6) | 5 (3, 8) | 4 (2, 8) |
| CCI, median (IQR) | 1 (0, 2) | 2 (1, 3) | 2 (0, 3) |
categorial variables reported as n (%)
continuous variables reported as median (interquartile range)
Table 2:
Meta-Analysis Results for Depression Polygenic Score, Utilization and Comorbidity Burden
| Outcome | Standardized PRS | 10th vs. 1st Decile PRS |
|---|---|---|
| Emergency Department Visits | 1.07 (1.05, 1.08)a | 1.22 (1.17, 1.29) |
| Inpatient Visits | 1.02 (1.01, 1.02) | 1.07 (1.03, 1.11) |
| Outpatient Visits | 1.00 (1.00, 1.00) | 1.00 (0.99, 1.01) |
| ECI | 1.01 (1.01, 1.01) | 1.03 (1.02, 1.05) |
| CCI | 1.02 (1.01, 1.02) | 1.06 (1.04, 1.08) |
Adjusted Risk Ratio (95% CI) from a Negative Binomial regression model
Figure 1.
Meta-Analysis Results of the Association between 10th and 1st Decile of MDD Polygenic Score and Healthcare Utilization
Figure 2.
Meta-Analysis Results of the Association between 10th and 1st Decile of MDD Polygenic Score and Comorbidity Burden
Table 3:
Meta-Analysis Results for Depression Polygenic Score Post-Diagnosis of Depression
| Outcome | Standardized PRS | 10th vs. 1st Decile PRS |
|---|---|---|
| Emergency Department Visits | 1.13 (1.10, 1.15)a | 1.56 (1.41, 1.72) |
| Inpatient Visits | 1.04 (1.02, 1.06) | 1.23 (1.12, 1.36) |
| Outpatient Visits | 1.03 (1.02, 1.05) | 1.16 (1.08, 1.24) |
Adjusted Risk Ratio (95% CI) per SD increase in PGS from a Negative Binomial regression model
DISCUSSION
It is well-established that psychiatric PGS are robustly associated with psychiatric disorders and that they also exhibit cross-disorder associations (including with a range of non-psychiatric disorders). However, less is known about their role as indices of healthcare burden more generally. Here, we examined this issue by meta-analyzing data from EHR-linked biobanks across three large and geographically diverse healthcare systems in the PsycheMERGE network. Several notable findings emerged from these analyses. First, we found that a PGS for MDD was significantly (though modestly) associated with ED and inpatient visits as well as two widely used indices of comorbidity burden (ECI and CCI). These findings suggest that, in real-world settings, polygenic contributions to depression may be biomarkers for healthcare resource utilization and comorbidity burden, in the absence of a diagnose of depression. Following a depression diagnosis, the MDD PGS was more strongly associated with ED visit frequency and was also associated with outpatient and inpatient visit frequency, suggesting that increased genetic liability to depression may be a marker of adverse prognosis among affected individuals. In contrast, both the SCZ and BD PGS showed smaller associations with utilization and co-morbidity burden. The largest associations were with ED visits.
The link between MDD PGS and both healthcare visits and chronic disease comorbidity may reflect pleiotropic effects of genomic contributions to depression. Prior reports have linked genetic risk for depression with a range of medical conditions, including cardiovascular, metabolic, and immune-mediated disease32–35. Thus, individuals with greater genetic liability for depression might be expected to exhibit comorbidity with genetically correlated disorders for which they might also be more likely to seek care. Elevated healthcare resource utilization might also reflect a greater burden of subthreshold psychiatric and somatic symptoms that precede a formal depression diagnosis. We also find that utilization is even greater among those at higher levels of genetic risk after they receive a diagnosis of depression, suggesting that depression PGS may also be a marker of worsened course among these patients. It is unclear why similar associations were not observed with PGS for SCZ or BD since these disorders have also been found to be genetically correlated with a range of psychiatric and medical syndromes. Future studies in larger samples may help clarify whether this represented Type II error.
Our study has several strengths, including a large sample size of over 110,000 individuals with both EHR and genomic data, and a median of 12 years of longitudinal EHR data. To avoid identifying patients that were screened for mental health we used the rule of the presence of a code on two or more OP visits or a code on an IP or ED visit to define the diagnosis of SMI. The three participating institutions also represent diverse patient populations in terms of both race/ethnicity as well as urban/rural representation.
The study also has several limitations. We cannot accurately ascertain onset of mental health conditions as patients might have had these conditions but not have been diagnosed, thus some healthcare visits may have occurred after the development of conditions of interest.
PGS for MDD was found to be modestly associated with increased healthcare utilization and comorbidity burden, in the absence of diagnosis. Our study also identified associations between MDD PGS and utilization following a diagnosis of depression. These findings suggest that depression genetic risk is associated with utilization and burden of chronic disease in real-world settings.
Supplementary Material
Acknowledgements
Supported by NIMH grant R01MH118233 (to Drs. Smoller and Davis).
Footnotes
Conflict of Interest:
JWS is a member of the Scientific Advisory Board of Sensorium Therapeutics (with equity), and has received grant support from Biogen, Inc. He is PI of a collaborative study of the genetics of depression and bipolar disorder sponsored by 23andMe for which 23andMe provides analysis time as in-kind support but no payments.
REFERENCES
- 1.Sporinova B, Manns B, Tonelli M, et al. Association of Mental Health Disorders With Health Care Utilization and Costs Among Adults With Chronic Disease. JAMA Netw Open. Aug 2 2019;2(8):e199910. doi: 10.1001/jamanetworkopen.2019.9910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon GE, Unutzer J. Health care utilization and costs among patients treated for bipolar disorder in an insured population. Psychiatr Serv. Oct 1999;50(10):1303–8. doi: 10.1176/ps.50.10.1303 [DOI] [PubMed] [Google Scholar]
- 3.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. Mar 2002;25(3):464–70. doi: 10.2337/diacare.25.3.464 [DOI] [PubMed] [Google Scholar]
- 4.Bock JO, Luppa M, Brettschneider C, et al. Impact of depression on health care utilization and costs among multimorbid patients--from the MultiCare Cohort Study. PLoS One. 2014;9(3):e91973. doi: 10.1371/journal.pone.0091973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tusa N, Koponen H, Kautiainen H, et al. The profiles of health care utilization among a non-depressed population and patients with depressive symptoms with and without clinical depression. Scand J Prim Health Care. Sep 2019;37(3):312–318. doi: 10.1080/02813432.2019.1639904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakharkar P, Mai T. Co-Occurring Depression and Associated Healthcare Utilization and Expenditure in Individuals with Respiratory Condition: A Population-Based Study. Pharmacy (Basel). Sep 25 2021;9(4)doi: 10.3390/pharmacy9040157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PH, Feng YA, Smoller JW. Pleiotropy and Cross-Disorder Genetics Among Psychiatric Disorders. Biol Psychiatry. Jan 1 2021;89(1):20–31. doi: 10.1016/j.biopsych.2020.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehby GL, Domingue BW, Wolinsky FD. Genetic Risks for Chronic Conditions: Implications for Long-term Wellbeing. J Gerontol A Biol Sci Med Sci. Mar 14 2018;73(4):477–483. doi: 10.1093/gerona/glx154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis J, Sealock J, Levinson RT, et al. Genetic risk for major depressive disorder and loneliness in sex-specific associations with coronary artery disease. Mol Psychiatry. Aug 2021;26(8):4254–4264. doi: 10.1038/s41380-019-0614-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhar AK, Barton DA. Depression and the Link with Cardiovascular Disease. Front Psychiatry. 2016;7:33. doi: 10.3389/fpsyt.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheutlin AB, Dennis J, Karlsson Linner R, et al. Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia in 106,160 Patients Across Four Health Care Systems. Am J Psychiatry. Oct 1 2019;176(10):846–855. doi: 10.1176/appi.ajp.2019.18091085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer MS, Miller CJ, Kim B, et al. Effectiveness of Implementing a Collaborative Chronic Care Model for Clinician Teams on Patient Outcomes and Health Status in Mental Health: A Randomized Clinical Trial. JAMA Netw Open. Mar 1 2019;2(3):e190230. doi: 10.1001/jamanetworkopen.2019.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer MS, Weaver K, Kim B, et al. The Collaborative Chronic Care Model for Mental Health Conditions: From Evidence Synthesis to Policy Impact to Scale-up and Spread. Med Care. Oct 2019;57 Suppl 10 Suppl 3(10 Suppl 3):S221–S227. doi: 10.1097/MLR.0000000000001145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray GK, Lin T, Austin J, McGrath JJ, Hickie IB, Wray NR. Could Polygenic Risk Scores Be Useful in Psychiatry?: A Review. JAMA Psychiatry. Feb 1 2021;78(2):210–219. doi: 10.1001/jamapsychiatry.2020.3042 [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. Nov 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 17.Karlson EW, Boutin NT, Hoffnagle AG, Allen NL. Building the Partners HealthCare Biobank at Partners Personalized Medicine: Informed Consent, Return of Research Results, Recruitment Lessons and Operational Considerations. J Pers Med. Jan 14 2016;6(1)doi: 10.3390/jpm6010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danciu I, Cowan JD, Basford M, et al. Secondary use of clinical data: the Vanderbilt approach. J Biomed Inform. Dec 2014;52:28–35. doi: 10.1016/j.jbi.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey DJ, Fetterolf SN, Davis FD, et al. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med. Sep 2016;18(9):906–13. doi: 10.1038/gim.2015.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu P, Gifford A, Meng X, et al. Mapping ICD-10 and ICD-10-CM Codes to Phecodes: Workflow Development and Initial Evaluation. JMIR Med Inform. Nov 29 2019;7(4):e14325. doi: 10.2196/14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro VM, Minnier J, Murphy SN, et al. Validation of electronic health record phenotyping of bipolar disorder cases and controls. Am J Psychiatry. Apr 2015;172(4):363–72. doi: 10.1176/appi.ajp.2014.14030423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. Mar 2019;22(3):343–352. doi: 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ripke SW J. T. R.; Donovan M. C. Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. MedRxiv. 2020;2020.09.12.20192922doi: 10.1101/2020.09.12.20192922 [DOI] [Google Scholar]
- 24.Stahl EA, Breen G, Forstner AJ, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. May 2019;51(5):793–803. doi: 10.1038/s41588-019-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. Jun 2021;53(6):817–829. doi: 10.1038/s41588-021-00857-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. Apr 16 2019;10(1):1776. doi: 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiber CZ A. Visualizing count data regressions using rootograms. The American Statistician. 2016;70(3):296–303. doi: 10.1080/00031305.2016.1173590 [DOI] [Google Scholar]
- 29.Team RC. R: A language and environment for statistical computing. Vienna, Austra: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 30.countreg: Count Data Regression. 2020. http://R-Forge.R-project.org/projects/countreg/ [Google Scholar]
- 31.Venables; WN RB. Modern Applied Statistics with S. Fourth ed. Springer; 2022. [Google Scholar]
- 32.Wong BC, Chau CK, Ao FK, et al. Differential associations of depression-related phenotypes with cardiometabolic risks: Polygenic analyses and exploring shared genetic variants and pathways. Depress Anxiety. Apr 2019;36(4):330–344. doi: 10.1002/da.22861 [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Wang Z, Georgakis MK, Lin H, Zheng L. Genetic Liability to Depression and Risk of Coronary Artery Disease, Myocardial Infarction, and Other Cardiovascular Outcomes. J Am Heart Assoc. Jan 5 2021;10(1):e017986. doi: 10.1161/JAHA.120.017986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wassertheil-Smoller S, Qi Q, Dave T, et al. Polygenic Risk for Depression Increases Risk of Ischemic Stroke: From the Stroke Genetics Network Study. Stroke. Mar 2018;49(3):543–548. doi: 10.1161/STROKEAHA.117.018857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glanville KP, Coleman JRI, O’Reilly PF, Galloway J, Lewis CM. Investigating Pleiotropy Between Depression and Autoimmune Diseases Using the UK Biobank. Biol Psychiatry Glob Open Sci. Jun 2021;1(1):48–58. doi: 10.1016/j.bpsgos.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.