Abstract
Background
The efficacy of adjuvant therapy for patients with cervical cancer with intermediate risk (CC‐IR) remains controversial. We examined the impact of adjuvant therapy on survival outcomes in patients with CC‐IR and evaluated the heterogeneous treatment effects (HTEs) of adjuvant therapies based on clinicopathologic characteristics.
Methods
We retrospectively analyzed a previous Japanese nationwide cohort of 6192 patients with stage IB–IIB cervical cancer who underwent radical hysterectomy. We created two pairs of propensity score‐matched treatment/control groups to investigate the treatment effects of adjuvant therapies: (1) adjuvant therapy versus non‐adjuvant therapy; (2) chemotherapy versus radiotherapy conditional on adjuvant therapy. Multivariate analyses with treatment interactions were performed to evaluate the HTEs.
Results
Among the 1613 patients with CC‐IR, 619 and 994 were in the non‐treatment and treatment groups, respectively. Survival outcomes did not differ between the two groups: 3‐year progression‐free survival (PFS) rates were 88.1% and 90.3% in the non‐treatment and treatment groups, respectively (p = 0.199). Of the patients in the treatment group, 654 and 340 received radiotherapy and chemotherapy, respectively. Patients who received chemotherapy had better PFS than those who received radiotherapy (3‐year PFS, 90.9% vs. 82.9%, p = 0.010). Tumor size was a significant factor that affected the treatment effects of chemotherapy; patients with large tumors gained better therapeutic effects from chemotherapy than those with small tumors.
Conclusion
Adjuvant therapy is optional for some patients with CC‐IR; however, chemotherapy can be recommended as adjuvant therapy, particularly for patients with large tumors.
Keywords: adjuvant chemotherapy, adjuvant radiotherapy, cervical cancer, cohort studies, propensity score, treatment outcome
1. INTRODUCTION
Cervical cancer is a common gynecologic malignancy worldwide. In Japan, 10,879 women were newly diagnosed with cervical cancer in 2019, and 2887 patients died of the disease in 2020. 1 , 2 Standard therapeutics for International Federation of Gynaecology and Obstetrics (FIGO) 2018 stage IB–IIA cervical cancer are radical hysterectomy and definitive radiation‐based therapies. 3 Based on the postoperative pathological diagnosis, patients are categorized into three groups according to recurrence risk: high‐risk with lymph node metastasis (LNM) or parametrium invasion; intermediate‐risk with deep interstitial invasion, large size, or lymphovascular space invasion (LVSI); and low‐risk without any recurrence risk. 4 For patients in the high‐risk group, adjuvant chemoradiotherapy (CRT) is recommended to prevent recurrence. 5 , 6 However, adjuvant radiation‐based therapies sometimes cause severe life‐threatening toxicities compared to surgery alone. 6 , 7 Therefore, adjuvant chemotherapies have been attracting attention as alternative strategies to radiation‐based adjuvant therapies. 8 , 9 Currently, a randomized Phase III trial comparing adjuvant chemotherapy and CRT in high‐risk group patients is being conducted in Japan. 8 However, not only types of adjuvant therapy but the indications of adjuvant therapies for patients with cervical cancer with intermediate‐risk (CC‐IR) group remain controversial. 7 , 9 , 10 , 11 Based on a prospective randomized control trial from the Gynecologic Oncology Group, adjuvant radiotherapy significantly improved progression‐free survival (PFS), whereas a significant impact on overall survival (OS) was not confirmed. 7 Particularly, the prognoses of patients with CC‐IR worsened as the number of risk factors increased. 12 , 13 Moreover, a previous study demonstrated that pelvic radiotherapy improved the survival outcomes of patients with CC‐IR with two or more risk factors. 14 According to Japanese clinical practice guidelines for cervical cancer, adjuvant therapies are proposed based on recurrence risk factors 15 ; however, the indications and types of adjuvant therapies for CC‐IRs differ among institutions. 16 , 17
Due to the wide variety of patient and tumor characteristics observed in CC‐IR, the effectiveness of adjuvant therapy might vary among patients. Therefore, we focused on the heterogeneous treatment effects (HTEs) of adjuvant therapies in patients with CC‐IR. HTE analysis focuses on investigating different treatment effects in individuals or subgroups in a population. 18 , 19 , 20 Therefore, by conducting a HTE analysis, it may become possible to identify CC‐IR subgroups that benefit from adjuvant therapies. This study aimed to examine the impact of adjuvant therapies on the survival outcomes of patients with CC‐IR and identify groups that particularly benefit from adjuvant therapies using an HTE analysis. Simultaneously, we also focused on the HTEs of chemotherapy compared with radiation‐based therapies.
2. METHODS
2.1. Eligibility
This study was a secondary analysis of the Japanese Gynecologic Oncology Group (JGOG) dataset (JGOG1072S) generated by a previous study investigating the prognosis of cervical cancer patients who received radical hysterectomy. 13 , 21 The JGOG1072S study was a nationwide, large‐scale retrospective observational study conducted in 116 JGOG‐designated institutions. The survey collected consecutive cases of FIGO 2008 stage IB–IIB cervical cancer treated with primary radical hysterectomy between January 1, 2004, and December 31, 2008. The survey period for data collection was from October 1, 2012, to February 28, 2013. Institutional review board approval was obtained from Tottori University, which served as the host institution, and the JGOG‐participating institutions reviewed the protocol.
From the JGOG1072S database, we extracted patients with CC‐IR based on the Japanese Society of Gynecologic Oncology guidelines as follows. 15 First, we excluded patients with distant metastasis, those who received neoadjuvant chemotherapy, and those with incomplete data. CC‐IR was defined as follows: cervical cancer without pathological LNM or parametrium infiltration and with one or more recurrent risk factors, including positive LVSI, large size (≥4 cm), and deep stromal invasion to the outer half (excluding low‐risk cases). Institutional review board approval for this study was obtained from the University of Tokyo (approval number: 2021078NI‐2) and Tokyo Metropolitan Komagome Hospital (approval number: 2749). The institutional review board granted an opt‐out recruitment approach and waived the need for obtaining written informed consent from each patient. We adhered to the Declaration of Helsinki.
2.2. Variables
The data collected included age, institution, pathology, tumor size, the presence or absence of LNM, parametrial tumor involvement, stromal invasion to the outer half, LVSI, uterine corpus invasion, vaginal invasion, PFS and OS rates, and the context of adjuvant therapy. Age was categorized as <40, 40 to <50, 50 to <60, 60 to <70, and ≥70 years; tumor size was categorized as <2 cm, 2 to <4 cm, and ≥4 cm. The pathological information consisted of histological evaluation (squamous cell carcinoma [SCC] or non‐SCC). Institutions were categorized into two groups: high‐volume and non‐high‐volume centers. High‐volume centers were defined as the top 10 institutions in terms of registered cases since the number of enrollments at these institutions accounted for one fourth of the total enrollment.
Patients with CC‐IR were categorized into subgroups according to the presence or absence of adjuvant therapy, namely, treatment and non‐treatment (control) (Figure 1A). Patients in the treatment subgroup were further classified based on the type of adjuvant therapy; those who underwent radiotherapy‐based therapies, including CRT and radiotherapy, were categorized into the radiotherapy subgroup (control), while those who underwent chemotherapy were categorized into the chemotherapy subgroup (Figure 1B). None of the patients in the chemotherapy subgroup received radiation‐based therapy in their primary cervical cancer treatment.
FIGURE 1.
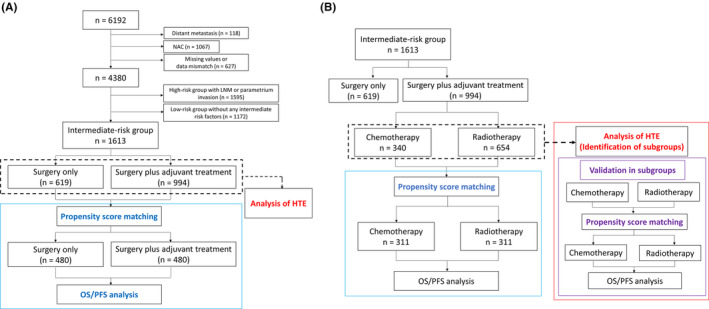
Flowchart of study selection and statistical analyses. (A) Comparison between the treatment and non‐treatment groups in patients with cervical cancer with intermediate risks. (B) Comparison between the radiation and chemotherapy groups in those in the treatment group. HTE, heterogeneous treatment effect; LNM, lymph node metastasis; NAC, neoadjuvant chemotherapy; OS, overall survival; PFS, progression‐free survival.
2.3. Statistical analyses
The Kaplan–Meier method was used in each subgroup to estimate PFS and OS following propensity score matching. Propensity scores, including the probability of receiving adjuvant treatment among patients with CC‐IR and the probability of receiving chemotherapy among the treatment groups, were estimated using logistic regression based on all clinicopathologic characteristics previously presented. Matching was performed using the “Matching” package in R (R Foundation for Statistical Computing). 22 Standardized differences for the covariates were calculated to assess the comparability of the matched cohorts; a standardized difference <0.1 was considered to support the assumption of balance between the cohorts. The statistical difference between the curves was determined for each comparison using a log‐rank test.
HTEs were estimated using Cox proportional hazard regression models that considered interactions between treatment indicators and each covariate, as previously reported. 18 Statistically significant coefficients of the interaction terms demonstrated the heterogeneous impact of adjuvant therapy on survival outcomes.
First, PFS and OS were compared between the treatment and non‐treatment groups using a propensity score‐matched cohort (treatment vs. non‐treatment) (Figure 1A). Second, a Cox proportional hazard regression model that considered the interaction with adjuvant therapy (treatment) was used to evaluate the HTEs of adjuvant therapy (Figure 1A).
PFS and OS were compared between the chemotherapy and radiotherapy groups to evaluate therapeutic effects according to the type of adjuvant therapy (Figure 1B). Subsequently, a Cox proportional hazard regression model that considered the interaction with adjuvant treatments was used to evaluate the HTEs of chemotherapy (treatment) compared with radiotherapy (control). After identifying subgroups in which chemotherapy was more (or less) effective than radiotherapy, background characteristics were adjusted between the chemotherapy and radiotherapy groups by propensity score matching (Figure 1B). OS and PFS were compared between the chemotherapy and radiotherapy groups in each subgroup. Statistical significance was defined as p < 0.05. All analyses were conducted using R (version 4.2.0).
3. RESULTS
3.1. Comparison between the treatment and non‐treatment groups
Among the 6192 patients in the original cohort, 1613 were enrolled in this analysis (Figure 1A). Among them, 619 patients did not receive any adjuvant therapy (non‐treatment group), whereas 994 patients received either radiotherapy‐based adjuvant treatments or chemotherapy (treatment group) after hysterectomy. The baseline characteristics of the patients before and after matching are presented in Table S1. Indications for adjuvant therapies were based on recurrence risk factors, including positive LVSI, large size (≥4 cm), and deep stromal invasion to the outer half. Other factors, including uterine corpus invasion and vaginal invasion, also affected the indications for adjuvant therapy. Of the 1613 patients with CC‐IR, 480 non‐treatment patients were matched with 480 treatment patients (Figure 1A). For all covariates, the absolute standardized difference was <0.1 after matching, suggesting sufficiently balanced treatment and non‐treatment groups (Table S1).
Kaplan–Meier curves based on adjuvant treatment after propensity score matching are shown in Figure 2A,B. Although patients with adjuvant treatment tended to have better PFS than those without adjuvant treatment, no significant differences were observed between the two groups: 3‐year PFS rates were 88.1% and 90.3% (p = 0.199, Figure 2A) and 5‐year OS rates were 94.2% and 94.0% in the non‐treatment and treatment groups, respectively (p = 0.627, Figure 2B).
FIGURE 2.
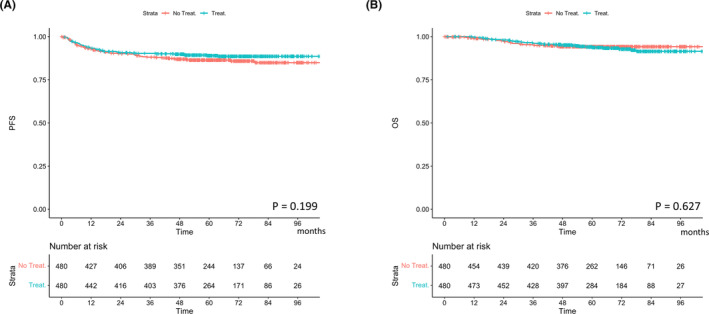
Cumulative incidence curves for (A) recurrence and (B) cervical cancer death based on the presence or absence of adjuvant treatment. OS, overall survival; PFS, progression‐free survival.
Subsequently, we conducted a HTE analysis to identify subgroups that could have a better prognosis with adjuvant treatment (Table 1A,B). “Baseline (non‐treatment)” represents the impact of each factor on patient prognosis in the absence of adjuvant treatment (non‐treatment group), and the “interaction term” represents changes in prognosis by adding adjuvant treatment with or without each factor. Therefore, a positive (negative) exponentiated coefficient for the interaction term of a factor could be interpreted as evidence of the factor's enhancing (diminishing) effects on the impact of adjuvant treatment on OS or PFS. The “baseline” OS and PFS were better for patients with SCC than for those with non‐SCC. On the other hand, the treatment effect of adding adjuvant treatment (shown as “interaction term”) did not change with or without each factor (Table 1A,B).
TABLE 1.
Heterogeneous treatment effect of adjuvant therapy after surgery.
| Variables | Baseline (non‐treatment) | Interaction terms | ||||
|---|---|---|---|---|---|---|
| Exp(coef) | 95% CI | p‐Values | Exp(coef) | 95% CI | p‐Values | |
| A. Overall survival | ||||||
| Age | ||||||
| 40 to <50 years | 0.527 | 0.157–1.770 | 0.300 | 1.246 | 0.316–4.914 | 0.752 |
| 50 to <60 years | 1.127 | 0.394–3.225 | 0.822 | 0.861 | 0.256–2.897 | 0.810 |
| 60 to <70 years | 1.179 | 0.348–3.990 | 0.791 | 1.009 | 0.251–4.041 | 0.989 |
| ≥70 years | 2.600 | 0.168–0.667 | 0.168 | 0.195 | 0.017–2.217 | 0.187 |
| Histology SCC | 0.426 | 0.193–0.942 | 0.035 | 1.365 | 0.545–3.419 | 0.506 |
| Size | ||||||
| 2 to <4 cm | 2.423 | 0.678–8.659 | 0.173 | 0.632 | 0.133–2.990 | 0.562 |
| ≥4 cm | 1.510 | 0.338–6.739 | 0.588 | 1.764 | 0.308–10.09 | 0.523 |
| LVSI positive | 1.412 | 0.586–3.401 | 0.441 | 1.998 | 0.648–6.158 | 0.227 |
| Cervical stromal invasion ≥1/2 | 1.555 | 0.632–3.821 | 0.335 | 0.833 | 0.288–2.408 | 0.736 |
| Uterine body invasion | 1.981 | 0.634–6.189 | 0.239 | 0.556 | 0.149–2.070 | 0.381 |
| Vaginal invasion | 1.993 | 0.774–5.134 | 0.152 | 0.706 | 0.241–2.063 | 0.524 |
| High‐volume center | 0.578 | 0.238–1.401 | 0.225 | 0.289 | 0.476–3.742 | 0.582 |
| B. Progression‐free survival | ||||||
| Age | ||||||
| 40 to <50 years | 0.615 | 0.306–1.235 | 0.172 | 1.923 | 0.836–4.422 | 0.124 |
| 50 to <60 years | 1.101 | 0.567–2.137 | 0.776 | 0.935 | 0.411–2.124 | 0.872 |
| 60 to <70 years | 1.765 | 0.887–3.515 | 0.106 | 0.692 | 0.288–1.659 | 0.409 |
| ≥70 years | 1.699 | 0.628–4.598 | 0.297 | 0.537 | 0.114–2.536 | 0.433 |
| Histology SCC | 0.534 | 0.327–0.873 | 0.012 | 1.054 | 0.579–1.920 | 0.863 |
| Size | ||||||
| 2 to <4 cm | 2.516 | 1.153–5.489 | 0.020 | 0.738 | 0.262–2.078 | 0.565 |
| ≥ 4 cm | 2.893 | 1.230–6.805 | 0.015 | 0.789 | 0.262–2.375 | 0.673 |
| LVSI positive | 1.345 | 0.309–0.939 | 0.2899 | 1.266 | 0.634–2.529 | 0.504 |
| Cervical stromal invasion ≥1/2 | 1.156 | 0.688–1.943 | 0.583 | 0.855 | 0.444–1.644 | 0.638 |
| Uterine body invasion | 1.389 | 0.635–3.042 | 0.411 | 0.992 | 0.396–2.487 | 0.987 |
| Vaginal invasion | 1.828 | 1.009–3.309 | 0.046 | 0.813 | 0.403–1.640 | 0.563 |
| High‐volume center | 0.539 | 0.309–0.939 | 0.029 | 1.066 | 0.529–2.145 | 0.859 |
Note: A. Overall survival; B. Progression‐free survival. “Baseline (non‐treatment)” represents the impact of each factor on patient prognosis in the absence of adjuvant treatment (non‐treatment group), and the “interaction term” represents changes in prognosis by adding adjuvant treatment with or without each factor. Therefore, a positive (negative) exponentiated coefficient for the interaction term of a factor could be interpreted as evidence of the factor's enhancing (diminishing) effects on the impact of adjuvant treatment on overall survival.
Abbreviations: CI, confidence interval; Exp(coef), exponentiated coefficient; LVSI, lymphovascular space invasion; SCC, squamous cell carcinoma.
Next, we compared the rates and types of adjuvant therapy based on the type of institution. No difference was found between high‐volume and non‐high‐volume centers in the rates or types of adjuvant therapies (Figure S1). Among the high‐volume centers, the frequency of adjuvant therapy differed between the institutions (Figure S2); however, at least 30% of the patients received adjuvant therapy in all institutions.
As previous studies have demonstrated that the prognosis of patients with CC‐IR worsens according to the number of recurrence risk factors, we subsequently analyzed the frequency of adjuvant therapy based on the number of recurrence risk factors. The frequency of adjuvant therapy increased with the number of recurrence risk factors: 43.5%, 74.8%, and 86.2% in those with one, two, and three risk factors, respectively (Figure S3). In the matched cohort, 61% of the patients had one risk factor (tumor size, interstitial invasion, or LVSI), whereas the other 39% had two or more risk factors (Figure S4A,B).
3.2. Comparison between the chemotherapy and radiotherapy groups in the treatment group
Because most patients with more than two recurrent risk factors received adjuvant therapy, we next focused on the effect of chemotherapy on CC‐IR compared with radiotherapy‐based treatment. Of the 994 patients with CC‐IR in the treatment group, 654 and 340 received radiotherapy and chemotherapy, respectively. More than half of the regimens were taxane–platinum combinations, such as paclitaxel plus carboplatin or cisplatin (Table S2). Patient background characteristics were compared between the chemotherapy and radiotherapy groups (Table S3). Patients with non‐SCC histology tended to receive adjuvant chemotherapy rather than radiotherapy. In contrast, those with larger tumor sizes, uterine body invasion, and vaginal invasion tended to receive radiotherapy rather than chemotherapy.
Of the 994 patients in the treatment group, 311 patients in the chemotherapy group were matched with 311 patients in the radiotherapy group (Figure 1B). For all covariates, the absolute standardized difference was <0.1 after matching, suggesting sufficiently balanced chemotherapy and radiotherapy groups (Table S3). In both groups, 62% of the patients had two or more risk factors (Figure S5A,B); this proportion was significantly higher than that in the non‐treatment and treatment groups (39%).
Kaplan–Meier curves of PFS and OS of patients who received chemotherapy and radiotherapy are shown in Figure 3A,B. Patients in the chemotherapy group had better PFS and OS than those in the radiotherapy group: 3‐year PFS rates were 90.9% and 82.9%, respectively (p = 0.010), and 5‐year OS rates were 95.5% and 90.3%, respectively (p = 0.041).
FIGURE 3.
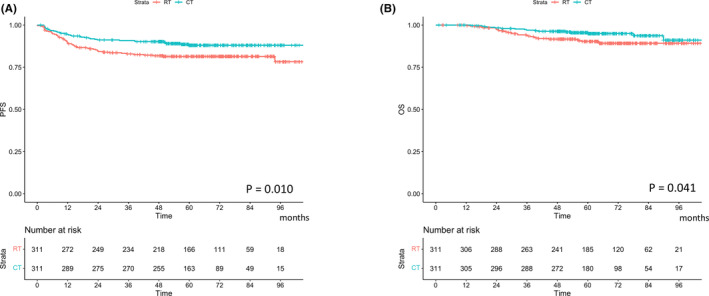
Cumulative incidence curves for (A) recurrence and (B) cervical cancer death based on the types of adjuvant therapy. OS, overall survival; PFS, progression‐free survival.
Subsequently, we conducted an HTE analysis to identify subgroups that could have a better prognosis with adjuvant chemotherapy than with adjuvant radiotherapy‐based treatments (Table 2A,B). “Baseline (radiation)” represents the impact of each factor on the prognosis of patients with adjuvant radiotherapy, and the “interaction term” represents changes in prognosis with adjuvant chemotherapy (compared with adjuvant radiotherapy) with or without each factor. The “baseline” PFS was better for patients with SCC or small tumors than for those with non‐SCC or large tumors. However, only tumor size significantly affected the treatment effect of adjuvant chemotherapy; the treatment effect of adjuvant chemotherapy was higher in patients with large tumors than that of adjuvant radiotherapy.
TABLE 2.
Heterogeneous treatment effect of adjuvant chemotherapy.
| Variables | Baseline (radiation) | Interaction terms | ||||
|---|---|---|---|---|---|---|
| Exp(coef) | 95% CI | p‐Values | Exp(coef) | 95% CI | p‐Values | |
| A. Overall survival | ||||||
| Age | ||||||
| 40 to <50 years | 0.553 | 0.259–1.182 | 0.127 | 1.684 | 0.383–7.415 | 0.491 |
| 50 to <60 years | 0.886 | 0.435–1.808 | 0.74 | 1.346 | 0.33–5.482 | 0.678 |
| 60 to <70 years | 0.98 | 0.455–2.11 | 0.959 | 1.8 | 0.387–8.38 | 0.454 |
| ≥70 years | 0.585 | 0.077–4.445 | 0.604 | 1.34 × 10−6 | n.c | 0.995 |
| Histology SCC | 0.581 | 0.33–1.023 | 0.06 | 0.726 | 0.236–2.233 | 0.576 |
| Size | ||||||
| 2 to <4 cm | 2.023 | 0.596–6.86 | 0.258 | 0.434 | 0.069–2.734 | 0.374 |
| ≥4 cm | 2.962 | 0.869–10.1 | 0.083 | 0.831 | 0.128–5.411 | 0.846 |
| LVSI positive | 2.16 | 1.009–4.623 | 0.047 | 4.239 | 0.484–37.13 | 0.192 |
| Cervical stromal invasion ≥1/2 | 1.255 | 0.635–2.481 | 0.513 | 1.116 | 0.323–3.856 | 0.862 |
| Uterine body invasion | 0.1.344 | 0.646–2.797 | 0.429 | 0.419 | 0.074–2.368 | 0.325 |
| Vaginal invasion | 1.471 | 0.833–2.596 | 0.184 | 0.527 | 0.129–2.142 | 0.37 |
| High‐volume center | 0.766 | 0.425–1.379 | 0.374 | 0.703 | 0.175–2.825 | 0.619 |
| B. Progression‐free survival | ||||||
| Age | ||||||
| 40 to <50 years | 1.022 | 0.59–1.768 | 0.939 | 1.484 | 0.549–4.012 | 0.436 |
| 50 to <60 years | 0.981 | 0.547–1.76 | 0.949 | 1.109 | 0.384–3.204 | 0.848 |
| 60 to <70 years | 1.289 | 0.698–2.38 | 0.417 | 0.614 | 0.163–2.317 | 0.472 |
| ≥ 70 years | 0.73 | 0.172–3.104 | 0.67 | 2.63 | 0.193–35.85 | 0.468 |
| Histology SCC | 0.509 | 0.335–0.773 | 0.002 | 0.973 | 0.439–2.158 | 0.947 |
| Size | ||||||
| 2 to <4 cm | 3.799 | 1.166–12.38 | 0.027 | 0.214 | 0.048–0.946 | 0.042 |
| ≥ 4 cm | 4.631 | 1.411–15.2 | 0.011 | 0.207 | 0.044–0.985 | 0.048 |
| LVSI positive | 1.356 | 0.829–2.217 | 0.226 | 2.727 | 0.848–8.774 | 0.092 |
| Cervical stromal invasion ≥1/2 | 0.918 | 0.566–1.489 | 0.729 | 1.42 | 0.593–3.401 | 0.431 |
| Uterine body invasion | 1.6 | 0.943–2.715 | 0.082 | 0.384 | 0.098–1.515 | 0.172 |
| Vaginal invasion | 1.479 | 0.966–2.263 | 0.072 | 0.633 | 0.236–1.693 | 0.362 |
| High‐volume center | 0.649 | 0.407–1.035 | 0.07 | 0.37 | 0.102–1.337 | 0.129 |
Note: A. Overall survival; B. Progression‐free survival. “Baseline (radiation)” represents the impact of each factor on the prognosis of patients with adjuvant radiotherapy, and the “interaction term” represents changes in prognosis with adjuvant chemotherapy (compared with adjuvant radiotherapy) with or without each factor. Therefore, a positive (negative) exponentiated coefficient for the interaction term of a factor could be interpreted as evidence of the factor's enhancing (diminishing) effects on the impact of adjuvant chemotherapy on overall survival and progression‐free survival.
Abbreviations: CI, confidence interval; Exp(coef), exponentiated coefficient; LVSI, lymphovascular space invasion; SCC, squamous cell carcinoma.
Subgroup analysis was conducted after propensity score matching in each subgroup to validate the treatment effect of chemotherapy compared with that of radiotherapy (Figure S6A,B). The background characteristics of patients with small tumor sizes (<2 cm) and large tumor sizes (≥2 cm) are summarized in Table S4A,B. After propensity score matching in each subgroup, the absolute standardized difference for all covariates except for age distribution was <0.1 after matching. In patients with large tumor sizes (≥2 cm), those who received chemotherapy had better PFS and OS than those who received radiotherapy‐based treatments: 3‐year PFS rates were 90.0% and 81.4% in the chemotherapy and radiotherapy‐based therapy groups, respectively (p = 0.003, Figure 4A), and 5‐year OS rates were 94.7% and 89.2%, respectively (p = 0.049, Figure 4B). In contrast, no difference was observed between the two groups in patients with small tumors (<2 cm): 3‐year PFS rates were 92.5% and 92.6% in the chemotherapy and radiotherapy‐based therapy groups, respectively (p = 0.441, Figure 4C), and 5‐year OS rates were 97.4% and 96.8%, respectively (p = 0.945, Figure 4D).
FIGURE 4.
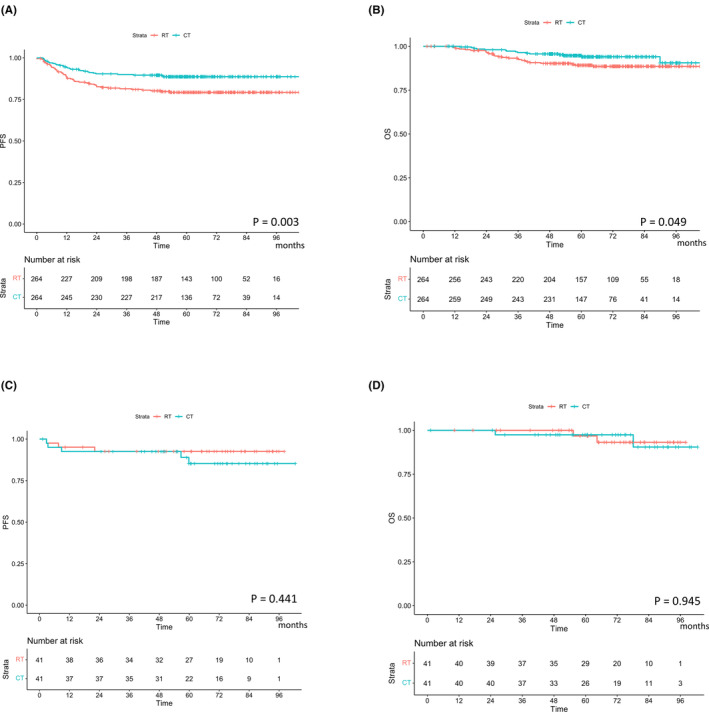
Cumulative incidence curves for recurrence and cervical cancer death based on the types of adjuvant therapy according to tumor sizes. (A) Recurrence in patients with large tumors (≥2 cm); (B) death from cervical cancer in patients with large tumors (≥2 cm); (C) recurrence in patients with small tumors (<2 cm); (D) death from cervical cancer in patients with small tumors (<2 cm). OS, overall survival; PFS, progression‐free survival.
4. DISCUSSION
In this study, we analyzed the significance of adjuvant therapies for patients with CC‐IR using a nationwide cohort database. Due to adjuvant therapy's selection bias, it has been difficult to perform unbiased analyses using single‐center data. However, because of the differences in treatment selection (for both indications and types of adjuvant therapy) between facilities, using a nationwide cohort database decreases the bias.
In the first analysis, we compared survival outcomes between patients with CC‐IR with and without adjuvant treatment and revealed that adjuvant treatment had no significant treatment effects on survival. The treatment and non‐treatment groups had similar 3‐year PFS rates (88.1% and 90.3%, respectively), which are similar to the rates reported previously in the treatment group. 7 There are two possible reasons for the good survival rate even in the non‐treatment group. One reason is that relatively low‐risk patients were included in the propensity score‐matched cohort (treatment vs. non‐treatment); approximately 60% of the patients had only one risk factor. The other reason relates to the surgical procedure for radical hysterectomy in Japan. Okabayashi radical hysterectomy is widely used in Japan, which corresponds to Type III hysterectomy of the Gynecologic Cancer Group, and has sufficient efficacy for local disease control. 23
The indications and the type of adjuvant therapy depend on the institutions. Therefore, we could compare the treatment effects between the radiotherapy and chemotherapy groups. Our results indicated that patients with CC‐IR who underwent adjuvant chemotherapy had a better prognosis than those who received adjuvant radiotherapy, with 3‐year PFS rates of 90.9% and 82.9%, respectively, which is better than those of previous reports. 7 , 12 , 14 Furthermore, we investigated the HTEs of adjuvant chemotherapy compared with adjuvant radiotherapy and identified tumor size as the most influential factor for differential treatment effects between adjuvant chemotherapy and radiotherapy. Adjuvant chemotherapy was more effective than adjuvant radiotherapy in patients with large tumors. Patients with tumors measuring <2 cm had a good prognosis, regardless of the type of adjuvant therapy. Considering that approximately 80% of the patients with small tumors (<2 cm) had only one recurrent risk factor (Figure S7A,B), the treatment effects of adjuvant therapy (regardless of the type of adjuvant therapy) may be limited in these patients.
Notably, adjuvant chemotherapy prolonged OS and improved PFS in patients with cervical cancer. Only definitive radiotherapy‐ and surgical‐based therapies are recognized as definitive therapeutics for cervical cancer, 4 and chemotherapy‐based therapies are not as definitive as radiotherapy; however, paclitaxel plus platinum‐based chemotherapy regimens have sufficient efficacy with a response rate of 30%–62% for advanced or recurrent cervical cancers. 24 , 25 , 26 , 27 Therefore, chemotherapy might be an alternative adjuvant therapy to radiotherapy for patients with CC‐IR who underwent Okabayashi radical hysterectomy.
This study had some limitations. First, even when using propensity score matching, we were uncertain about the effect of unobserved factors on disease recurrence or OS. Particularly, information regarding patients' general condition that could affect both the indication for adjuvant therapy and survival, including their performance status and complications, may have improved comparability between the groups. Therefore, a prospective randomized controlled study is warranted to validate the results of this study. Second, because only approximately one fifth of patients with two or more recurrent risk factors were in the non‐treatment group, most of the patients in the matched analysis had only one risk factor, and a limited number of those with two or more risk factors were included. Therefore, indications for adjuvant therapy should be carefully determined for patients with multiple risk factors. However, this study is still important because it confirmed that adjuvant therapy has no treatment effects on PFS and OS in patients with CC‐IR (especially those with one risk factor) who are treated at Japanese hospitals. Third, this was a retrospective study with missing data. Some survival events could have been underestimated because of insufficient prognostic follow‐up and short observational periods.
In conclusion, adjuvant therapy had no therapeutic effect for some patients; therefore, adjuvant therapy is optional for patients with CC‐IR. However, these results should be applied with caution to patients with multiple risk factors. Regarding the type of adjuvant therapy, chemotherapy may be recommended for patients with CC‐IR, particularly those with large tumor sizes. Further prospective randomized controlled studies are warranted to confirm these results.
AUTHOR CONTRIBUTIONS
Ayumi Taguchi: Conceptualization (lead); data curation (equal); investigation (equal); visualization (equal); writing – original draft (lead). Kosuke Kato: Data curation (equal); formal analysis (lead); investigation (equal); visualization (equal). Konan Hara: Conceptualization (equal); methodology (lead); supervision (equal); writing – review and editing (equal). Akiko Furusawa: Project administration (equal); writing – review and editing (equal). Yujiro Nakajima: Data curation (supporting); formal analysis (supporting). Chihiro Ishizawa: Writing – review and editing (equal). Michihiro Tanikawa: Writing – review and editing (equal). Kenbun Sone: Writing – review and editing (equal). Mayuyo Mori: Supervision (supporting); writing – review and editing (equal). Muneaki Shimada: Data curation (equal); supervision (equal); writing – review and editing (equal). Aikou Okamoto: Supervision (equal). Munetaka Takekuma: Supervision (equal); writing – review and editing (equal).
FUNDING INFORMATION
No funding.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Institutional review board approval for this study was obtained from the University of Tokyo (approval number: 2021078NI‐2) and Tokyo Metropolitan Komagome Hospital (approval number: 2749).
PATIENT CONSENT
The institutional review board granted an opt‐out recruitment approach and waived the need for obtaining written informed consent. We adhered to the Declaration of Helsinki.
Supporting information
Figures S1–S7.
Tables S1–S4.
ACKNOWLEDGMENTS
The authors would like to thank all participants of the JGOG. We would also like to thank Editage for the English language editing.
Taguchi A, Kato K, Hara K, et al. Heterogeneous treatment effects of adjuvant therapy for patients with cervical cancer in the intermediate‐risk group. Cancer Med. 2023;12:18557‐18567. doi: 10.1002/cam4.6460
Ayumi Taguchi and Kosuke Kato contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, MT, upon reasonable request.
REFERENCES
- 1. National Cancer Registry (Ministry of Health, Labour and Welfare), tabulated by National Cancer Center, Japan, Cancer Information Service. Accessed September 1, 2022. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html
- 2. Vital Statistics in Japan, tabulated by National Cancer Center, Japan, Cancer Information Service [cited September 1 2022]. https://ganjoho.jp/reg_stat/statistics/stat/cancer/17_cervix_uteri.html
- 3. Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet. 1997;350(9077):535‐540. doi: 10.1016/S0140-6736(97)02250-2 [DOI] [PubMed] [Google Scholar]
- 4. Miller C, Elkas JC. Cervical and Vaginal Cancer. In: Berk JS, ed. Berek & Novak's Gynecology. 5th ed. Lippincott Williams & Wilkins; 2012:1304‐1349. [Google Scholar]
- 5. Rosa DD, Medeiros LR, Edelweiss MI, Pohlmann PR, Stein AT. Adjuvant platinum‐based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. 2012;6(6):CD005342. doi: 10.1002/14651858.CD005342.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606‐1613. doi: 10.1200/JCO.2000.18.8.1606 [DOI] [PubMed] [Google Scholar]
- 7. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73(2):177‐183. doi: 10.1006/gyno.1999.5387 [DOI] [PubMed] [Google Scholar]
- 8. Furusawa A, Takekuma M, Mori K, et al. A randomized phase III trial of adjuvant chemotherapy versus concurrent chemoradiotherapy for postoperative cervical cancer: Japanese Gynecologic Oncology Group study (JGOG1082). Int J Gynecol Cancer. 2021;31(4):623‐626. doi: 10.1136/ijgc-2020-002344 [DOI] [PubMed] [Google Scholar]
- 9. Matsuo K, Nusbaum DJ, Matsuzaki S, et al. Utilization and outcomes of adjuvant systemic chemotherapy alone in high risk, early stage cervical cancer in the United States. Int J Gynecol Cancer. 2021;31(7):991‐1000. doi: 10.1136/ijgc-2021-002655 [DOI] [PubMed] [Google Scholar]
- 10. Bilek K, Ebeling K, Leitsmann H, Seidel G. Radical pelvic surgery versus radical surgery plus radiotherapy for stage Ib carcinoma of the cervix uteri. Preliminary results of a prospective randomized clinical study. Arch Geschwulstforsch. 1982;52(3):223‐229. [PubMed] [Google Scholar]
- 11. Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev. 2012;5(5):CD007583. doi: 10.1002/14651858.CD007583.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sartori E, Tisi G, Chiudinelli F, La Face B, Franzini R, Pecorelli S. Early stage cervical cancer: adjuvant treatment in negative lymph node cases. Gynecol Oncol. 2007;107(1):S170‐S174. doi: 10.1016/j.ygyno.2007.07.026 [DOI] [PubMed] [Google Scholar]
- 13. Shigeta S, Shimada M, Tsuji K, et al. Risk assessment in the patients with uterine cervical cancer harboring intermediate risk factors after radical hysterectomy: a multicenter, retrospective analysis by the Japanese Gynecologic Oncology Group. Int J Clin Oncol. 2022;27(9):1507‐1515. doi: 10.1007/s10147-022-02198-6 [DOI] [PubMed] [Google Scholar]
- 14. Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow‐up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65(1):169‐176. doi: 10.1016/j.ijrobp.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 15. Ebina Y, Mikami M, Nagase S, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019;24(1):1‐19. doi: 10.1007/s10147-018-1351-y [DOI] [PubMed] [Google Scholar]
- 16. Ikeda Y, Furusawa A, Kitagawa R, et al. Practice patterns of adjuvant therapy for intermediate/high recurrence risk cervical cancer patients in Japan. J Gynecol Oncol. 2016;27(3):e29. doi: 10.3802/jgo.2016.27.e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mikami M, Aoki Y, Sakamoto M, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (bulky tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer. 2014;24(7):1333‐1340. doi: 10.1097/IGC.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 18. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189‐2194. doi: 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 19. Kaito S, Nakajima Y, Hara K, et al. Heterogeneous impact of cytomegalovirus reactivation on nonrelapse mortality in hematopoietic stem cell transplantation. Blood Adv. 2020;4(6):1051‐1061. doi: 10.1182/bloodadvances.2019000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baum A, Scarpa J, Bruzelius E, Tamler R, Basu S, Faghmous J. Targeting weight loss interventions to reduce cardiovascular complications of type 2 diabetes: a machine learning‐based post‐hoc analysis of heterogeneous treatment effects in the Look AHEAD trial. Lancet Diabetes Endocrinol. 2017;5(10):808‐815. doi: 10.1016/S2213-8587(17)30176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuo K, Shimada M, Yokota H, et al. Effectiveness of adjuvant systemic chemotherapy for intermediate‐risk stage IB cervical cancer. Oncotarget. 2017;8(63):106866‐106875. doi: 10.18632/oncotarget.22437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J Stat Softw. 2011;42(7):1‐52. doi: 10.18637/jss.v042.i07 [DOI] [Google Scholar]
- 23. Marin F, Plesca M, Bordea CI, Moga MA, Blidaru A. Types of radical hysterectomies: from Thoma Ionescu and Wertheim to present day. J Med Life. 2014;7(2):172‐176. [PMC free article] [PubMed] [Google Scholar]
- 24. Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open‐label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33(19):2129‐2135. doi: 10.1200/JCO.2014.58.4391 [DOI] [PubMed] [Google Scholar]
- 25. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin‐containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(28):4649‐4655. doi: 10.1200/JCO.2009.21.8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore KN, Herzog TJ, Lewin S, et al. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IVB, recurrent or persistent cervical cancer. Gynecol Oncol. 2007;105(2):299‐303. doi: 10.1016/j.ygyno.2006.12.031 [DOI] [PubMed] [Google Scholar]
- 27. Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734‐743. doi: 10.1056/NEJMoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S7.
Tables S1–S4.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MT, upon reasonable request.


