Abstract
Purpose
The prognostic factors for diffuse large B‐cell lymphoma (DLBCL) have been fully explored, but prognostic information for bulky mass DLBCL patients is limited. This study aimed to analyze the prognostic value of MYC protein expression and other biological parameters in bulky mass DLBCL patients.
Methods
We defined a bulky mass as a maximum tumor diameter ≥7.5 cm and studied 227 patients with de novo bulky mass DLBCL.
Results
In all patients with bulky mass DLBCL, the 1‐year and 3‐year OS rates were 72.7% and 57.1%, respectively, and the 1‐year and 3‐year PFS rates were 52.0% and 42.5%, respectively. The MYC overexpression group (n = 140) showed significantly worse overall survival (OS; p = 0.019) and progression‐free survival (PFS; p = 0.001) than the non‐MYC overexpression group (n = 87). Subgroup analyses demonstrated that the MYC overexpression group was associated with inferior OS and PFS in the subgroups with the International Prognostic Index score of 3–5 (OS: p = 0.011; PFS: p < 0.001), Ann Arbor stage 3–4 (OS: p = 0.014; PFS: p < 0.001) and GCB subtype (OS: p = 0.014; PFS: p = 0.010). Consolidation radiotherapy improved OS and PFS in patients with bulky mass DLBCL (OS: p = 0.008; PFS: p = 0.004) as well as in those with MYC overexpression (OS: p = 0.001; PFS: p = 0.001). The prognostic value of MYC overexpression was maintained in a multivariate model adjusted for the International Prognostic Index.
Conclusion
MYC overexpression is a poor predictor for bulky mass DLBCL patients. Consolidation radiotherapy for residual disease after induction therapy may improve outcomes for patients with bulky mass DLBCL.
Keywords: diffuse large B‐cell lymphoma, bulky mass, immunohistochemistry, lymphoma, MYC, radiotherapy
Our study showed that MYC overexpression but not MYC/BCL2 double expression is a poor predictor for bulky mass DLBCL patients. Consolidation radiotherapy for residual disease after induction therapy may improve outcomes for patients with bulky mass DLBCL.
1. INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is a heterogeneous disease with distinct clinical, histological, and molecular characteristics. Rituximab combined with cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone (R‐CHOP) is recognized as the standard therapy. However, up to 40% of DLBCL patients exhibit refractory disease or disease relapse. Therefore, it is crucial to identify DLBCL subgroups with a poor prognosis. The International Prognostic Index (IPI) is commonly used to stratify the risk prognosis of DLBCL, but it does not capture biological parameters, which may lead to inaccurate risk stratification. An increasing number of studies have demonstrated the prognostic effect of MYC and BCL2 gene or protein levels. The MYC gene is located on chromosome 8q24 and has been reported to regulate up to 10% of genes within the human genome. 1 The deregulation of MYC drives many carcinogenic processes involving proliferation, differentiation, and metabolism. 2 , 3 , 4 Notably, DLBCL patients with MYC/BCL2 double expression (DE) have been reported to have significantly poor survival. 5 , 6 , 7 , 8 , 9 Therefore, the prognostic role of biological parameters in DLBCL should be underlined.
Moreover, DLBCL patients with bulky mass are recognized as a distinct group with inferior survival outcomes. 10 , 11 , 12 However, the prognostic role of clinical and biological parameters in patients with bulky mass DLBCL has been little explored. Therefore, this study aimed to investigate the effect of biological parameters for bulky mass DLBCL patients and further explore the prognostic value of different treatment strategies for bulky mass DLBCL.
2. MATERIALS AND METHODS
2.1. Patients
We reviewed formalin‐fixed, paraffin‐embedded (FFPF) biopsies from 227 patients with de novo bulky mass DLBCL at the First Affiliated Hospital of Zhengzhou University between February 2018 and December 2021. The diagnosis of DLBCL was based on the 2008 WHO classification criteria. 13 A bulky mass was defined as a maximum tumor diameter (MTD) ≥7.5 cm. Patients with the following were excluded: (1) primary central nervous system lymphoma; (2) primary mediastinal large B‐cell lymphoma; and (3) DLBCL transformed from low‐grade B‐cell lymphoma. Clinical data were obtained by consulting hospitalization records and through telephone interviews. All patients received R‐CHOP or intensive‐dose therapy such as rituximab, etoposide, prednisone, cyclophosphamide, and doxorubicin (R‐EPOCH) as the first‐line induction therapy. Those without appropriate clinical information were excluded.
2.2. Immunohistochemistry (IHC) and FISH
All tissues were fixed in 3.7% neutral formaldehyde, routinely dehydrated, paraffin‐embedded, and serially sectioned to 2–3 μm thick for H&E and immunohistochemical staining. The EnVision two‐step method was used for immunohistochemistry. Monoclonal antibodies against MYC (MXB, Fuzhou, China), BCL2 (ZSGB, Beijing, China), BCL6 (ZSGB), and Ki‐67 (Gene Tech, Shanghai, China) were used. The cutoff scores defined as protein overexpression were 40% for MYC (Figure 1), 50% for BCL2, and 50% for BCL6, as reported in previous studies. 7 , 14 , 15 , 16 , 17 , 18 The cell‐of‐origin (COO) subtypes based on the HANS algorithm were classified as GCB and non‐GCB and were determined by the immunohistochemical expression of CD10, BCL6, and MUM1.
FIGURE 1.
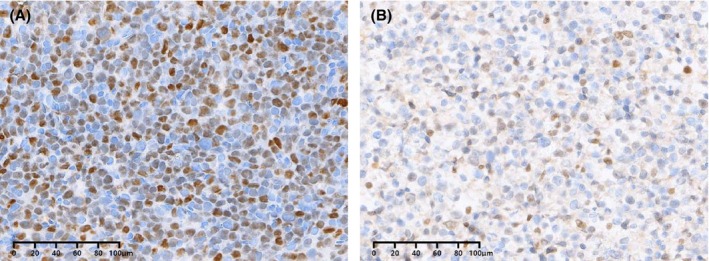
MYC protein expression measured by immunohistochemistry in bulky mass DLBCL. MYC staining pattern is nuclear. (A) MYC protein expression ≥40%; (B) MYC protein expression <40%.
FISH analysis for MYC, BCL2, and BCL6 was performed based on a tissue microarray (TMA) using dual‐colour break‐apart probes (Abbott Molecular, Des Plaines, IL, USA). The signals from 100 interphase nuclei were analyzed. The cases with break‐apart signals >10% of nuclei were considered positive for the presence of a translocation.
2.3. Statistical analysis
Overall survival (OS) was measured from the date of diagnosis until death of any cause or the last follow‐up. Progression‐free disease (PFS) was measured from the date of diagnosis until the date of disease progression, relapse, or death of any cause. Response assessment was defined as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). 19 The overall response rate (ORR) was defined as the proportion of patients who achieved CR or PR to therapy. Survival analyses were performed with the Kaplan–Meier method and were compared using the log‐rank test. Pearson's χ2 test or Fisher's exact test was used to assess differences in categorical variables. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model. Statistical analysis was performed using SPSS version 23.0. A p value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Clinicopathological characteristics
The baseline characteristics of patients with bulky mass DLBCL are presented in Table 1. There were 91 females and 136 males with a median age of 56 years (range, 18–89). A total of 89 (39.2%) patients were 60 years or older. A total of 159 (70.0%) patients had ≥2 extranodal sites of disease. Twenty‐five (11.5%) patients had bone marrow involvement, and 13 (5.8%) had central nervous system involvement by lymphoma. A total of 164 (72.2%) patients had stage 3–4 disease according to the Ann Arbor staging classification, and 125 (55.3%) had an IPI score of 3–5. Sixty‐six (29.1%) patients had comorbidities, such as hypertension, coronary heart disease, and cerebrovascular disease.
TABLE 1.
Clinicopathologic features of bulky mass DLBCL according to MYC expression.
| Characteristics | Total (n = 227) | Non‐MYC overexpression (n = 87) | MCY overexpression (n = 140) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 91 (40.1%) | 38 (43.7%) | 53 (37.9%) | 0.384 |
| Male | 136 (59.9%) | 49 (56.3%) | 87 (62.1%) | |
| Age | ||||
| <60 | 138 (60.8%) | 58 (66.7%) | 80 (57.1%) | 0.153 |
| ≥60 | 89 (39.2%) | 29 (33.3%) | 60 (42.9%) | |
| Performance status | ||||
| ECOG <2 | 132 (58.1%) | 55 (63.2%) | 77 (55.0%) | 0.222 |
| ECOG ≥2 | 95 (41.9%) | 32 (36.8%) | 63 (45.0%) | |
| Ann Arbor stage | ||||
| 1–2 | 63 (27.8%) | 30 (34.5%) | 33 (23.6%) | 0.074 |
| 3–4 | 164 (72.2%) | 57 (65.5%) | 107 (76.4%) | |
| IPI | ||||
| 0–2 | 101 (44.7%) | 39 (44.8%) | 62 (44.6%) | 0.974 |
| 3–5 | 125 (55.3%) | 48 (55.2%) | 77 (55.4%) | |
| NA | 1 | 0 | 1 | |
| COO | ||||
| GCB | 63 (28.1%) | 23 (27.4%) | 40 (28.6%) | 0.848 |
| Non‐GCB | 161 (71.9%) | 61 (72.6%) | 100 (71.4%) | |
| NA | 3 | 3 | 0 | |
| Extranodal sites ≥2 | ||||
| NO | 68 (30.0%) | 30 (34.5%) | 38 (27.1%) | 0.240 |
| YES | 159 (70.0%) | 57 (65.5%) | 102 (72.9%) | |
| B symptom | ||||
| NO | 152 (67.0%) | 62 (71.3%) | 90 (64.3%) | 0.277 |
| YES | 75 (33.0%) | 25 (28.7%) | 50 (35.7%) | |
| BM involvement | ||||
| NO | 192 (88.5%) | 76 (91.6%) | 116 (86.6%) | 0.262 |
| YES | 25 (11.5%) | 7 (8.4%) | 18 (13.4%) | |
| NA | 10 | 3 | 7 | |
| CNS involvement | ||||
| NO | 210 (94.2%) | 81 (94.2%) | 129 (94.2%) | 0.994 |
| YES | 13 (5.8%) | 5 (5.8%) | 8 (5.8%) | |
| NA | 4 | 1 | 3 | |
| MTD | ||||
| <10 cm | 113 (49.8%) | 47 (54.0%) | 66 (47.1%) | 0.313 |
| ≥10 cm | 114 (50.2%) | 40 (46.0%) | 74 (52.9%) | |
| LDH | ||||
| Normal | 31 (14.0%) | 14 (16.7%) | 17 (12.3%) | 0.365 |
| Elevated | 191 (86.0%) | 70 (83.3%) | 121 (87.7%) | |
| NA | 5 | 3 | 2 | |
| β2‐MG | ||||
| Normal | 132 (59.8%) | 54 (63.5%) | 78 (56.5%) | 0.301 |
| Elevated | 91 (40.8%) | 31 (36.5%) | 60 (43.5%) | |
| NA | 0 | 2 | 2 | |
| MYC translocation | ||||
| NO | 164 (93.7) | 59 (96.7%) | 105 (92.1%) | 0.231 |
| YES | 11 (6.3%) | 2 (3.3%) | 9 (7.9%) | |
| NA | 52 | 26 | 26 | |
| BCL2 translocation | ||||
| NO | 165 (94.8%) | 59 (96.7%) | 106 (93.8%) | 0.407 |
| YES | 9 (5.2%) | 2 (3.3%) | 7 (6.2%) | |
| NA | 53 | 26 | 27 | |
| BCL6 translocation | ||||
| NO | 130 (74.7%) | 47 (77.0%) | 83 (73.5%) | 0.602 |
| YES | 44 (25.3%) | 14 (23.0%) | 30 (26.5%) | |
| NA | 53 | 26 | 27 | |
Abbreviations: BM, bone marrow; CNS, central nervous system; COO, cell‐of‐origin; GCB, Germinal Center B‐cell like; IPI, International Prognostic Index; LDH, lactic dehydrogenase; MTD, maximum tumor diameter; NA, not available; OS, overall survival; PFS, progression‐free survival; β2‐MG, β2‐microglobulin.
All patients (n = 227) received immunochemotherapy as the first‐line induction treatment: 180 (79.3%) patients received R‐CHOP, and 47 (20.7%) patients received R‐EPOCH. Of these 227 patients, 14 (6.2%) patients underwent surgery before induction treatment, 37 (16.3%) patients underwent consolidation radiotherapy (RT) for the residual disease after induction treatment, and 10 (4.4%) received autologous hematopoietic stem cell transplantation after CR was achieved. The baseline characteristics were similar between the non‐MYC overexpression (n = 87) group and MYC overexpression (n = 140) group.
3.2. MYC overexpression status and survival outcome
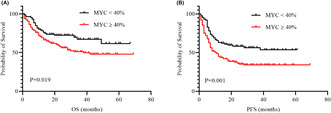
In all patients with bulky mass DLBCL, the 1‐year and 3‐year OS rates were 72.7% and 57.1%, respectively, and the 1‐year and 3‐year PFS rates were 52.0% and 42.5%, respectively, with a median follow‐up duration of 23 months (range, 1–69). All patients (n = 227) had MYC status confirmed by immunohistochemistry at the time of initial diagnosis. A total of 140 of 227 (61.7%) patients had MYC overexpression. The MYC overexpression group had a significantly worse OS than the non‐MYC overexpression group, with 3‐year OS rates of 51.1% and 67.3%, respectively (p = 0.019; Figure 2A). Similarly, patients with MYC overexpression demonstrated a significantly worse PFS than those with non‐MYC overexpression, with 3‐year PFS rates of 34.1% and 56.3%, respectively (p = 0.001; Figure 2B).
FIGURE 2.
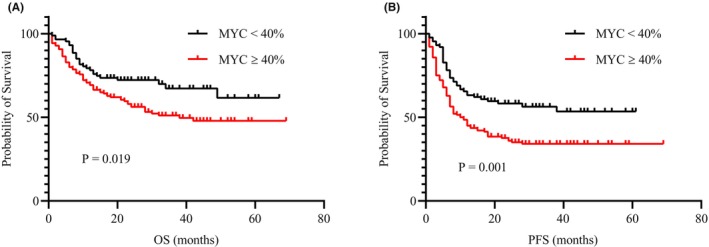
Survival analysis according to MYC expression in the entire cohort. (A) OS and (B) PFS.
Moreover, compared with the non‐MYC overexpression group, patients with MYC overexpression exhibited a worse ORR to first‐line induction treatment (p = 0.005, 69.0% vs. 50.0%). As of the date of follow‐up, a greater proportion of patients in the MYC overexpression group compared with the non‐MYC overexpression group experienced disease relapse and progression (p = 0.002, 31.0% vs. 52.1%).
3.3. Survival outcomes according to MYC overexpression status in the IPI, stage, and COO subtype subgroups
Further survival analysis was investigated according to the MYC overexpression status based on the IPI, stage, and COO subtype subgroups. MYC overexpression status was significantly associated with inferior OS and PFS in the subgroup with IPI score of 3–5 (OS; p = 0.011, PFS; p < 0.001; Figure 3C, D) and in the Ann Arbor stage 3–4 subgroup (OS; p = 0.014, PFS; p < 0.001; Figure 3G, H) but not in the subgroup with IPI score of 0–2 or in the Ann Arbor stage 1–2 subgroup (p > 0.05; Figure 3A, B, E, F). In addition, MYC overexpression status was significantly associated with inferior OS in patients with the GCB subtype (p = 0.014; Figure 3K) but not in those with the non‐GCB subtype (p = 0.191; Figure 3I). Inferior PFS was observed in both patients with the GCB subtype (p = 0.010; Figure 3L) and those with the non‐GCB subtype (p = 0.035; Figure 3J). Furthermore, compared with the non‐MYC overexpression group, the MYC overexpression group exhibited a worse ORR and higher disease relapse and progression rates in the subgroups with IPI score of 3–5, Ann Arbor stage 3–4, and GCB subtype (p < 0.05) but not in the subgroups with IPI score of 0–2, Ann Arbor stage 1–2, and non‐GCB subtype (p > 0.05).
FIGURE 3.
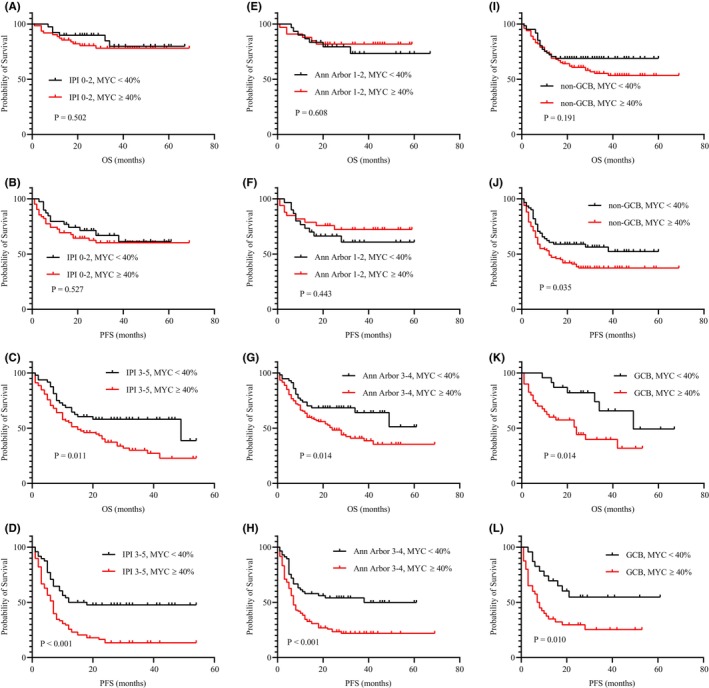
Survival analysis according to MYC expression in different subgroups. (A, B) OS and PFS in IPI score of 0–2 subgroup; (C, D) OS and PFS in IPI score of 3–5 subgroup; (E, F) OS and PFS in Ann Arbor stage 1–2 subgroup; (G, H) OS and PFS in Ann Arbor stage 3–4 subgroup; (I, J) OS and PFS in non‐GCB subgroup; (K, L) OS and PFS in GCB subgroup.
3.4. Prognostic significance between MYC expression and different treatment strategies
With respect to the impact of various treatment strategies on survival among bulky mass DLBCL patients, we did not observe a significant difference in survival outcomes between patients who did or did not receive dose‐intensive immunohistochemistry treatment such as R‐EPOCH, surgery, and autologous transplantation. By contrast, consolidation RT was associated with a better OS (p = 0.008) and PFS (p = 0.004) in all patients with bulky mass DLBCL. Of pivotal importance, we identified that RT was associated with a preferable OS (p = 0.001) and PFS (p = 0.001) in patients with MYC overexpression. However, RT showed no significant differences in OS (p = 0.704) and PFS (p = 0.523) within the non‐MYC overexpression group.
3.5. DE and DH status and survival outcomes
BCL2 overexpression was detected in 151 of 194 (77.8%) patients, BCL6 in 112 of 146 (76.7%) patients, and MYC/BCL2 DE in 106 of 194 (54.6%) patients. By contrast to the results of previous studies, compared with non‐MYC/BCL2 DE status, MYC/BCL2 DE status did not correlate with poorer OS (p = 0.318) and PFS (p = 0.114). The MYC, BCL2, and BCL6 cytogenetic statuses were confirmed by FISH in 175, 174, and 174 patients, respectively. MYC rearrangement was detected in 11 of 175 (6.3%) patients, BCL2 rearrangement in nine of 174 (5.2%) and BCL6 rearrangement in 44 of 174 (19.4%). MYC/BCL2 or MYC/BCL6 double hit (DH) was present in two of 173 (1.1%) patients. MYC rearrangement, BCL2 rearrangement, BCL6 rearrangement, and DH status did not correlate with a significantly worse OS and PFS (p > 0.05).
3.6. Univariate and multivariate analyses of survival outcomes
Potential prognostic factors for bulky mass DLBCL were analyzed in univariate and multivariate analyses. Univariate analysis demonstrated that age ≥60 years, Ann Abor stage 3–4, IPI score of 3–5, elevated β2‐microglobulin level, comorbidities and MYC overexpression showed significant associations with both a poor OS and a poor PFS (p < 0.05, Table 2). In the multivariate analysis, MYC overexpression had a significant prognostic impact on OS (vs. non‐MYC overexpression group; HR, 1.679; 95% CI, 1.049–2.688, p = 0.031) and PFS (vs. non‐MYC overexpression group; HR, 1.864; 95% CI, 1.255–2.770, p = 0.002) after adjusting for the IPI score (Table 3).
TABLE 2.
Univariate analysis of OS and PFS of bulky mass DLBCL.
| Variables | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Male (vs. female) | 1.047 (0.693 ~ 1.580) | 0.828 | 1.123 (0.788 ~ 1.600) | 0.522 |
| Age ≥60 years | 2.822 (1.871 ~ 4.257) | <0.001 | 2.106 (1.489 ~ 2.978) | <0.001 |
| Ann Arbor Stage 3–4 | 3.046 (1.692 ~ 5.483) | <0.001 | 2.893 (1.793 ~ 4.669) | <0.001 |
| IPI 3–5 | 4.377 (2.636 ~ 7.270) | <0.001 | 2.850 (1.938 ~ 4.191) | <0.001 |
| GCB (vs. non‐GCB) | 1.275 (0.825 ~ 1.970) | 0.274 | 1.203 (0.825 ~ 1.753) | 0.337 |
| LDH elevated | 1.399 (0.725 ~ 2.700) | 0.317 | 1.652 (0.930 ~ 2.935) | 0.087 |
| β2‐MG elevated | 2.299 (1.513 ~ 3.492) | <0.001 | 2.169 (1.524 ~ 3.087) | <0.001 |
| MTD ≥10 cm | 1.246 (0.829 ~ 1.871) | 0.290 | 1.372 (0.969 ~ 1.942) | 0.074 |
| Comorbidity | 1.917 (1.266 ~ 2.904) | 0.002 | 1.479 (1.027 ~ 2.129) | 0.035 |
| MYC/BCL2 DE | 1.292 (0.848 ~ 1.969) | 0.232 | 1.349 (0.922 ~ 1.972) | 0.124 |
| MYC overexpression | 1.687 (1.079 ~ 2.637) | 0.022 | 1.824 (1.249 ~ 2.664) | 0.002 |
Abbreviations: CI, confidence interval; DE, double expression; HR, hazard ratio.
TABLE 3.
Multivariate analysis of OS and PFS of bulky mass DLBCL.
| Variables | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age ≥60 years | 1.324 (0.798 ~ 2.199) | 0.277 | 1.146 (0.737 ~ 1.783) | 0.546 |
| Ann Arbor Stage 3–4 | 1.645 (0.849 ~ 3.226) | 0.139 | 1.865 (1.093 ~ 3.183) | 0.022 |
| IPI score of 3–5 | 2.868 (1.527 ~ 5.386) | 0.001 | 1.977 (1.202 ~ 3.250) | 0.007 |
| β2‐MG elevated | 1.403 (0.902 ~ 2.182) | 0.133 | 1.378 (0.941 ~ 2.019) | 0.100 |
| Comorbidity | 1.444 (0.923 ~ 2.258) | 0.108 | 1.168 (0.789 ~ 1.728) | 0.438 |
| MYC overexpression | 1.679 (1.049 ~ 2.688) | 0.031 | 1.864 (1.255 ~ 2.770) | 0.002 |
4. DISCUSSION
Bulky mass is a significant adverse prognostic factor for DLBCL; however, studies regarding the effect of biological parameters on bulky mass DLBCL are limited. Therefore, this study aimed to supplement the data in this field.
Many studies have shown that MYC plays a pivotal role in the occurrence and development of lymphoma. MYC deregulation can occur at the level of gene transcription, mRNA stability, and protein modification. 20 However, FISH can only be used to detect MYC rearrangement at the gene level. Some findings have suggested that MYC protein expression can also occur via alternative nontranslocation‐based mechanisms in lymphoma lacking rearrangement of the MYC gene. 7 , 21 For example, miRNAs may regulate MYC protein expression. Leucci et al. found that the miRNA hsa‐miR‐34b, which downregulated in lymphoma without an MYC rearrangement, was inversely related to MYC protein expression in a dose‐dependent manner. 22 Onnis et al. identified another miRNA, hsa‐miR‐9, that positively controlled MYC protein expression in lymphoma without an MYC rearrangement. 23 An increased MYC gene copy number has been shown to be associated with an increased MYC protein expression level in DLBCL. 24 , 25 Furthermore, an in vitro experiment demonstrated that MYC protein expression in DLBCL was probably associated with the MYC‐oncogenic effect regardless of MYC rearrangements. 26 Given the above discoveries, it is not surprising that MYC protein expression can still be displayed in the absence of detectable MYC rearrangement.
Many studies have demonstrated that MYC protein overexpression is relevant to inferior outcomes within the entire DLBCL cohort, in accordance with its central role in the regulation of thousands of genes. 6 , 21 , 27 , 28 Consistent with the above conclusions, this study also confirmed that MYC protein overexpression was associated with inferior OS and PFS and exhibited a worse response to treatment in patients with bulky mass DLBCL. Moreover, MYC protein overexpression retained its significant prognostic value after adjusting for the IPI score. We further investigated the survival outcomes of MYC protein expression status in different subgroups. Our study found that MYC overexpression was a factor for poor prognosis only in the subgroups with IPI score of 3–5 and Ann Arbor stage 3–4 but not in the subgroups with IPI score of 0–2 and Ann Arbor stage 1–2. This suggests that MYC protein expression is an excellent prognostic indicator for bulky mass DLBCL with high‐risk factors. Another interesting finding was that MYC overexpression was related to poor OS and PFS in the GCB subtype but not in the non‐GCB subtype, which was in contrast to some studies of DLBCL cohorts. 6 Burkitt's lymphoma (BL) harbors a dysregulation of MYC, and the majority of its COO subtype is the GCB subtype. 2 , 3 Dave et al. showed that one DLBCL case had a probability of 66% for the diagnosis of BL, which might represent a rare genetic overlap between DLBCL and BL. 2 Therefore, we speculate that bulky mass DLBCL with both MYC overexpression and the GCB subtype might have similar biological specificity to BL. In conclusion, MYC overexpression is a poor predictor for bulky mass DLBCL patients, especially those with high‐risk factors and the GCB subtype.
Regarding the effect of other biological parameters in patients with bulky mass DLBCL, this study found that the MYC/BCL2 DE status failed to confer a significant prognostic difference in OS and PFS, contrary to several studies of an entire DLBCL cohort. 5 , 6 , 7 , 8 , 9 The most likely reasons for this discrepancy were that this study targeted the bulky mass cohort, with heterogeneity that differed from the entire DLBCL cohort. In addition, we found that DH also had no impact on survival in bulky mass DLBCL patients, but this might not be a statistically significant conclusion due to the insufficient amount of data and therefore should be treated with caution.
The inferior response to traditional therapy and poor survival outcomes highlight that alternative therapeutic strategies for bulky mass DLBCL patients are warranted. Therefore, we further explored the impact of different treatment strategies on the prognosis of bulky mass DLBCL. Several studies have confirmed the prognostic effect of dose‐intensified immunochemotherapy regimens on different subgroups of DLBCL, and the conclusions were mixed. Some studies have shown that R‐EPOCH or other dose‐intensified regimens improved the prognosis of certain DLBCL subgroups, such as DE, DT/TH, high‐risk young, or high Ki‐67 expression subgroups. 29 , 30 , 31 , 32 , 33 For instance, Dunleavy et al. showed that DA‐EPOCH‐R produced durable remission in patients with MYC‐rearranged aggressive B‐cell lymphomas. 34 However, the Alliance/CALGB 50303 study identified that DA‐EPOCH‐R failed to show its advantages over R‐CHOP in OS and PFS, including the high‐risk IPI subgroup and other subgroups, and grade 3/4 treatment‐related toxicity was more common in the DA‐EPOCH‐R group. 35 Our study found that a dose‐intensive regimen such as R‐EPOCH did not improve the prognosis of patients with bulky mass DLBCL or with MYC overexpression. Moreover, our study found that surgery to relieve tumor burden prior to first‐line induction therapy and autologous hematopoietic stem cell transplantation did not improve the outcomes of patients with bulky mass DLBCL. In recent years, an increasing number of new drugs have been explored for application in DLBCL. Grzegorz et al. showed that the addition of lenalidomide to R‐CHOP did not improve prognosis in the bulky mass subgroup compared to R‐CHOP. 36 The POLARIX study also demonstrated that polatuzumab vedotin, an antibody‐drug conjugate targeting the B‐cell surface antigen receptor CD79b, in combination with R‐CHP did not present a clear benefit in patients who had bulky disease compared to R‐CHOP. 37 By contrast, our study indicated significant improvements in OS and PFS among patients with bulky mass DLBCL who received consolidation RT to residual disease after induction therapy. Further subgroup analysis also showed that RT improved OS and PFS in the MYC overexpression subgroup. Tzankov et al. also showed that RT improved the survival of DLBCL, and this improvement was more profound in patients with MYC deregulation. 38 This further illustrated that RT may overcome MYC‐related treatment resistance. Many studies have also confirmed the positive role of RT in DLBCL, particularly in bulky mass populations. 10 , 16 , 17 , 18
We acknowledge that this study had some limitations. Clinical information obtained by retrospective retrieval of medical records was not entirely complete, and there was selection bias. This study was conducted at a single center. Despite these limitations, the prognostic value of MYC protein expression proposed in this study was assessed in a relatively large cohort, and this study was the first to target the bulky mass population. FISH technology is laborious and fails to provide any information about deregulation at the gene transcriptional and translational levels. MYC protein expression determined by immunohistochemistry is readily performed in most laboratories and represents a promising tool for stratifying risk in daily practice. Therefore, its value as a predictive marker should not be underestimated.
In conclusion, this study has shown that MYC overexpression is a poor predictor for bulky mass DLBCL patients, and its adverse prognostic effect is more pronounced in the high‐risk population and those with the GCB subtype. Consolidation RT for residual disease after induction therapy improves outcomes for bulky mass DLBCL patients as well as those with MYC overexpression. Further investigation and prospective studies in patients with bulky mass DLBCL are warranted.
AUTHOR CONTRIBUTIONS
yanjie wang: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Donglin Liu: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Xudong Zhang: Conceptualization (equal); funding acquisition (equal); supervision (equal). Mingzhi Zhang: Conceptualization (equal); funding acquisition (equal); supervision (equal). Shenglei Li: Data curation (equal); methodology (equal); validation (equal). Xiaoyan Feng: Data curation (equal); methodology (equal); validation (equal). Meng Dong: Data curation (equal); methodology (equal); validation (equal). Shanshan Ma: Data curation (equal). Siyu Qian: Data curation (equal). Zeyuan Wang: Data curation (equal). Yue Zhang: Data curation (equal). Pengyuan Wang: Data curation (equal). Shuhao Mei: Data curation (equal). Qingjiang Chen: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Informed consent for the collection of medical information was obtained from all patients. The present study was conducted according to the Helsinki declaration and was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University (Approval number: 2022‐KY‐0869‐001).
ACKNOWLEDGEMENTS
This study was supported by the National Nature Science Foundation of China (Grant No. 82070210) and the Major Medical Scientific and Technological Project of Henan Province (Grant No. SBGJ202001008).
Wang Y, Liu D, Zhang X, et al. MYC overexpression but not MYC/BCL2 double expression predicts survival in bulky mass diffuse large B‐cell lymphoma patients. Cancer Med. 2023;12:18568‐18577. doi: 10.1002/cam4.6463
Yanjie Wang and Donglin Liu contributed equally to this work
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author.
REFERENCES
- 1. Fernandez PC, Frank SR, Wang L, et al. Genomic targets of the human c‐Myc protein. Genes Dev. 2003;17(9):1115‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354(23):2431‐2442. [DOI] [PubMed] [Google Scholar]
- 3. Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354(23):2419‐2430. [DOI] [PubMed] [Google Scholar]
- 4. Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B‐cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28(20):3360‐3365. [DOI] [PubMed] [Google Scholar]
- 5. Han B, Kim S, Koh J, et al. Immunophenotypic landscape and prognosis of diffuse large B‐cell lymphoma with MYC/BCL2 double expression: an analysis of a prospectively Immunoprofiled cohort. Cancers (Basel). 2020;12(11):3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ambrosio MR, Lazzi S, Bello GL, et al. MYC protein expression scoring and its impact on the prognosis of aggressive B‐cell lymphoma patients. Haematologica. 2019;104(1):e25‐e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452‐3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green TM, Young KH, Visco C, et al. Immunohistochemical double‐hit score is a strong predictor of outcome in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460‐3467. [DOI] [PubMed] [Google Scholar]
- 9. Staiger AM, Ziepert M, Horn H, et al. Clinical impact of the cell‐of‐origin classification and the MYC/ BCL2 dual expresser status in diffuse large B‐cell lymphoma treated within prospective clinical trials of the German high‐grade non‐Hodgkin's lymphoma study group. J Clin Oncol. 2017;35(22):2515‐2526. [DOI] [PubMed] [Google Scholar]
- 10. Rajasooriyar C, Tey J, Wong LC, et al. A multi‐institutional analysis of diffuse large B‐cell lymphoma (DLBCL) treated with consolidative radiotherapy and the impact of cell‐of‐origin on outcomes. Radiol Oncol. 2019;53(4):473‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP‐like chemotherapy with or without rituximab in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: 6‐year results of an open‐label randomised study of the MabThera international trial (MInT) group. Lancet Oncol. 2011;12(11):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 12. Pfreundschuh M, Ho AD, Cavallin‐Stahl E, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good‐prognosis diffuse large‐B‐cell lymphoma treated with CHOP‐like chemotherapy with or without rituximab: an exploratory analysis of the MabThera international trial group (MInT) study. Lancet Oncol. 2008;9(5):435‐444. [DOI] [PubMed] [Google Scholar]
- 13. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification Of Tumours Of Haematopoietic And Lymphoid Tissues. WHO Press; 2008. [Google Scholar]
- 14. Li S, Desai P, Lin P, et al. MYC/BCL6 double‐hit lymphoma (DHL): a tumour associated with an aggressive clinical course and poor prognosis. Histopathology. 2016;68(7):1090‐1098. [DOI] [PubMed] [Google Scholar]
- 15. Valera A, López‐Guillermo A, Cardesa‐Salzmann T, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98(10):1554‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jegadeesh N, Rajpara R, Esiashvili N, et al. Predictors of local recurrence after rituximab‐based chemotherapy alone in stage III and IV diffuse large B‐cell lymphoma: guiding decisions for consolidative radiation. Int J Radiat Oncol Biol Phys. 2015;92(1):107‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B‐cell lymphoma treated with R‐CHOP chemotherapy. J Clin Oncol. 2010;28(27):4170‐4176. [DOI] [PubMed] [Google Scholar]
- 18. Shi Z, Das S, Okwan‐Duodu D, et al. Patterns of failure in advanced stage diffuse large B‐cell lymphoma patients after complete response to R‐CHOP immunochemotherapy and the emerging role of consolidative radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):569‐577. [DOI] [PubMed] [Google Scholar]
- 19. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slack GW, Gascoyne RD. MYC and aggressive B‐cell lymphomas. Adv Anat Pathol. 2011;18(3):219‐228. [DOI] [PubMed] [Google Scholar]
- 21. Kluk MJ, Chapuy B, Sinha P, et al. Immunohistochemical detection of MYC‐driven diffuse large B‐cell lymphomas. PLoS One. 2012;7(4):e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leucci E, Cocco M, Onnis A, et al. MYC translocation‐negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol. 2008;216(4):440‐450. [DOI] [PubMed] [Google Scholar]
- 23. Onnis A, De Falco G, Antonicelli G, et al. Alteration of microRNAs regulated by c‐Myc in Burkitt lymphoma. PLoS One. 2010;5(9):e12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stasik CJ, Nitta H, Zhang W, et al. Increased MYC gene copy number correlates with increased mRNA levels in diffuse large B‐cell lymphoma. Haematologica. 2010;95(4):597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Testoni M, Kwee I, Greiner TC, et al. Gains of MYC locus and outcome in patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Br J Haematol. 2011;155(2):274‐277. [DOI] [PubMed] [Google Scholar]
- 26. Li W, Gupta SK, Han W, et al. Targeting MYC activity in double‐hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B‐cell lymphoma. Blood. 2013;121(12):2253‐2263. [DOI] [PubMed] [Google Scholar]
- 28. Ziepert M, Lazzi S, Santi R, et al. A 70% cut‐off for MYC protein expression in diffuse large B cell lymphoma identifies a high‐risk group of patients. Haematologica. 2020;105(11):2667‐2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pedersen M, Gang AO, Brown P, et al. Real world data on young patients with high‐risk diffuse large B‐cell lymphoma treated with R‐CHOP or R‐CHOEP ‐ MYC, BCL2 and BCL6 as prognostic biomarkers. PLoS One. 2017;12(10):e0186983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miura K, Takahashi H, Nakagawa M, et al. Ideal dose intensity of R‐CHOP in diffuse large B‐cell lymphoma. Expert Rev Anticancer Ther. 2022;22(6):583‐595. [DOI] [PubMed] [Google Scholar]
- 31. Huang JJ, Xia Y, Wang Y, et al. A comparison of R‐EPOCH and R‐CHOP as a first‐line regimen in de novo DLBCL patients with high Ki‐67 expression in a single institution. Oncotarget. 2016;7(27):41242‐41250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molina TJ, Canioni D, Copie‐Bergman C, et al. Young patients with non‐germinal center B‐cell‐like diffuse large B‐cell lymphoma benefit from intensified chemotherapy with ACVBP plus rituximab compared with CHOP plus rituximab: analysis of data from the Groupe d'Etudes des Lymphomes de l'Adulte/lymphoma study association phase III trial LNH 03‐2B. J Clin Oncol. 2014;32(35):3996‐4003. [DOI] [PubMed] [Google Scholar]
- 33. Récher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B‐cell lymphoma (LNH03‐2B): an open‐label randomised phase 3 trial. Lancet. 2011;378(9806):1858‐1867. [DOI] [PubMed] [Google Scholar]
- 34. Dunleavy K, Fanale MA, Abramson JS, et al. Dose‐adjusted EPOCH‐R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B‐cell lymphoma with MYC rearrangement: a prospective, multicentre, single‐arm phase 2 study. Lancet Haematol. 2018;5(12):e609‐e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bartlett NL, Wilson WH, Jung SH, et al. Dose‐adjusted EPOCH‐R compared with R‐CHOP as frontline therapy for diffuse large B‐cell lymphoma: clinical outcomes of the phase III intergroup trial Alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nowakowski GS, Hong F, Scott DW, et al. Addition of Lenalidomide to R‐CHOP Improves Outcomes in Newly Diagnosed Diffuse Large B‐Cell Lymphoma in a Randomized Phase II US Intergroup Study ECOG‐ACRIN E1412. J Clin Oncol. 2021;39(12):1329‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab Vedotin in previously untreated diffuse large B‐cell lymphoma. N Engl J Med. 2021;386(4):351‐363. [DOI] [PubMed] [Google Scholar]
- 38. Tzankov A, Xu‐Monette ZY, Gerhard M, et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B‐cell lymphoma patients treated with rituximab‐CHOP. Mod Pathol. 2014;27(7):958‐971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.


