Abstract
C-8-methoxy fluoroquinolones were more lethal than C-8-bromine, C-8-ethoxy, and C-8-H derivatives for Staphylococcus aureus, especially when topoisomerase IV was resistant. The methoxy group also increased lethality against wild-type cells when protein synthesis was inhibited. These properties encourage refinement of C-8-methoxy fluoroquinolones to kill staphylococci.
Fluoroquinolones are antibacterial agents that trap gyrase and topoisomerase IV on DNA (6). Current derivatives are only marginally effective against Staphylococcus aureus, largely because this bacterium readily acquires resistance mutations (6, 9, 11). In S. aureus, topoisomerase-based resistance occurs stepwise, with moderate levels arising from a single mutation in the primary target of the drug, topoisomerase IV, and higher levels from the accumulation of an additional mutation in gyrase (7, 8, 17). Since other forms of resistance are also rendering many therapeutic agents ineffective against S. aureus, we sought to improve fluoroquinolone action.
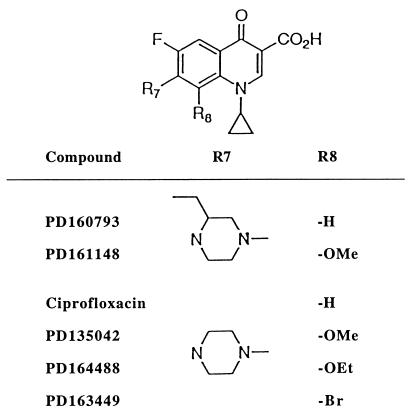
Several studies direct attention to substituents at the C-8 position of fluoroquinolones (R8 in Fig. 1). A computational approach indicated that C-8 fluorine or alkoxy groups, when coupled with an N-1 cyclopropyl moiety, would increase bacteriostatic activity (15). This was the case for 15 of 16 bacterial species subsequently tested (4). Another study showed that a C-8 chlorine increased activity against Pseudomonas aeruginosa, especially with strains containing a quinolone-resistant gyrase (14). The C-8 chlorine also increased activity against gyrase purified from the mutants. In a third example, a C-8 methoxy group increased lethality with Escherichia coli, particularly for a gyrase mutant (21). Fluoroquinolones with C-8 substitutents have also shown promise against gram-positive organisms (1, 3, 13), including fluoroquinolone-resistant clinical isolates of S. aureus (12). The present report compares C-8 substituents for enhancement of fluoroquinolone lethality with S. aureus carrying wild-type and characterized resistance alleles of parC (topoisomerase IV) and gyrA (gyrase).
FIG. 1.
Fluoroquinolone structures.
S. aureus strains (7) included ciprofloxacin-resistant, first-step mutants 2-2 and 2-11, derived from RN4220, and resistant second-step mutants 2-32C128B and 11C128B, derived from 2-2 and 2-11, respectively. Ciprofloxacin and new fluoroquinolones PD161148, PD160793, PD135042, PD164488, and PD163449 (20) were prepared as 10-mg/ml solutions in 0.1 N NaOH. To measure fluoroquinolone lethality, cells were cultured with vigorous shaking at 37°C in CY medium (18), fluoroquinolone was added for 2 h during exponential growth, and the fraction of surviving cells was determined by plating cells on GL agar (18) lacking drug. Bacteriostatic activity was measured as ID50, the fluoroquinolone concentration required to inhibit growth to half that observed in the absence of drug. For this measurement, overnight cultures were diluted 100-fold into CY medium containing various concentrations of fluoroquinolone; after overnight incubation at 37°C, untreated controls reached stationary phase while treated cultures grew to varying extents, as estimated by culture turbidity (A600).
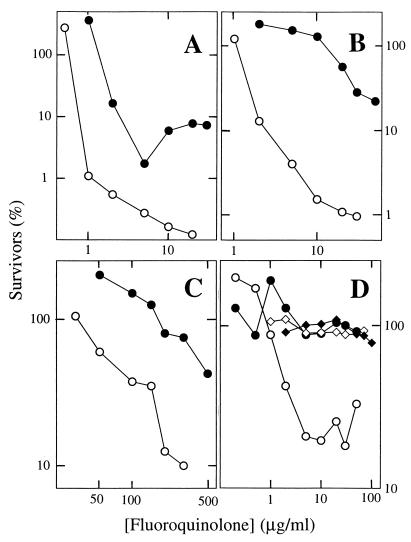
A C-8-methoxy fluoroquinolone (PD161148), which is effective against E. coli (21), was about fivefold more lethal than a C-8-H control (PD160793) against wild-type S. aureus when the concentration required to kill 99% of the cells (99% lethal dose [LD99]) was measured (Fig. 2A). The difference between the compounds was about 10-fold for a parC mutant (strain 2-2) when LD50 was measured (the C-8-H compound was not effective enough for LD99 comparisons [Fig. 2B]) and about 7-fold for a parC gyrA double mutant (strain 2-32C1128B [Fig. 2C]). The reduced effect in the double mutant suggests that at least part of the C-8-methoxy effect is directed against gyrase.
FIG. 2.
Lethal action of fluoroquinolones. Aliquots from exponentially growing cultures of S. aureus were removed after incubation with the indicated concentrations of fluoroquinolones for 2 h, diluted, and plated on drug-free agar for determination of viable cells. (A) Strain RN4220 (wild type); (B) strain 2-2 (parC Cipr); (C) strain 2-32C-128B (parC Cipr gyrA Cipr); (D) strain RN4220 (wild type; circles) or 2-2 (parC Cipr; diamonds) treated with 20 μg of chloramphenicol per ml in addition to the indicated concentrations of fluoroquinolones. Drugs were PD161148 (C-8-methoxy; open symbols) and PD 160793 (C-8-hydrogen; filled symbols). Experiments were repeated three times with similar results.
Chloramphenicol protects bacteria from the lethal action of the quinolones (5), presumably by blocking the induction of a protein responsible for releasing DNA breaks from quinolone-topoisomerase-DNA complexes (2). This protective effect occurred with wild-type S. aureus (compare Fig. 2A and D, filled circles). The effect was less complete for the C-8-methoxy compound PD161148 (Fig. 2D); thus, C-8-methoxy fluoroquinolones are more likely than C-8-H derivatives to kill nongrowing cells. A parC mutation blocked the chloramphenicol-insensitive activity of the C-8-methoxy compound (Fig. 2D, strain 2-2), indicating that fluoroquinolone action against its secondary target, gyrase, is sensitive to chloramphenicol. A comparable effect has been observed with E. coli (2, 16). There the secondary target is topoisomerase IV.
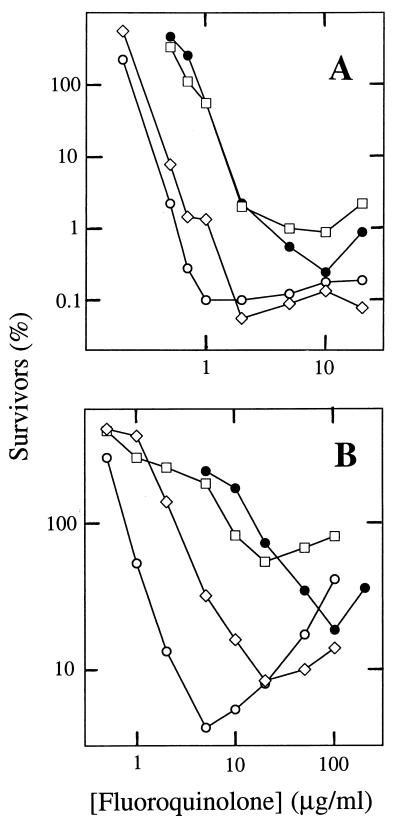
We next compared the effects of several C-8 substituents when added to ciprofloxacin. A methoxy group increased lethal activity (LD50) against wild type and a parC mutant (strain 2-2) by factors of 4 and 30, respectively (compare circles in Fig. 3). A C-8-ethoxy compound behaved much like ciprofloxacin, and a C-8-bromine compound was intermediate (Fig. 3). A C-8-fluorine compound was previously shown to fall between C-8-methoxy and C-8-H compounds (12). Thus, the methoxy group is the most effective.
FIG. 3.
Effects of C-8 variation on fluoroquinolone bactericidal activity. S. aureus wild type (A) (strain RN4220) and a parC mutant (B) (strain 2-2) were treated with the indicated concentrations of ciprofloxacin (C-8-hydrogen; filled circles), PD135042 (C-8-methoxy; open circles), PD164488 (C-8-ethoxy; squares), or PD163449 (C-8-bromine; diamonds) as described in the legend to Fig. 2.
We postulated that the quinolones act in two steps (2). First, drug-topoisomerase-DNA complexes block DNA synthesis and eventually cell growth. This step is reversible. Lethal events arise subsequently when double-strand DNA breaks are released from the complexes. Thus, two compounds can have similar potencies for growth inhibition but different abilities to kill cells. Such a situation was seen with E. coli (21). With S. aureus, the C-8-methoxy substituent increased both bacteriostatic action (lowered ID50 [Table 1]) and bactericidal action (Fig. 2 and 3).
TABLE 1.
Bacteriostatic activities of fluoroquinolones against S. aureus mutants
| Fluoroquinolone(s) | Bacteriostatic activity (ID50 [μg/ml])a for S. aureus strain:
|
||||
|---|---|---|---|---|---|
| RN4220 (wild type) | 2-11 (parC) E84Kb | 2-2 (parC) S80Y | 11C128B (parC gyrA) E84K S84L | 2-32C128B (parC gyrA) S80Y S84L | |
| PD160793 (C-8-H) | 0.8 | 1.8 | 2.3 | 38 | 37 |
| PD161148 (C-8-methoxy) | 0.4 | 0.7 | 0.7 | 18 | 23 |
| PD160793/PD161148 | 2.1 | 2.6 | 3.2 | 2.1 | 1.6 |
| Ciprofloxacin | 0.5 | 3.5 | 3.5 | 260 | 400 |
| PD135042 (C-8-methoxy) | 0.16 | 0.36 | 0.36 | 6.2 | 14 |
| Ciprofloxacin/PD135042 | 3.1 | 9.7 | 9.7 | 42 | 30 |
Determinations were made from three experiments that successively narrowed the range of fluoroquinolone concentrations required to determine ID50.
Mutational changes are indicated by the wild-type amino acid followed by the codon number and then the mutant amino acid. Abbreviations: E, glutamic acid; K, lysine; L, leucine; S, serine; Y, tyrosine.
Ciprofloxacin had roughly as much bacteriostatic activity as the other C-8-H compound (PD160793) with wild-type cells; however, it was less effective against resistant mutants (Table 1, rows 1 and 4). Ciprofloxacin lacks the ethyl moiety found on the piperizinyl ring of PD160793. But when a C-8-methoxy group was added to each, the ciprofloxacin derivative was two to three times more bacteriostatic (Table 1, rows 2 and 5) and twice as lethal, at least for wild-type cells (compare LD90s in Fig. 2A and 3A). It may be useful to survey C-8-methoxy fluoroquinolones for additional structural improvements.
The striking effect of the C-8-methoxy group is especially evident when S. aureus carries a preexisting parC mutation or when E. coli carries a gyrA mutation (21). Fluoroquinolones that effectively kill first-step resistant mutants could greatly lower the ability of wild-type populations to acquire resistance if two mutations were required for resistance (for a discussion, see references 17, 19, and 21). Since the C-8-methoxy group also makes fluoroquinolones more effective against nongrowing cells (Fig. 2D), it should reduce the pool from which new mutants arise (10). Thus, C-8-methoxy fluoroquinolones are promising leads toward obtaining more-effective agents to control S. aureus infections.
Acknowledgments
We thank Marila Gennaro and Samuel Kayman for critical comments on the manuscript and J. Crouzet for S. aureus strains.
This work was supported by NIH grant AI35257.
REFERENCES
- 1.Bauernfeind A. Comparative in-vitro activities of the new quinolone, Bay y 3118, and ciprofloxacin, sparfloxacin, tosufloxacin, CI960, and CI-990. J Antimicrob Chemother. 1993;31:505–522. doi: 10.1093/jac/31.4.505. [DOI] [PubMed] [Google Scholar]
- 2.Chen C-R, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Yoder S, Huband M, Roland G, Courtney C. In vitro and in vivo activities of clinafloxacin, CI-990 (PD 131112), and PD 138312 versus enterococci. Antimicrob Agents Chemother. 1995;39:2123–2127. doi: 10.1128/aac.39.9.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coll R, Gargallo-Viola D, Tudela E, Xicota M A, Llovera S, Guinea J. Antibacterial activity and pharmacokinetics of four new 7-azetidinyl fluoroquinolones. Antimicrob Agents Chemother. 1996;40:274–277. doi: 10.1128/aac.40.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deitz W H, Cook T M, Goss W A. Mechanism of action of nalidixic acid on Escherichia coli. III. Conditions required for lethality. J Bacteriol. 1966;91:768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher L M, Oram M, Sreedharan S. DNA gyrase: mechanism and resistance to 4-quinolone antibacterial agents. In: Andoh T, editor. Molecular biology of DNA topoisomerases. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 145–155. [Google Scholar]
- 10.Foster P. Nonadaptive mutations occur on the F′ episome during adaptive mutation conditions in Escherichia coli. J Bacteriol. 1997;179:1550–1554. doi: 10.1128/jb.179.5.1550-1554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper D, Wolfson J. Mechanisms of bacterial resistance to quinolones. In: Hooper D, Wolfson J, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1993. pp. 97–118. [Google Scholar]
- 12.Ito T, Matsumoto M, Nishino T. Improved bactericidal activity of Q-35 against quinolone-resistant staphylococci. Antimicrob Agents Chemother. 1995;39:1522–1525. doi: 10.1128/aac.39.7.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Otsuki M, Nishino T. In vitro antibacterial activity of Q-35, a new fluoroquinolone. Antimicrob Agents Chemother. 1992;36:1708–1714. doi: 10.1128/aac.36.8.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura A, Hoshino K, Kimura Y, Hayakawa I, Sato K. Contribution of the C-8 substituent of DU-6859a, a new potent fluoroquinolone, to its activity against DNA gyrase mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1467–1471. doi: 10.1128/aac.39.7.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klopman G, Fercu D, Li J-Y, Rosenkranz H S, Jacobs M R. Antimycobacterial quinolones: a comparative analysis of structure-activity and structure-cytotoxicity relationships. Res Microbiol. 1996;147:86–96. doi: 10.1016/0923-2508(96)80209-9. [DOI] [PubMed] [Google Scholar]
- 16.Lewin C, Howard B, Smith J. Protein- and RNA-synthesis independent bactericidal activity of ciprofloxacin that involves the A subunit of DNA gyrase. J Med Microbiol. 1991;34:19–22. doi: 10.1099/00222615-34-1-19. [DOI] [PubMed] [Google Scholar]
- 17.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novick R P, Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972;68:285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- 19.Pan X-S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez J P, Gogliotti R D, Domagala J M, Gracheck S J, Huband M D, Sesnie J A, Cohen M A, Shapiro M A. The synthesis, structure-activity, and structure-side effect relationships of a series of 8-alkoxy- and 5-amino-8-alkoxyquinolone antibacterial agents. J Med Chem. 1995;38:4478–4487. doi: 10.1021/jm00022a013. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Xu C, Domagala J, Drlica K. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc Natl Acad Sci USA. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]