Abstract
Background
We conducted a review of current literature to examine the effects of smoking and smoking cessation on shoulder arthroplasty surgery.
Methods
A literature search was performed using the search terms “shoulder arthroplasty AND [smoke OR smoking OR tobacco OR nicotine].” Studies included English-language clinical outcomes studies on anatomic total shoulder arthroplasty (TSA), reverse TSA, and partial shoulder arthroplasty with evidence levels 1 through 4. Descriptive statistics calculated in the included studies were used during the analysis. Categorical variables were reported as proportions, while continuous variables were reported as means with minimum to maximum absolute ranges.
Results
Twenty-four studies were included and analyzed. Following TSA, patients who quit smoking at least 1 month preoperatively had improved outcomes compared to current smokers. Current smokers had statistically significant higher pain scores or opioid use. Five studies found increased rates of revision surgery in smokers. Smokers were significantly (p < 0.05) more likely to have increased rates of surgical, wound, superficial, and deep surgical site complications.
Discussion
Former smokers had lower complication rates and visual analog scale scores when compared to current users. A period of four weeks or more of preoperative smoking cessation is recommended.
Level of Evidence
Level III, Systematic Review.
Keywords: intraoperative complications, postoperative complications, shoulder replacement arthroplasty, smoking, tobacco smoking
Introduction
The incidence of anatomic total shoulder arthroplasty (TSA) and reverse TSA has increased by approximately 9% per year in the United States. 1 Over two-thirds of TSAs are performed on patients 65 years or older; thus, the demand for these procedures will grow with the aging US population in future decades. 2 Outcomes are generally excellent, with 95% of patients reporting positive patient satisfaction, pain relief, and improved function. 3 However, intraoperative complications (usually fractures) have been reported in 3% of cases, 4 while postoperative complications (usually glenoid loosening and humeral head subluxation) occurred in 8% of cases. 3
Identifying risk factors that affect postoperative outcomes for patients is a growing interest in today's healthcare landscape. In 2018, 14% of United States adults were current cigarette smokers; about $170 billion is spent annually on smoking-related medical care for adults, and $156 billion is lost every year due to decreased productivity. 5 As the leading cause of preventable death, smoking kills over 7 million people a year globally, of which 480,000 are in the United States. 6 For total joint arthroplasty procedures, hospital costs are about $5000 higher for smoking patients than nonsmoking patients. 7
For surgical patients, smoking is the single most important risk factor for developing postoperative complications. 8 A randomized clinical trial found that a preoperative six- to eight-week smoking cessation program substantially reduced postoperative morbidity. 9 Another retrospective study found that smoking increased the risk of surgical-site infections and wound complications after shoulder arthroplasty, but medical complications (cardiac arrest, myocardial infarction, deep vein thrombosis, pulmonary embolism, septic shock, pulmonary complications, renal injury, and death) were not increased. 10
Although studies have been conducted on the relationship between shoulder surgery complications and smoking, there are very few systematic reviews of related literature. Existing systematic reviews that discuss the subject of smoking and arthroplasty excluded shoulder arthroplasty. We hypothesized that smoking negatively affects outcomes of anatomic TSA, reverse TSA, and partial shoulder arthroplasty (PSA, also known as hemiarthroplasty) and increases complications. Furthermore, we hypothesize that former smokers would have lower complication rates than current users, providing evidence that cessation is associated with better outcomes.
Methods
Methodology and reporting of data in this systematic review were performed over available literature using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol (Figure 1). This review was registered with the PROSPERO database (registration number: CRD42021235823). The search strategy was based on “shoulder arthroplasty AND [smoke OR smoking OR tobacco OR nicotine]” using the PubMed, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Ovid Medline, and Cochrane Library databases. Studies were included if they met the following criteria: English-language clinical outcome studies after anatomic TSA, reverse TSA, and PSA with levels of evidence I (randomized controlled trials), II (cohort studies), III (case–control studies), and IV (case series); stemless shoulder arthroplasty and resurfacing hemiarthroplasty studies; basic science studies examining anatomic TSA, reverse TSA, and PSA; and studies that investigated smoking as a risk factor for increased shoulder complications or shoulder revision surgery. Total shoulder arthroplasty is defined as the replacement of portions of the shoulder joint. Partial arthroplasty/hemiarthroplasty involves replacing the upper humerus with a metal implant and leaving the glenoid intact. Studies not written in English, level V evidence (expert opinion), clinical and basic science studies analyzing surgeries outside of shoulder arthroplasty, and studies not reporting shoulder arthroplasty outcomes for tobacco smokers were excluded.
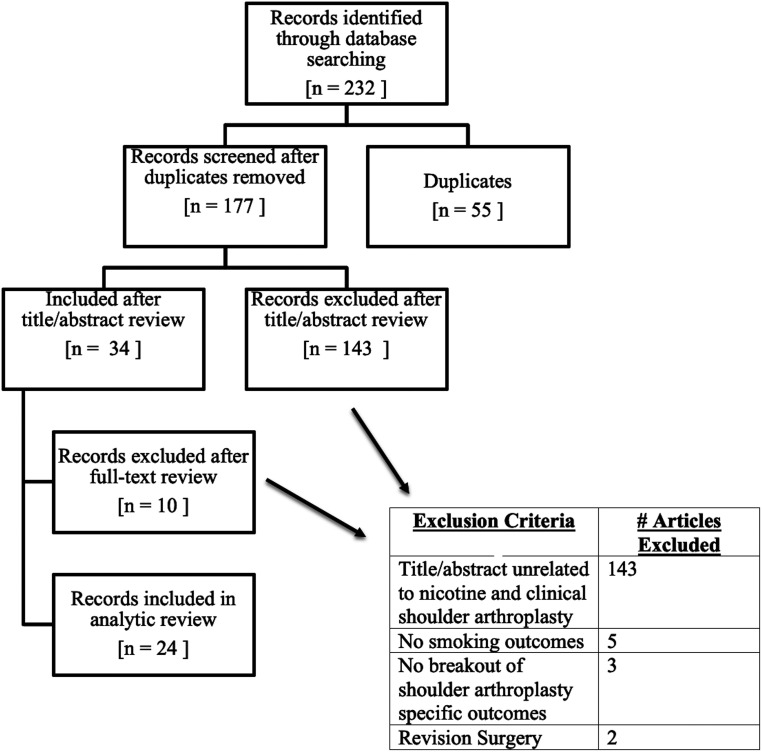
Figure 1.
PRISMA flowchart.
The initial search yielded 232 articles (55 duplicates, 177 screened, and 34 full-text reviews). Of these, 24 studies met the inclusion criteria and were analyzed. These studies included one basic science study, two cohort studies (Level of Evidence II), 14 case–control studies (Level of Evidence III), and seven case series (Level of Evidence IV). Descriptive statistics calculated in the included studies were used during the analysis. Categorical variables were reported as proportions, while continuous variables were reported as means with minimum to maximum absolute ranges. All studies used statistical analyses with p < 0.05 as the statistically significant threshold.
Results
Pain and opioid use
Eight studies analyzing pain and opioid use found statistically significant higher subjective pain scores or opioid use in smokers compared to nonsmokers following TSA (Table 1).11–18 The two studies investigating visual analog scale (VAS) scores for reverse TSA found significantly higher scores in smokers than nonsmokers (p < .05).13,18 The other two studies investigating VAS scores after anatomic TSA found significantly higher scores for active smokers (p < .05).15,16 A notable finding was that former smokers had lower VAS scores than nonsmokers and active smokers, suggesting that smoking cessation leads to significant pain reduction postoperatively (p < .05).13,15,16
Table 1.
Pain and opioid use.
| Level of evidence | Author(s) | Journal | Number of patients, mean age (years) | Type of arthroplasty | Pertinent findings |
|---|---|---|---|---|---|
| III | Best et al 11 (2021) | Orthopedics | 5676 (57) | Anatomic TSA | Smokers had significantly increased postoperative opioid dependence (OR, 1.3 [95% CI, 1.0–1.7], p = .034). |
| III | Kolade et al 12 (2020) | Journal of Shoulder and Elbow Surgery | 622 (68) | Anatomic TSA & reverse TSA | Inpatient opioid consumption was measured in morphine milligram equivalents (MME). Active smokers had higher MME compared to never smokers (38.2 and 24.9, respectively; p = .021). Former smokers had MME of 31.6 (p = .3) when compared to active smokers. Expected decrease in inpatient opioid use postoperatively was lower in active smokers than former smokers (p = .03) and never smokers (p = .01). Longer duration of smoking (more pack-years) showed lower reduction of opioid consumption (p = .03). |
| III | Walters et al 13 (2020) | Journal of Shoulder and Elbow Surgery | 186 (70) | Reverse TSA | Postoperative active smokers (2.5) experienced less pain relief than nonsmokers (1.8) and former smokers (1.0) (P = .014). |
| III | Khazi et al 14 (2020) | Journal of Shoulder and Elbow Surgery | 12,038 (NR) | anatomic TSA & reverse TSA | Smokers at 12 months postoperatively had increased opioid dependence for anatomic TSA (OR, 1.36 [95% CI, 1.11–1.66], p = .0023), but not for reverse TSA (OR, 1.17 [95% CI, 0.96–1.41], p = .119). |
| III | Walters et al 15 (2018) | Current Orthopaedic Practice | 102 (66) | Anatomic TSA | Smokers averaged a VAS score 3.9 times higher than nonsmokers (1.3) and former smokers (1.0). Smokers to nonsmokers, p = .04. |
| III | Wells et al 16 (2018) | Journal of Shoulder and Elbow Surgery | 163 (61) | Anatomic TSA | Cumulative OME use 12 weeks postoperatively was higher in smokers (2348 mg) than nonsmokers (1637 mg) and former smokers (1623 mg) (p < .003). Average OME/day was higher in smokers than non- and former smokers (p < .003). Preoperative VAS scores were higher in smokers than non- and former smokers (p < .001) for both. Mean reduction of VAS scores was significantly lower in smokers compared to non- and former smokers (2.8 versus 4 and 4.3, p < .02). |
| IV | Martusiewicz et al 17 (2020) | Journal of Shoulder and Elbow Surgery | 50 (63) | Anatomic TSA | Smokers consumed 211.6 more MEUs than nonsmokers (SE, 92.3; P = .03), while former smokers consumed 110.9 more MEUs than nonsmokers (SE, 50.2; p = .03). |
| III | Friedman et al 18 (2019) | Journal of Shoulder and Elbow Surgery | 2421 (NR) | Anatomic TSA & reverse TSA | Smokers had increased VAS scores by 0.81 compared to nonsmokers (p < .005) for reverse TSA. Anatomic TSA findings were nonsignificant. |
CI: confidence interval; MEU: morphine equivalent units; MME: morphine milligram equivalents; NR: not reported; OME: oral morphine equivalents; OR: odds ratio; SE: standard error; TSA: total shoulder arthroplasty; VAS: visual analog scale.
Kolade et al. 12 reported that smoking was a modifiable risk factor for increased inpatient opioid use following TSA. Active smokers had an average morphine milligram equivalents (MMEs) of 38.2, compared to patients who had never smoked (MME 24.9, p = 0.021) and former smokers (MME 31.6, p = 0.3). The expected decrease in inpatient opioid use postoperatively was lower in active smokers than in former smokers (p = .03) and nonsmokers (p = .01). Longer duration of smoking (more pack-years) also showed a lower than expected reduction in opioid consumption (p = .03).
The four studies evaluating postoperative opioid dependence following anatomic TSA all found a significant increase for smokers compared to nonsmokers.11,14,16,17 Only one study evaluated postoperative opioid dependence following reverse TSA and found no significant difference among smoker categories. 14
Hospital-related outcomes
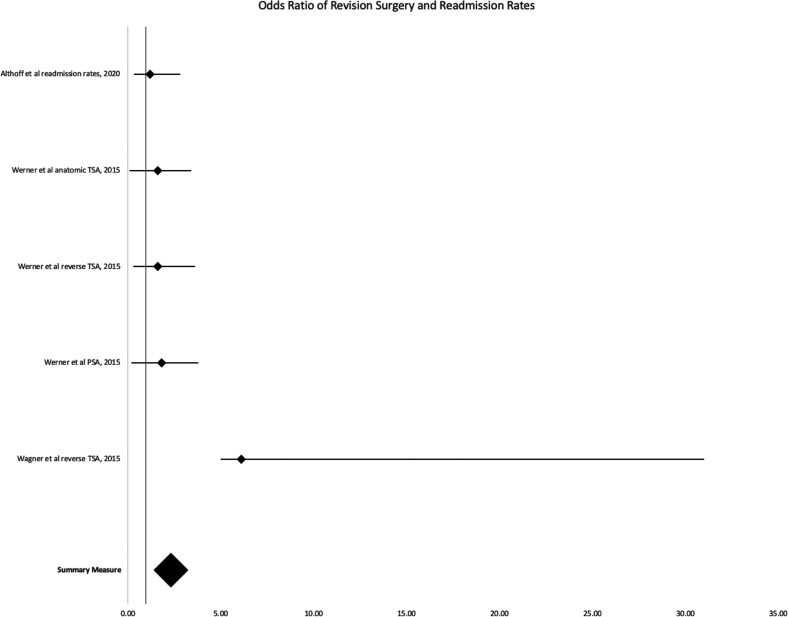
Of the six studies analyzing smoking and revision TSA, five found increased rates of revision surgery in smokers (Table 2 and Figure 2).15,19–23 Four studies, shown in Figure 2, have a combined odds ratio of 1.59 (95% CI, 1.30–1.89). Walters et al. 15 was the only study to claim no difference in revision surgery rates for smokers; these authors also noted they routinely preferred nonoperative management over revision surgery in patients who smoked.
Table 2.
Hospital-related outcomes.
| Level of evidence | Author(s) | Journal | Number of patients, mean age (years) | Type of arthroplasty | Pertinent findings |
|---|---|---|---|---|---|
| III | Althoff et al 10 (2020) | Journal of Shoulder and Elbow Surgery | 14,465 (NR) | anatomic TSA & reverse TSA | No significant difference in 30-day hospital readmission rates between smokers and nonsmokers undergoing TSA (OR, 1.2 [95% CI, 0.885–1.626], p = .241). |
| IV | Leong et al 19 (2020) | HSS Journal | 824 (NR) | NR | Smokers had significantly higher need for revision surgery (OR 8.1, p < .001). |
| IV | Samuelsen et al 20 (2017) | Journal of Shoulder and Elbow Surgery | 63 (60) | Reverse TSA | Smokers had significantly higher need for revision surgery (p < .001) |
| III | Werner et al 21 (2015) | Journal of Shoulder and Elbow Surgery | 22,138 (NR) | Anatomic TSA, reverse TSA, and PSA | Smokers had significantly higher rates of early (< 1 year) revision surgery than nonsmokers for anatomic TSA (OR, 1.6 [95% CI, 1.5–1.8], p < .0001), reverse TSA (OR, 1.6 [95% CI, 1.3–2.0], p < .0001), and PSA (OR, 1.8 [95% CI, 1.6–2.0], p < .0001). |
| II | Schwartz et al 22 (2020) | Bone & Joint Journal | 210,786 (NR) | NR | Smokers had increased 30- and 90-day readmission rates compared to nonsmokers (p = .025 and p = .001, respectively). Smokers also had significantly higher rates of revision surgery within 90 days (p < .001). |
| IV | Matar et al 24 (2021) | Journal of Shoulder and Elbow Surgery | 50 (63) | Anatomic TSA & reverse TSA | HCAHPS satisfaction scores were significantly higher for nonsmokers compared to smokers (77.7 ± 22 and 59.6 ± 5.2, p = .03). There was no significant difference in CG-CAHPS satisfaction scores between smokers and nonsmokers. |
| III | Wagner et al 23 (2015) | Journal of Bone & Joint Surgery | 143 (69) | Reverse TSA | Smoking was identified as a statistically significant risk factor for revision surgery (HR, 6.11 [95% CI, 1.11–24.9], p < .04). |
CG-CAHPS: Clinician and Group Consumer Assessment of Healthcare Providers and Systems; CI: confidence interval; HCAHPS: Hospital Consumer Assessment of Healthcare Providers and Systems; HR: hazard ratio; NR: not reported; OR: odds ratio; PSA: partial shoulder arthroplasty; TSA: total shoulder arthroplasty.
Figure 2.
Forest plot 1: odds ratio of revision surgery and readmission rates.
Although Althoff et al. 10 found no significant difference in 30-day hospital readmission rates between smokers and nonsmokers undergoing TSA (P = 0.241), they did observe that the percentage of smokers readmitted was greater than nonsmokers (0.6% vs. 0.3%, respectively). Schwartz et al. 22 noted smokers had increased 30- and 90-day readmission rates following shoulder arthroplasty compared to nonsmokers (p = 0.025 and p = 0.001, respectively).
Consumer Assessment of Healthcare Providers and Systems (CAHPS) scores are used by CMS for financial reimbursement, so it is important for healthcare providers to manage factors that influence them. Surveys on patient perspectives of their care showed Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) satisfaction scores were significantly higher for nonsmokers compared to smokers following shoulder arthroplasty (p = 0.03). 24 There was no significant difference in Clinician and Group Consumer Assessment of Healthcare Providers and Systems (CG-CAHPS) satisfaction scores between smokers and nonsmokers. 24
Measures of shoulder function
Smoking was not found to have deleterious effects on postoperative range of motion (ROM) improvement in three studies (Table 3).13,18,25 Friedman et al. 18 found smoking to be significantly associated with decreased internal rotation scores for reverse TSA patients (p = 0.002).
Table 3.
Measures of shoulder function.
| Level of evidence | Author(s) | Journal | Number of patients, mean age (years) | Type of arthroplasty | Pertinent findings |
|---|---|---|---|---|---|
| III | Walters et al 13 (2020) | Journal of Shoulder and Elbow Surgery | 186 (70) | Reverse TSA | Smokers did not significantly improve postoperatively for internal and external rotation (p = 0.31 and p = 0.83, respectively). Nonsmokers and former smokers had significant (p < 0.05) improvements postoperatively for all forward flexion and internal/external rotation. Nonsmokers had nonsignificant improvement in external rotation. |
| III | Polce et al 26 (2021) | Journal of Shoulder and Elbow Surgery | 204 (66) | Anatomic TSA & reverse TSA | A two-year follow-up, patient-reported outcomes survey found that smokers had lower rates of achieving the PASS than nonsmokers after anatomic TSA: PASS on ASES (OR, 0.5 [95% CI, 0.279–0.896], p = .022), PASS for Constant-Murley (OR, 0.48 [95% CI, 0.251–0.909], p = .027). The PASS for SANE in anatomic TSA was nonsignificant for smokers. The reverse TSA cohort had nonsignificant PASS score differences for smokers in the ASES, SANE, and Constant–Murley surveys. |
| III | Walters et al 15 (2018) | Current Orthopaedic Practice | 102 (66) | Anatomic TSA | No significant difference in smokers for ROM and SANE scores. The mean ASES score in smokers is significantly lower (62) than nonsmokers (79) and former smokers (84) (p = .0007), indicating worse postoperative shoulder function. |
| III | Wells et al 16 (2018) | Journal of Shoulder and Elbow Surgery | 163 (61) | Anatomic TSA | LOS was not significantly longer in smokers than former and nonsmokers (1.21 days vs. 0.95 vs. 1.07). |
| IV | Levy et al 25 (2016) | Journal of Shoulder and Elbow Surgery | 230 (70) | Anatomic TSA | Smokers did not have statistically different ROM postoperatively from nonsmokers (R, 0.078; p = .23). |
| III | Friedman et al 18 (2019) | Journal of Shoulder and Elbow Surgery | 2421 (NR) | Anatomic TSA & reverse TSA | Smoking had significant effects on reverse TSA scores postoperatively. It negatively influenced the following postoperative scores: internal rotation scores by 0.7 points (p = .002), ASES scores by 8.7 points (p = .001), Shoulder Function Score by 0.6 points (p = .033), SST score by 1.2 points (p = .001), and UCLA score by 1.6 points (p = .02). Tobacco use did not have significant effects on postoperative reverse TSA scores for abduction, forward flexion, and external rotation. Smoking did not have significant effects postoperatively for any anatomic TSA shoulder scores. |
ASES: American Shoulder and Elbow Surgeons Shoulder Score; LOS: length of stay; NR: not reported; PASS: Patient Acceptable Symptomatic State; ROM: range of motion; SANE: Single Assessment Numeric Evaluation; SST: Simple Shoulder Test; TSA: total shoulder arthroplasty; UCLA: University of California Los Angeles.
Smoking was found as an independent risk factor for reduced Patient Acceptable Symptom State (PASS) achievement on American Shoulder and Elbow Surgeons (ASES) shoulder score for anatomic TSA by two-thirds of the studies,15,18,26 and half of the studies for reverse TSA.18,26 Two studies evaluated PASS achievement for Single Assessment Numerical Evaluation and found no statistical difference due to smoking status.15,26 The single study looking at PASS for Constant–Murley found significantly lower PASS scores for anatomic TSA but not reverse TSA. 26
Although the length of hospital stay was longer for current smokers than for former and nonsmokers (1.21 days, 0.95, and 1.07 days, respectively), it was not a statistically significant difference. 16
Friedman et al. 18 noted that shoulder function score, Simple Shoulder Test score, and University of California Los Angeles postoperative scores were not significantly different for smokers undergoing anatomic TSA. However, all these scores were significantly lower in smokers undergoing reverse TSA. The study by Friedman et al. 18 had a mean follow-up of 49 months, while Polce et al. 26 completed their follow-up at the two-year mark, which may contribute to the differences in shoulder function scores.
Postoperative complications
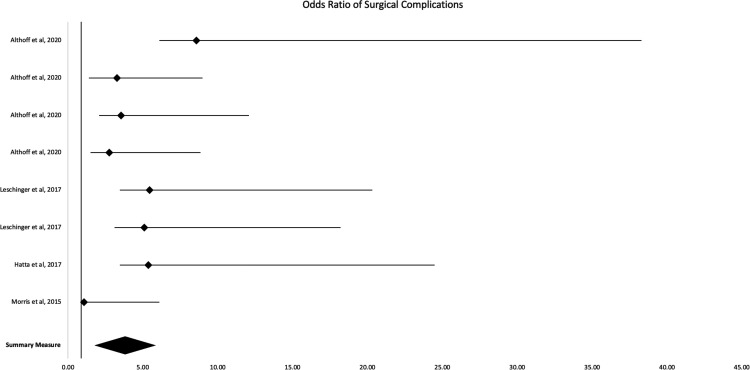
Mixed results were found regarding postoperative TSA complications (Table 4 and Figure 3). Four studies, shown in Figure 3, have a combined odds ratio of 2.92 (95% CI, 1.80–4.04). Patients who smoke were significantly (p < 0.05) more likely to have increased rates of surgical, wound, superficial, and deep surgical site complications. 10 Two studies found supporting evidence that smokers have significantly increased rates of wound complications following shoulder arthroplasty.10,22 Roach et al. 27 found a higher incidence of positive culture on subscapularis tagging sutures compared to nonsmokers and former smokers, indicating an increased risk of infection for smokers.
Table 4.
Postoperative complications.
| Level of evidence | Author(s) | Journal | Number of patients, mean age (years) | Type of arthroplasty | Pertinent findings |
|---|---|---|---|---|---|
| III | Althoff et al. 10 (2020) | Journal of Shoulder and Elbow Surgery | 14,465 (NR) | Anatomic TSA & reverse TSA | Smoking was not significantly associated with higher rates of medical complications (cardiac arrest or MI, pulmonary complication, renal injury, UTI, DVT or PE, sepsis, or death). Smokers had increased rates of wound complications (OR, 8.586 [95% CI, 2.483–29.69], p = .001), and surgical complications (OR, 3.259 [95% CI, 1.861–5.709], p < .001), including superficial surgical-site infection (OR, 3.537 [95% CI, 1.464–8.542], p = .005) and deep surgical-site infection (OR, 2.749 [95% CI, 1.238–6.104], p = .013). |
| Basic science | Roach et al 27 (2019) | Journal of Shoulder and Elbow Surgery | 50 (70) | Anatomic TSA & reverse TSA | Current smokers had significantly higher risk of positive culture on subscapularis tagging suture compared to nonsmokers and former smokers (OR, 12.3, [95% CI, 1.14–132.9], p = .038). |
| III | Walters et al 15 (2018) | Current Orthopaedic Practice | 102 (66) | Anatomic TSA | Smokers were found to have higher complication rates following anatomic TSA compared to nonsmokers and former smokers (36%, 15%, and 7% respectively; p = .05). No significant difference was found for periprosthetic radiolucency between the groups. |
| III | Wells et al 16 (2018) | Journal of Shoulder and Elbow Surgery | 163 (61) | Anatomic TSA | No statistically significant difference was found between complication rates following anatomic TSA among current smokers (18%), nonsmokers (11%, p = .35), and former smokers (8%, p = .28). |
| IV | Leschinger et al 29 (2017) | Journal of Shoulder and Elbow Surgery | 275 (68) | Anatomic TSA | Smokers had significantly increased risk for Category I complications (intraoperative complications and complications not requiring revision: nerve palsy, implant dislocation, humeral fracture, glenoid fracture, temporary glenohumeral dislocation, intraoperative bleeding, and implant instability) compared to nonsmokers following anatomic TSA (OR, 5.44 [95% CI, 1.99–14.88], p = .002). No difference was found in rates of Category II complications (complications needing soft-tissue revision: recurrent glenohumeral dislocation, wound infection, and contractures at shoulder) between the smoking and nonsmoking groups. The overall complication rates between smokers and nonsmokers who underwent anatomic TSA was significantly different (OR, 5.08 [95% CI, 1.96–13.11], p = .002). |
| II | Hatta et al 28 (2017) | Journal of Shoulder and Elbow Surgery | 1614 (69) | Anatomic TSA & reverse TSA | Univariate analysis of complication rates between smokers and nonsmokers who underwent TSA showed a statistically significant difference (HR, 1.75 [95% CI, 1.28–2.75], p = .013); however, multivariable-adjusted analyses failed to show a significant difference between the smoking and nonsmoking groups. Univariate analysis showed significantly more postoperative fractures among the smoking group compared to the nonsmoking group (HR, 4.5 [95% CI, 1.09–30.21], p = .037), while multivariate analysis did not show a significant difference between the groups. There was also no significant difference in rates of intraoperative fractures between the groups. The rate of periprosthetic infection in patients who underwent TSA was significantly higher for the smoking group compared to the nonsmoking group with both univariate analysis (HR, 5.22 [95% CI, 1.92–18.23], p < .001) and multivariate analysis (HR, 5.36 [95% CI, 1.91–19.11], p < .001). |
| IV | Samuelsen et al 20 (2017) | Journal of Shoulder and Elbow Surgery | 63 (60) | Reverse TSA | Smokers had significantly higher rates of complications (p = .01) and postoperative dislocation (p = .04) compared to nonsmokers. |
| III | Morris et al 31 (2015) | Journal of Shoulder and Elbow Surgery | 301 (68) | Reverse TSA | The rate of periprosthetic infection in patients who underwent reverse TSA was not significantly higher for smokers compared to nonsmokers (OR, 1.07 [95% CI, 0.11–5.02], p = .999). |
| IV | Otto et al 30 (2013) | Journal of Shoulder and Elbow Surgery | 265 (75 [median]) | Reverse TSA | c2 analysis did not show a significant difference in scapular fracture rates after reverse TSA between smoking and nonsmoking patients. |
| III | Singh et al 32 (2017) | Journal of Shoulder and Elbow Surgery | 84 (66.9) | Hemiarthroplasty | Smokers did not have a significant difference in anatomic tuberosity healing compared to nonsmokers (OR for union, 1.21; p = .73). |
| II | Schwartz et al 22 (2020) | Bone & Joint Journal | 210,786 (NR) | NR | Smokers had significantly increased rates of wound complications, infection, prosthesis instability, postoperative pneumonia, postoperative myocardial infarction (p < .001 for all), and postoperative sepsis (p = .001). |
| III | Wagner et al 23 (2015) | Journal of Bone & Joint Surgery | 143 (69) | Reverse TSA | Smoking significantly increased glenoid lucency (p < .01), but did not significantly influence the graft resorption, humeral lucency, or scapular notching. |
CI: confidence interval; DVT: deep vein thrombosis; HR: hazard ratio; MI: myocardial infarction; NR: not reported; OR: odds ratio; PE: pulmonary embolism; TSA: total shoulder arthroplasty; UTI: urinary tract infection.
Figure 3.
Forest plot 2: odds ratio of surgical complications.
General complication rates for reverse TSA in association with smokers were found by one study to be significantly higher (p = 0.01). 20 Hatta et al. 28 found that smoking was only significant for anatomic TSA/reverse TSA during univariate analysis, but not during multivariate analysis (p = 0.013 vs. p = 0.11, respectively). Two-thirds of studies strictly analyzing anatomic TSA complication rates found that smokers had significantly higher rates.15,16,29
Hatta et al. 28 also found that smoking was only significant for anatomic TSA/reverse TSA during univariate analysis for postoperative fractures, but not during multivariate analysis (p = 0.037 vs. p = 0.068, respectively). One other study evaluating scapular fractures after reverse TSA found smokers did not have increased rates of postoperative fractures. 30 Lastly, Hatta et al. 28 found periprosthetic infections were significantly increased in smokers for univariate and multivariate analysis (p < 0.001). This was supported by Schwartz et al 22 ; conversely, Morris et al. 31 looked at only reverse TSA and found opposing data.
Radiographic outcome data by Wagner et al. 23 found that smokers had increased glenoid lucency (p < 0.01), while Walters et al 15 found no significant difference in periprosthetic radiolucency. Singh et al. 32 found that smokers did not have a significant difference in anatomic tuberosity healing compared to nonsmokers. Schwartz et al. 22 ultimately found that smokers were significantly more likely to have instability of the prosthesis, postoperative pneumonia, sepsis, and myocardial infarction (p < 0.001). However, Althoff et al. 10 found no significant difference in smoking status and medical complications such as cardiac arrest or myocardial infarction, pulmonary complication, renal injury, urinary tract infection, urinary tract infection, deep vein thrombosis, pulmonary embolism, sepsis, or death.
Discussion
The goal of reviewing current literature on TSA postoperative outcomes was to identify methods for decreasing complication rates. Our review identifies tobacco smoking as a modifiable risk factor closely associated with worse postoperative outcomes, making it a prime target for intervention. Implementation of preoperative smoking cessation protocol will result in improved patient outcomes and reduced costs (associated with complications) to providers. There are some factors to consider prior to surgery on patients with a history of smoking: smoking status may influence the surgical approach, and outcomes may be improved by smoking cessation preoperatively.
Our data show that, when deciding between reverse TSA or anatomic TSA, the smoking status may warrant consideration. Polce et al 26 found that patient-reported ASES outcome scores were significantly lower in the smoking group compared to the nonsmoking group for anatomic TSA, but were not significantly different between smokers and nonsmokers for reverse TSA. The same study also found a similar result for patient-reported outcome scores on the Constant–Murley assessment. A study by Walters et al. 13 suggested that reverse TSA may yield more favorable outcomes than anatomic TSA in patients with a history of smoking, possibly attributable to reverse TSA circumventing the rotator cuff, which is especially affected by smoking. However, in direct contradiction, Friedman et al. 18 found no negative association between tobacco use on anatomic TSA patient outcomes but observed a negative influence on ROM and passive ROM scores following reverse TSA. Currently, there is not enough evidence to indicate the superiority of one procedure over the other in terms of postoperative outcomes for patients who smoke. Further investigation is required to identify which technique yields the least complications for the subset of TSA patients that consume nicotine products.
That smokers have inferior postoperative outcomes compared to nonsmokers is well-documented; however, smoking cessation prior to TSA can significantly reduce negative outcomes. Patients with a history of smoking who quit at least one month prior to TSA had improved outcomes compared to current smokers, with the largest improvement in the rate of periprosthetic fractures. 28 Longer preoperative smoking cessation for former smokers was associated with postoperative outcomes comparable to nonsmokers.15,16 Walters et al. 15 found that two-month smoking cessation prior to TSA lowered the risk of complications, pain, and dysfunction to nonsmoker levels. In another study, Wells et al. 16 found patients that quit smoking at least three months prior to anatomic TSA had postoperative outcomes similar to nonsmokers.
A similar review investigating the potential benefits of smoking cessation prior to all types of total joint arthroplasty found results congruent to ours. 7 Although there are limited data regarding preoperative smoking cessation models and the quitting time required to yield improved postoperative outcomes, 33 information on preoperative smoking cessation models indicates that significant improvements in postoperative outcomes occur when patients stop smoking at least four weeks prior to surgery, and outcomes can be further improved with longer quit times.34–36 Our findings specific to TSA parallel those of McConaghy et al. 7 Based on the studies analyzed in our review, the clinical recommendation for a four-week minimum preoperative smoking cessation program prior to total joint arthroplasty is applicable for most patients prior to TSA. Although benefits may be seen with as little as one month of smoking cessation, patients should be encouraged to abstain from smoking for as long as possible prior to TSA given the additional reductions in negative postoperative outcomes seen with longer quit times.
The primary strength of this review is the clinically relevant guidance that can be gained from our observations. However, a few limitations should also be recognized. The large breadth of included articles meant there was little homogeneity in methodologies. The lack of standardization for smoking definitions introduces ambiguity when discussing smoking outcomes. Among the studies analyzed in this review, most did not provide the criteria used to categorize patients as current, former, or nonsmokers. Those that did provide criteria were usually not standardized, which complicated analysis. The Centers for Disease Control and Prevention's National Health Interview Survey provides standardized and generalizable criteria for a current smoker as “an adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes” and a former smoker as “an adult who has smoked at least 100 cigarettes in his or her lifetime but who had quit smoking at the time of interview.” 37 Additionally, the definition of former smokers can be further refined by standardizing a quantifiable quit time, or the length of time passed since last tobacco consumption. Of the studies included, only Hatta et al. 28 and Wells et al. 16 quantified minimum quit time to be considered a former smoker as one month and three months prior to surgery, respectively. Additionally, no studies stratified smokers based on the quantity of tobacco consumption. This leaves unanswered questions regarding the dose–response effect of smoking and whether higher consumption requires longer smoking cessation to receive the same protective effects. Currently, the CDC NHIS divides current smokers into “everyday smokers” and “someday smokers” to increase specificity regarding the quantity smoked; however, a numerical classification of cigarettes or packs per day would be more advantageous to draw conclusions about the quantity of tobacco consumption. 37 Regardless of variations in study methods, a regular result was that preoperative smoking cessation was correlated with better outcomes following TSA. Many of the studies may be affected by recall bias since they depended on patients to report smoking cessation timelines. Due to possible recall bias and lack of homogeneous methodology, a large-scale, prospectively controlled study over TSA postoperative outcomes would provide more evidence.
This review demonstrates that smoking is significantly associated with poor clinical outcomes following shoulder arthroplasty. Current literature shows that smoking is associated with numerous postoperative complications, increased opioid use, and worse shoulder function improvement scores. Smoking, likely mediated by nicotine, interferes with the postoperative recovery process. Smoking directly impairs the immune system and causes increased susceptibility to infection. 38 Specifically, nicotine is immunosuppressive via inhibition of the innate immune system, which is important in preventing postoperative wound infection. 39 Nicotine use also impairs wound healing by reducing collagen synthesis.40–42 Since nicotine is one of the main harmful ingredients of cigarettes, the use of nicotine replacement therapy, electronic cigarettes, or “vapes” likely produces similarly inferior postoperative outcomes. Furthermore, there is large variability between the nicotine content of different electronic cigarettes, which are not always accurately dosed. 43 Electronic cigarettes are still a newer form of nicotine consumption that needs to be studied longitudinally for us to have a comprehensive picture of their deleterious impact on postoperative recovery. Importantly, former smokers had lower complication rates and VAS scores when compared to current users. To improve postoperative outcomes following preoperative smoking cessation, healthcare providers should work with all patients who actively smoke tobacco products at the time of initial consult to identify a preoperative smoking cessation plan that is achievable for at least one month prior to surgery.
Footnotes
Authors’ Note: Work for this study was performed at The University of Texas Medical Branch, Galveston, TX.
Contributorship: SAK: conceptualization, formal analysis, investigation, methodology, project administration, writing – original draft, writing – review and editing. RKP: formal analysis, investigation, methodology, writing – original draft, writing – review and editing. JSS: conceptualization, methodology, project administration, supervision, writing – original draft, writing – review and editing. All authors approved the final version.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JSS reports educational support from Arthrex, Medinc of Texas, and DJO/Encore.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: JSS.
ORCID iD: Jeremy S Somerson https://orcid.org/0000-0001-7272-5922
References
- 1.Day JS, Lau E, Ong KL, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010; 19: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011; 93: 2249–2254. [DOI] [PubMed] [Google Scholar]
- 3.Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg 2002; 11: 130–135. [DOI] [PubMed] [Google Scholar]
- 4.Cowling PD, Holland P, Kottam L, et al. Risk factors associated with intraoperative complications in primary shoulder arthroplasty. Acta Orthop 2017; 88: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Smoking and tobacco use fast facts, https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm (accessed January 9, 2021).
- 6.National Center for Chronic Disease P, Health Promotion Office on S and Health. Reports of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US), 2014. [Google Scholar]
- 7.McConaghy K, Kunze KN, Murray T, et al. Smoking cessation initiatives in total joint arthroplasty: an evidence-based review. JBJS Rev 2021; 9: e21.00009. DOI: 10.2106/jbjs.Rvw.21.00009. [DOI] [PubMed] [Google Scholar]
- 8.Møller AM, Pedersen T, Villebro N, et al. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br 2003; 85: 178–181. [DOI] [PubMed] [Google Scholar]
- 9.Møller AM, Villebro N, Pedersen T, et al. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002; 359: 114–117. [DOI] [PubMed] [Google Scholar]
- 10.Althoff AD, Reeves RA, Traven SA, et al. Smoking is associated with increased surgical complications following total shoulder arthroplasty: an analysis of 14,465 patients. J Shoulder Elbow Surg 2020; 29: 491–496. [DOI] [PubMed] [Google Scholar]
- 11.Best MJ, Harris AB, Bansal A, et al. Predictors of long-term opioid use after elective primary total shoulder arthroplasty. Orthopedics 2021; 44: 58–63. [DOI] [PubMed] [Google Scholar]
- 12.Kolade OO, Ghosh N, Fernandez L, et al. Study of variations in inpatient opioid consumption after total shoulder arthroplasty: influence of patient- and surgeon-related factors. J Shoulder Elbow Surg 2020; 29: 508–515. [DOI] [PubMed] [Google Scholar]
- 13.Walters JD, George LW, 2nd, Walsh RN, et al. The effect of current and former tobacco use on outcomes after primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2020; 29: 244–251. [DOI] [PubMed] [Google Scholar]
- 14.Khazi ZM, Lu Y, Patel BH, et al. Risk factors for opioid use after total shoulder arthroplasty. J Shoulder Elbow Surg 2020; 29: 235–243. [DOI] [PubMed] [Google Scholar]
- 15.Walters J, George L, Wan J, et al. Tobacco use results in inferior outcomes after anatomic total shoulder arthroplasty. Curr Orthop Pract 2018; 30: 1. [Google Scholar]
- 16.Wells DB, Holt AM, Smith RA, et al. Tobacco use predicts a more difficult episode of care after anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 23–28. [DOI] [PubMed] [Google Scholar]
- 17.Martusiewicz A, Khan AZ, Chamberlain AM, et al. Outpatient narcotic consumption following total shoulder arthroplasty. JSES Int 2020; 4: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman RJ, Eichinger J, Schoch B, et al. Preoperative parameters that predict postoperative patient-reported outcome measures and range of motion with anatomic and reverse total shoulder arthroplasty. JSES Open Access 2019; 3: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong NL, Sumner S, Gowd A, et al. Risk factors and complications for revision shoulder arthroplasty. HSS J 2020; 16: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuelsen BT, Wagner ER, Houdek MT, et al. Primary reverse shoulder arthroplasty in patients aged 65 years or younger. J Shoulder Elbow Surg 2017; 26: e13–e17. [DOI] [PubMed] [Google Scholar]
- 21.Werner BC, Burrus MT, Begho I, et al. Early revision within 1 year after shoulder arthroplasty: patient factors and etiology. J Shoulder Elbow Surg 2015; 24: e323–e330. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz AM, Farley KX, Boden SH, et al. The use of tobacco is a modifiable risk factor for poor outcomes and readmissions after shoulder arthroplasty. Bone Joint J 2020; 102–b: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 23.Wagner E, Houdek MT, Griffith T, et al. Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am 2015; 97: 1653–1660. [DOI] [PubMed] [Google Scholar]
- 24.Matar RN, Shah NS, Vincent JC, et al. Factors that influence inpatient satisfaction after shoulder arthroplasty. J Shoulder Elbow Surg 2021; 30: e165–e172. [DOI] [PubMed] [Google Scholar]
- 25.Levy JC, Ashukem MT, Formaini NT. Factors predicting postoperative range of motion for anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 55–60. [DOI] [PubMed] [Google Scholar]
- 26.Polce EM, Wolfson TS, Skallerud WK, et al. Establishing thresholds for achievement of clinically significant satisfaction at two years following shoulder arthroplasty: the patient acceptable symptomatic state. Semin Arthrop: JSES 2021; 31: 159–170. [Google Scholar]
- 27.Roach R, Yu S, Pham H, et al. Microbial colonization of subscapularis tagging sutures in shoulder arthroplasty: a prospective, controlled study. J Shoulder Elbow Surg 2019; 28: 1848–1853. [DOI] [PubMed] [Google Scholar]
- 28.Hatta T, Werthel JD, Wagner ER, et al. Effect of smoking on complications following primary shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: –6. [DOI] [PubMed] [Google Scholar]
- 29.Leschinger T, Raiss P, Loew M, et al. Total shoulder arthroplasty: risk factors for intraoperative and postoperative complications in patients with primary arthritis. J Shoulder Elbow Surg 2017; 26: e71–e77. [DOI] [PubMed] [Google Scholar]
- 30.Otto RJ, Virani NA, Levy JC, et al. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg 2013; 22: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 31.Morris BJ, O’Connor DP, Torres D, et al. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 161–166. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Padilla M, Nyberg EM, et al. Cement technique correlates with tuberosity healing in hemiarthroplasty for proximal humeral fracture. J Shoulder Elbow Surg 2017; 26: 437–442. [DOI] [PubMed] [Google Scholar]
- 33.Ring J, Shoaib A, Shariff R. Smoking cessation advice in limb reconstruction: an opportunity not to be missed. Injury 2017; 48: 345–348. [DOI] [PubMed] [Google Scholar]
- 34.Wong J, Lam DP, Abrishami A, et al. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth 2012; 59: 268–279. [DOI] [PubMed] [Google Scholar]
- 35.Tønnesen H, Nielsen PR, Lauritzen JB, et al. Smoking and alcohol intervention before surgery: evidence for best practice. Br J Anaesth 2009; 102: 297–306. [DOI] [PubMed] [Google Scholar]
- 36.Mills E, Eyawo O, Lockhart I, et al. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med 2011; 124: 144–154. e148. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. National Health Interview Survey: adult tobacco use information, https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm (2017, accessed October 6, 2021).
- 38.Hersey P, Prendergast D, Edwards A. Effects of cigarette smoking on the immune system. Follow-up studies in normal subjects after cessation of smoking. Med J Aust 1983; 2: 425–429. [PubMed] [Google Scholar]
- 39.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res 2008; 57: 497–503. [DOI] [PubMed] [Google Scholar]
- 40.Knuutinen A, Kokkonen N, Risteli J, et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol 2002; 146: 588–594. [DOI] [PubMed] [Google Scholar]
- 41.Ramp WK, Lenz LG, Galvin RJ. Nicotine inhibits collagen synthesis and alkaline phosphatase activity, but stimulates DNA synthesis in osteoblast-like cells. Proc Soc Exp Biol Med 1991; 197: 36–43. [DOI] [PubMed] [Google Scholar]
- 42.Jorgensen LN, Kallehave F, Christensen E, et al. Less collagen production in smokers. Surgery 1998; 123: 450–455. [PubMed] [Google Scholar]
- 43.Srbinoska M, Kavrakovski Z, Rafajlovska V, et al. Determined and declared nicotine content in refill liquids for electronic cigarettes marketed in north Macedonia. Arh Hig Rada Toksikol 2019; 70: 130–133. [DOI] [PubMed] [Google Scholar]