Abstract
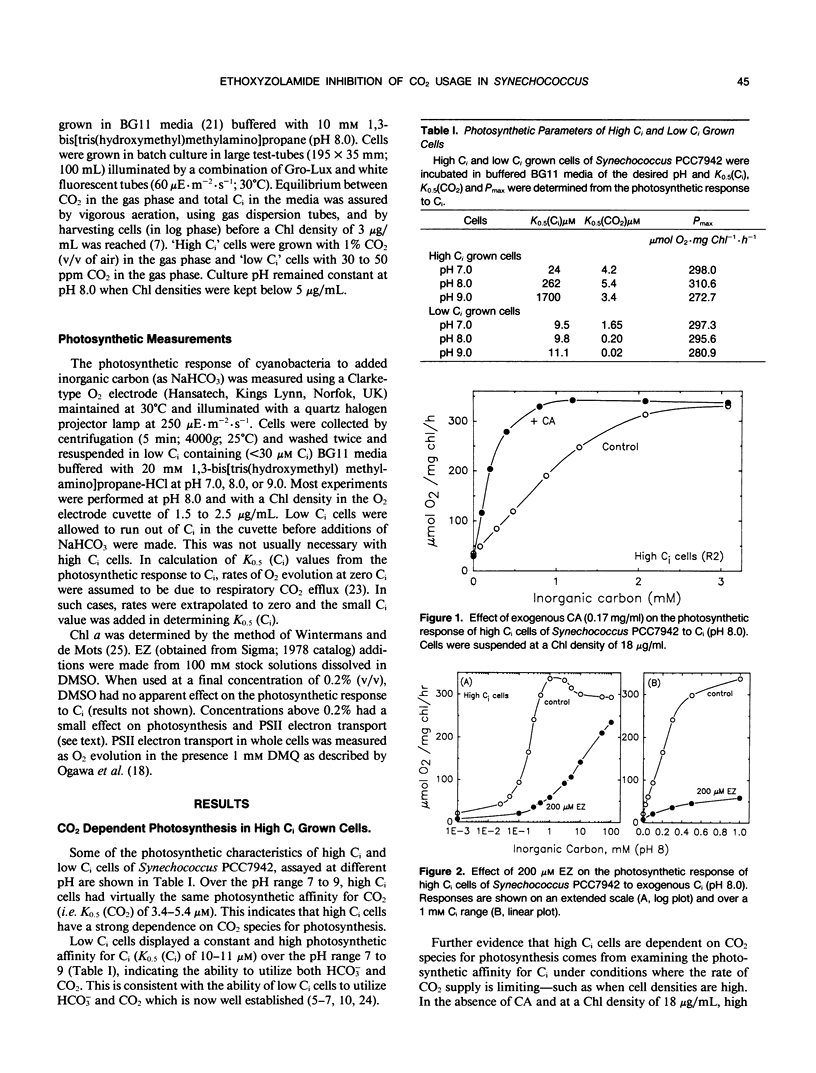
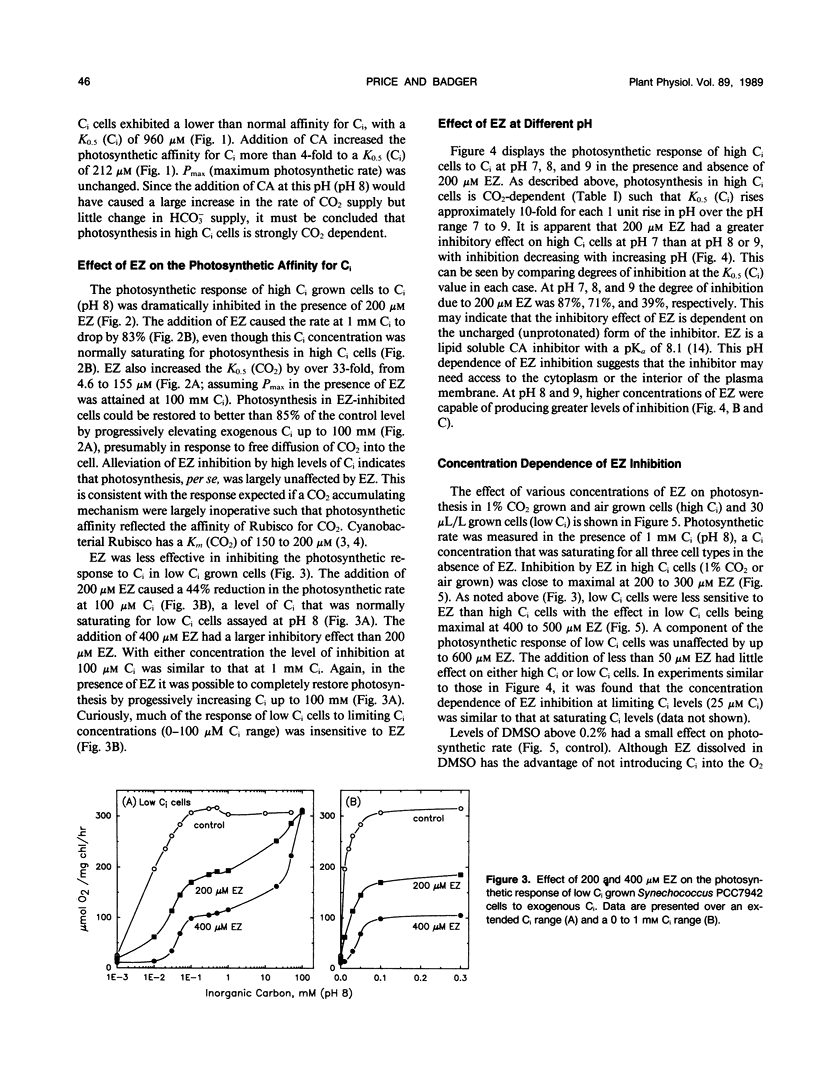
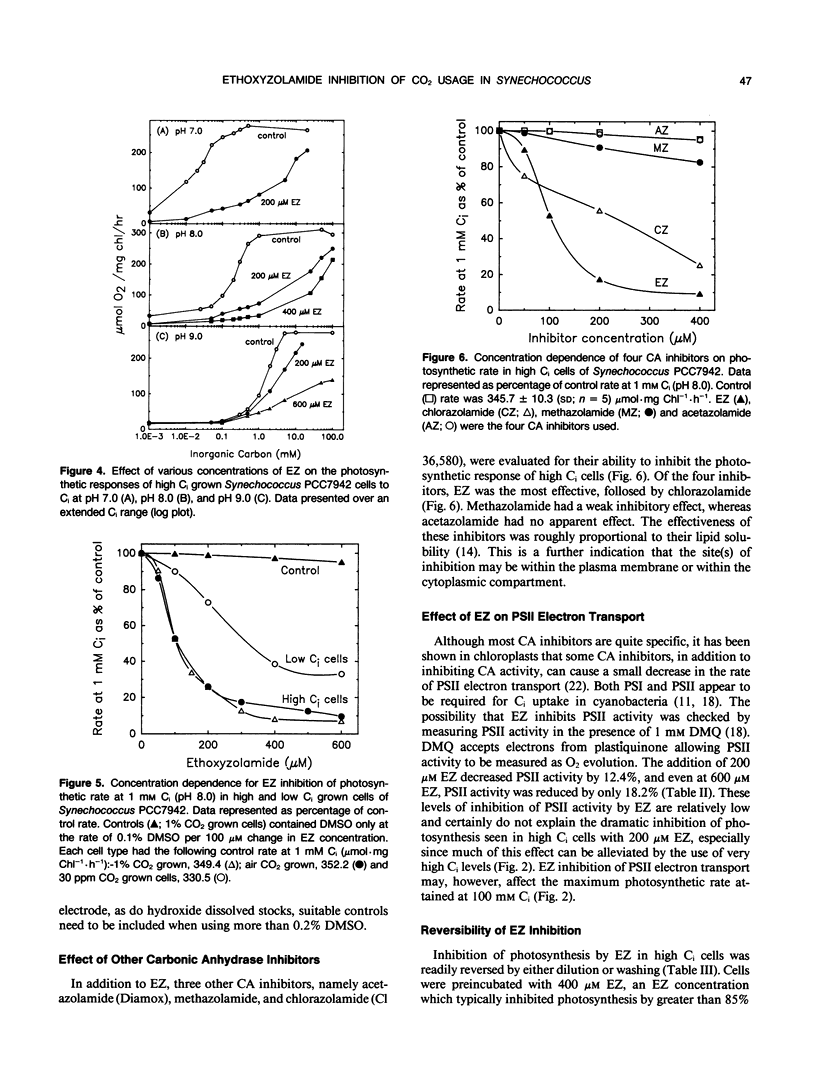
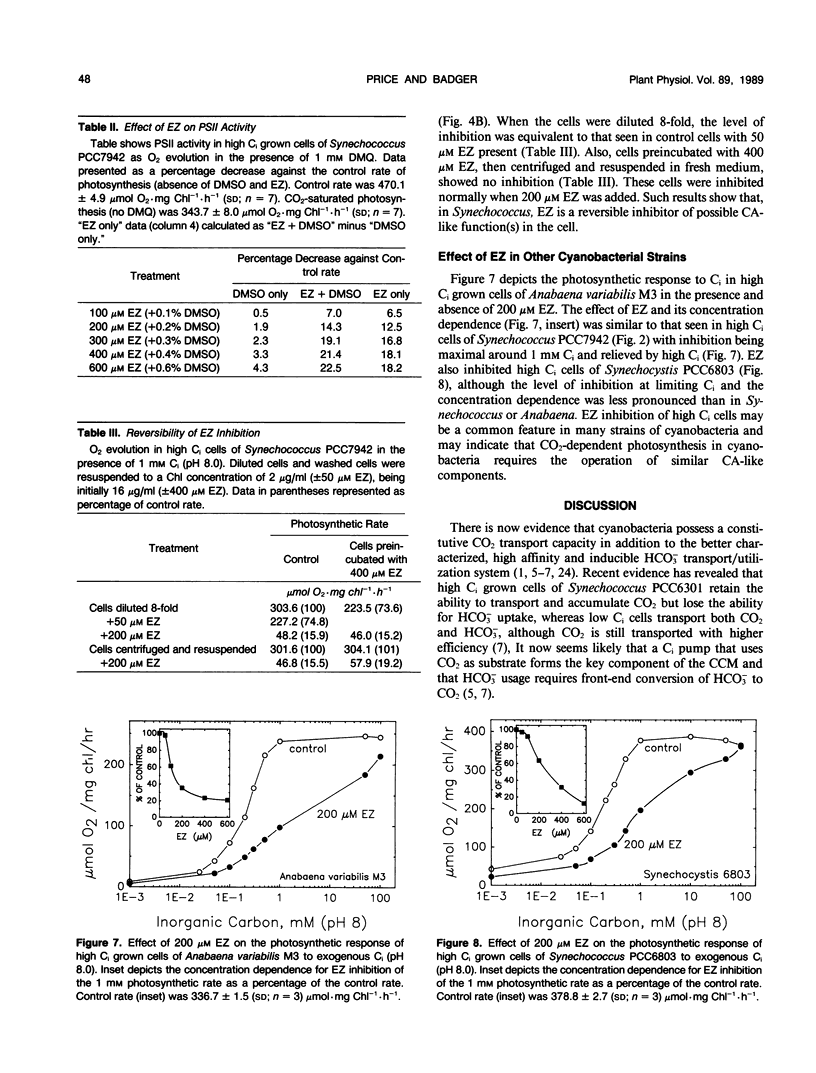
Cells of the cyanobacterium, Synechococcus PCC7942, grown under high inorganic carbon (Ci) conditions (1% CO2; pH 8) were found to be photosynthetically dependent on exogenous CO2. This was judged by the fact that they had a similar photosynthetic affinity for CO2 (K0.5[CO2] of 3.4-5.4 micromolar) over the pH range 7 to 9 and that the low photosynthetic affinity for Ci measured in dense cell suspensions was improved by the addition of exogenous carbonic anhydrase (CA). The CA inhibitor, ethoxyzolamide (EZ), was shown to reduce photosynthetic affinity for CO2 in high Ci cells. The addition of 200 micromolar EZ to high Ci cells increased K0.5(CO2) from 4.6 micromolar to more than 155 micromolar at pH 8.0, whereas low Ci cells (grown at 30 microliters CO2 per liter of air) were less sensitive to EZ. EZ inhibition in high and low Ci cells was largely relieved by increasing exogenous Ci up to 100 millimolar. Lipid soluble CA inhibitors such as EZ and chlorazolamide were shown to be the most effective inhibitors of CO2 usage, whereas water soluble CA inhibitors such as methazolamide and acetazolamide had little or no effect. EZ was found to cause a small drop in photosystem II activity, but this level of inhibition was not sufficient to explain the large effect that EZ had on CO2 usage. High Ci cells of Anabaena variabilis M3 and Synechocystis PCC6803 were also found to be sensitive to 200 micromolar EZ. We discuss the possibility that the inhibitory effect of EZ on CO2 usage in high Ci cells of Synechococcus PCC7942 may be due to inhibition of a `CA-like' function associated with the CO2 utilizing Ci pump or due to inhibition of an internal CA activity, thus affecting CO2 supply to ribulose bisphosphate carboxylase-oxygenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Abel K. M. Kinetics and subunit interactions of ribulose bisphosphate carboxylase-oxygenase from the cyanobacterium, Synechococcus sp. J Biol Chem. 1981 Aug 25;256(16):8445–8451. [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys. 1980 Apr 15;201(1):247–254. doi: 10.1016/0003-9861(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Zenvirth D., Marcus Y., Omata T., Ogawa T. Energization and activation of inorganic carbon uptake by light in cyanobacteria. Plant Physiol. 1987 Jun;84(2):210–213. doi: 10.1104/pp.84.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Schwarz R., Friedberg D., Kaplan A. High CO(2) Requiring Mutant of Anacystis nidulans R(2). Plant Physiol. 1986 Oct;82(2):610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Mayo W. P., Williams T. G., Birch D. G., Turpin D. H. Photosynthetic Adaptation by Synechococcus leopoliensis in Response to Exogenous Dissolved Inorganic Carbon. Plant Physiol. 1986 Apr;80(4):1038–1040. doi: 10.1104/pp.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaneda T., Omata T. A Mutant of Synechococcus PCC7942 Incapable of Adapting to Low CO(2) Concentration. Plant Physiol. 1987 Jul;84(3):711–715. doi: 10.1104/pp.84.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wolpert T. J., Macko V., Acklin W., Arigoni D. Molecular Features Affecting the Biological Activity of the Host-Selective Toxins from Cochliobolus victoriae. Plant Physiol. 1988 Sep;88(1):37–41. doi: 10.1104/pp.88.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


