Abstract
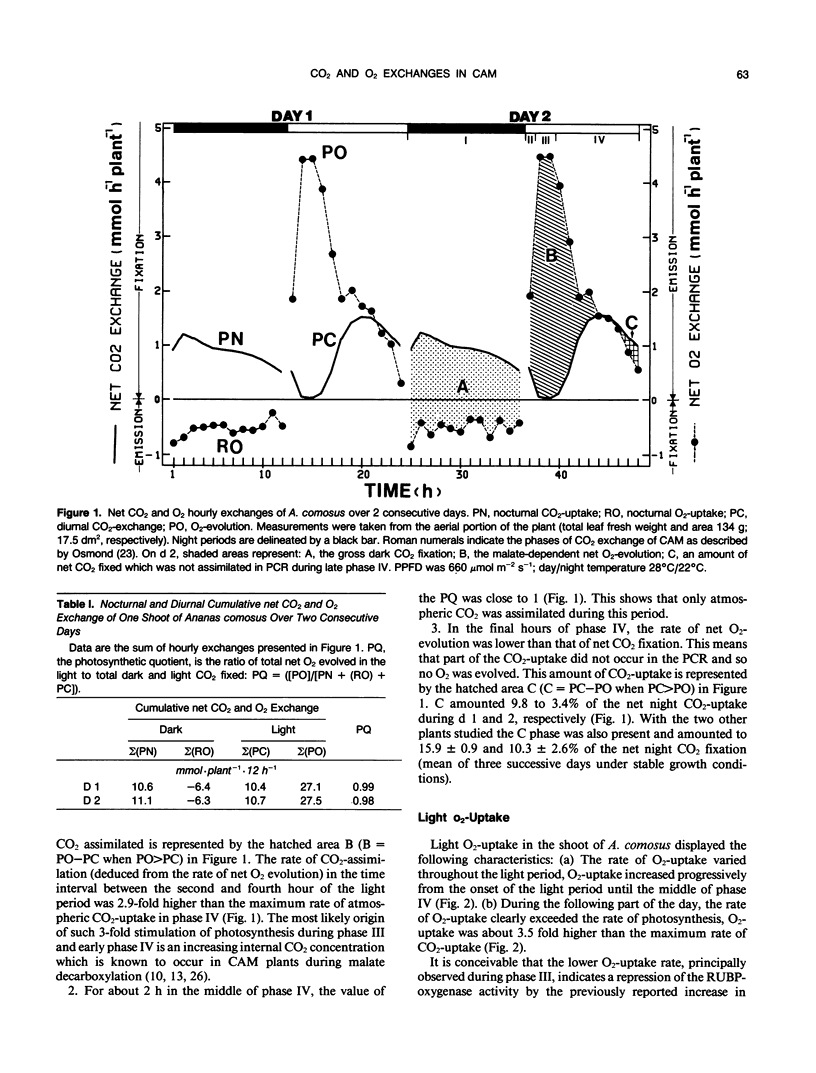
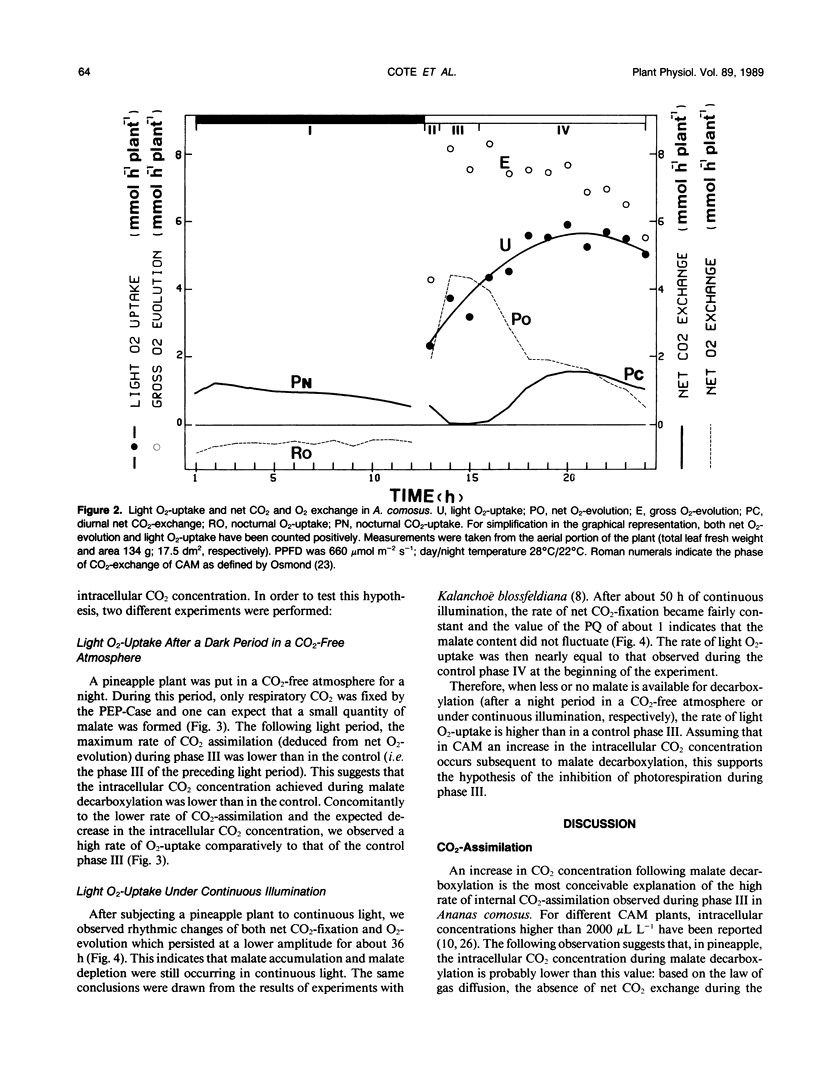
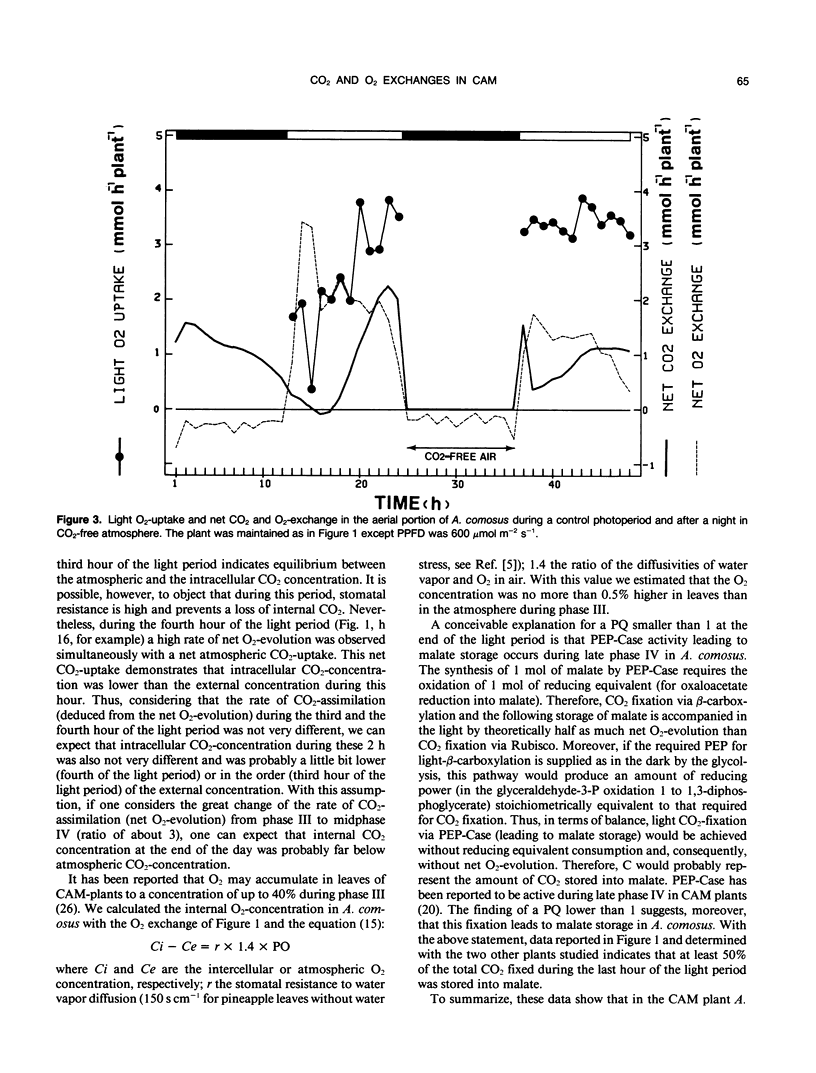
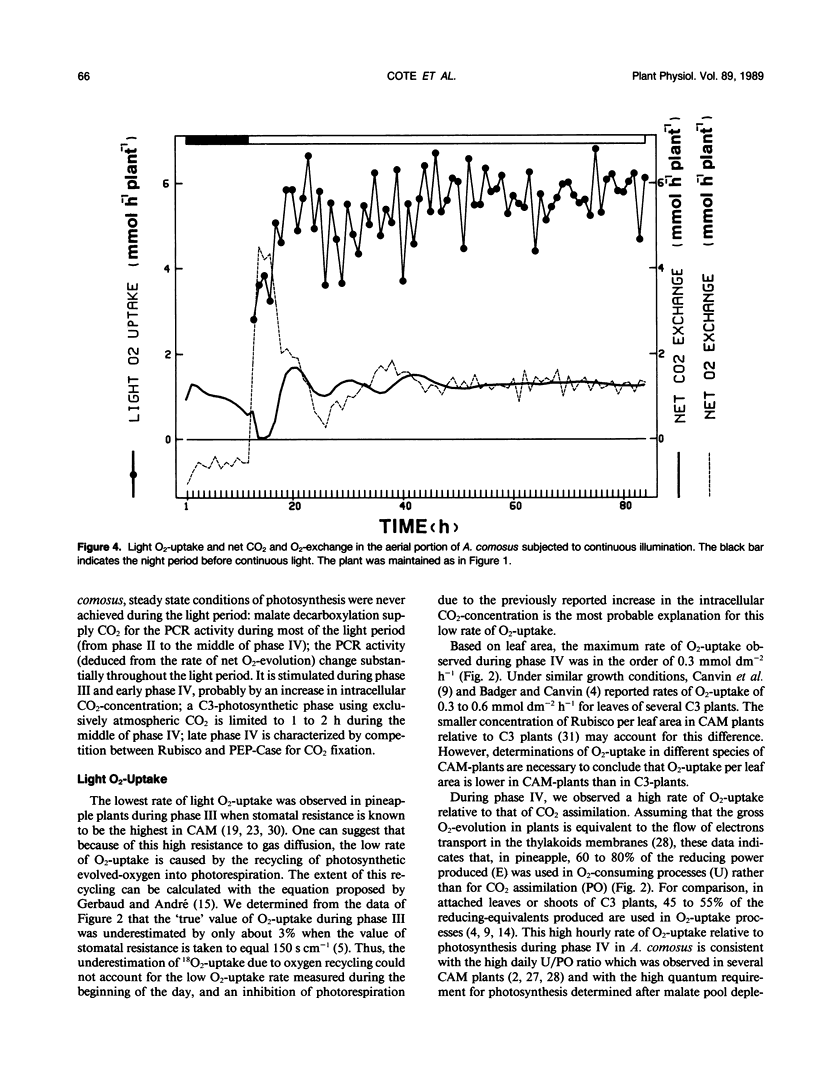
Photosynthesis and light O2-uptake of the aerial portion of the CAM plant Ananas comosus (L.) merr. were studied by CO2 and O2 gas exchange measurements. The amount of CO2 which was fixed during a complete day-night cycle was equal to the amount of total net O2 evolved. This finding justifies the assumption that in each time interval of the light period, the difference between the rates of net O2-evolution and of net light atmospheric CO2-uptake give the rates of malate-decarboxylation-dependent CO2 assimilation. Based upon this hypothesis, the following photosynthetic characteristics were observed: (a) From the onset of the light to midphase IV of CAM, the photosynthetic quotient (net O2 evolved/net CO2 fixed) was higher than 1. This indicates that malate-decarboxylation supplied CO2 for the photosynthetic carbon reduction cycle during this period. (b) In phase III and early phase IV, the rate of CO2 assimilation deduced from net O2-evolution was 3 times higher than the maximum rate of atmospheric CO2-fixation during phase IV. A conceivable explanation for this stimulation of photosynthesis is that the intracellular CO2-concentration was high because of malate decarboxylation. (c) During the final hours of the light period, the photosynthetic quotient decreased below 1. This may be the result of CO2-fixation by phosphoenolpyruvate-carboxylase activity and malate accumulation. Based upon this hypothesis, the gas exchange data indicates that at least 50% of the CO2 fixed during the last hour of the light period was stored as malate. Light O2-uptake determined with 18O2 showed two remarkable characteristics: from the onset of the light until midphase IV the rate of O2-uptake increased progressively; during the following part of the light period, the rate of O2-uptake was 3.5 times higher than the maximum rate of CO2-uptake. When malate decarboxylation was reduced or suppressed after a night in a CO2-free atmosphere or in continuous illumination, the rate of O2-uptake was higher than in the control. This supports the hypothesis that the low rate of O2-uptake in the first part of the light period is due to the inhibition of photorespiration by increased intracellular CO2 concentration because of malate decarboxylation. In view of the law of gas diffusion and the kinetic properties of the ribulose-1,5-bisphosphate carboxylase/oxygenase, O2 and CO2 gas exchange suggest that at the end of the light period the intracellular CO2 concentration was very low. We propose that the high ratio of O2-uptake/CO2-fixation is principally caused by the stimulation of photorespiration during this period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W. Relationships between Stomatal Behavior and Internal Carbon Dioxide Concentration in Crassulacean Acid Metabolism Plants. Plant Physiol. 1979 Jun;63(6):1029–1032. doi: 10.1104/pp.63.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews C. E., Vines H. M., Black C. C. Postillumination burst of carbon dioxide in crassalacean Acid metabolism plants. Plant Physiol. 1975 Apr;55(4):652–657. doi: 10.1104/pp.55.4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. An evaluation of the recycling in measurements of photorespiration. Plant Physiol. 1987 Apr;83(4):933–937. doi: 10.1104/pp.83.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. Photosynthesis and photorespiration in whole plants of wheat. Plant Physiol. 1979 Nov;64(5):735–738. doi: 10.1104/pp.64.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradshahi A., Vines H. M., Black C. C. Carbon Dioxide Exchange and Acidity Levels in Detached Pineapple, Ananas comosus (L.), Merr., Leaves during the Day at Various Temperatures, Oxygen and Carbon Dioxide Concentrations. Plant Physiol. 1977 Feb;59(2):274–278. doi: 10.1104/pp.59.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Edwards G. E. Quantum Requirement for Photosynthesis in Sedum praealtum during Two Phases of Crassulacean Acid Metabolism. Plant Physiol. 1980 Sep;66(3):463–465. doi: 10.1104/pp.66.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K. Carbon Dioxide and Water Vapor Exchange in the Crassulacean Acid Metabolism Plant Kalanchoë pinnáta during a Prolonged Light Period: METABOLIC AND STOMATAL CONTROL OF CARBON METABOLISM. Plant Physiol. 1980 Nov;66(5):917–921. doi: 10.1104/pp.66.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]


