Abstract
Percutaneous coronary intervention (PCI) has evolved significantly over the past four decades. Since its inception, in-stent restenosis (ISR)—the progressive reduction in vessel lumen diameter after PCI—has emerged as the main complication of the procedure. Although the incidence of ISR has reduced from 30% at 6 months with bare-metal stents to 7% at 4 years with drug-eluting stents (DESs), its occurrence is relevant in absolute terms because of the dimensions of the population treated with PCI. The aim of this review is to summarize the emerging understanding of the biological pathways that underlie ISR. In-stent restenosis is associated with several factors, including patient-related, genetic, anatomic, stent, lesion, and procedural characteristics. Regardless of associated factors, there are common pathophysiological pathways involving molecular phenomena triggered by the mechanical trauma caused by PCI. Such biological pathways are responses to the denudation of the intima during balloon angioplasty and involve inflammation, hypersensitivity reactions, and stem cell mobilization particularly of endothelial progenitor cells (EPCs). The results of these processes are either vessel wall healing or neointimal hyperplasia and/or neo-atherosclerosis. Unravelling the key molecular and signal pathways involved in ISR is crucial to identify appropriate therapeutic strategies aimed at abolishing the ‘Achille’s heel’ of PCI. In this regard, we discuss novel approaches to prevent DES restenosis. Indeed, available evidence suggests that EPC-capturing stents promote rapid stent re-endothelization, which, in turn, has the potential to decrease the risk of stent thrombosis and allow the use of a shorter-duration dual antiplatelet therapy.
Keywords: In-stent restenosis, Bare-metal stents, Drug-eluting stents, Endothelial progenitor cells, Hypersensitivity, Inflammation, Percutaneous coronary intervention
Graphical Abstract
Graphical Abstract.
Introduction
Percutaneous coronary intervention (PCI) is an effective and widely used treatment for patients with coronary artery disease. Despite the fact that the side effects of the procedure have progressively reduced thanks to extensive scientific work, in-stent restenosis (ISR) remains one of the main limitations of PCI, leading to the recurrence of exertional angina pectoris or acute coronary syndromes.1
The incidence of ISR was reported to be up to 30% in the bare-metal stent (BMS) era but has substantially declined with the development of drug-eluting stents (DESs).2 However, a low rate of ISR after DES still exists, and its overall occurrence is not negligible because of the size of the population receiving PCI.3 Most importantly, ISR should not be regarded as a benign complication of PCI, as it is associated with the occurrence of acute coronary syndrome in nearly 50% of cases.3,4
The aim of this review is to update the state of knowledge on the biological pathways underlying ISR. We believe that a full understanding of previously unrecognized mechanisms of ISR has the potential to improve the outcome of PCI.
Pathology of in-stent restenosis
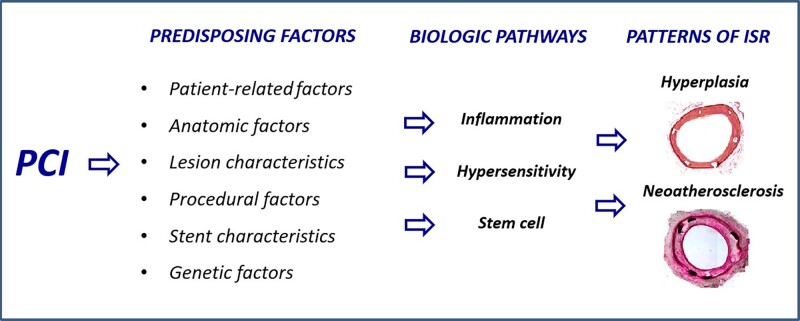
Since the beginning of the stenting era, pathologists accurately defined the histological consequences of the endothelial damage caused by balloon dilatation and stent implantation.5 Percutaneous coronary intervention triggers two distinct pathological processes that eventually lead to ISR. The first one is excessive neointimal hyperplasia, which usually begins soon after PCI and has a timeframe of up to 12 months (Figure 1); the second is neo-atherosclerosis, which typically occurs after a minimum of 12 months (Figure 2).6
Figure 1.
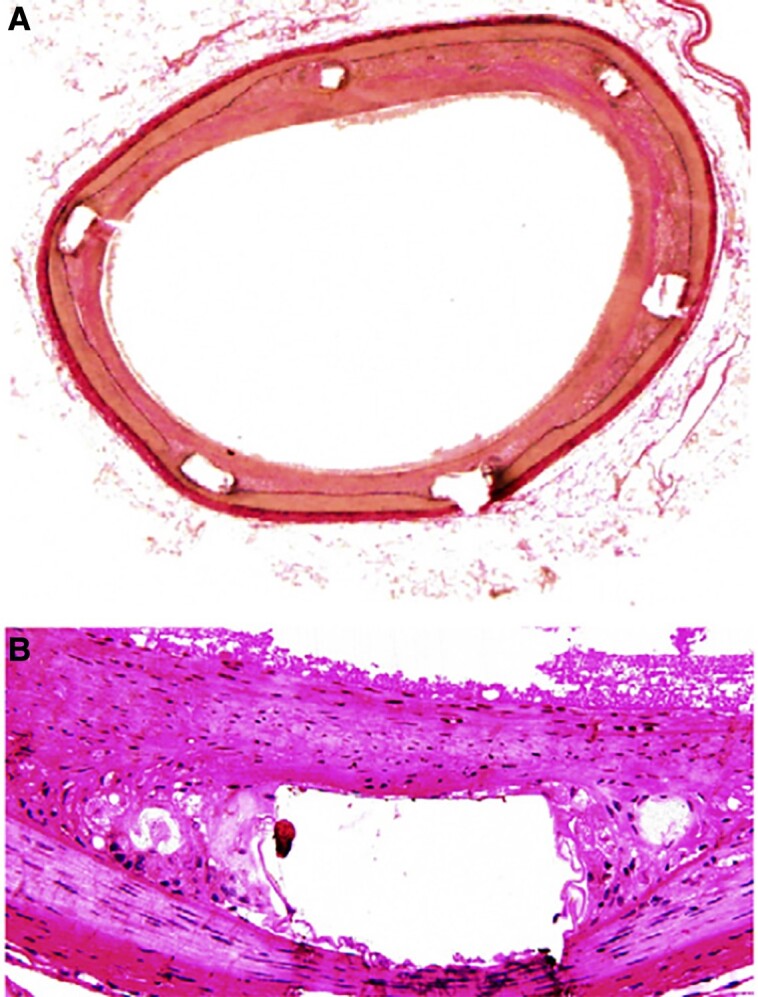
The histology of neointimal hyperplasia. (A) van Gieson’s elastin–stained cross-section of the stented segments. Intima hyperplasia is limited in the segments treated with bevacizumab stent and more pronounced in the arterial segments treated with control stent (×40). (B) Representative photomicrographs with haematoxylin and eosin staining at the stented segments, showing the difference in the neointima hyperplasia between the two groups (×200). Reproduced with permission from Stefanadis et al.51
Figure 2.
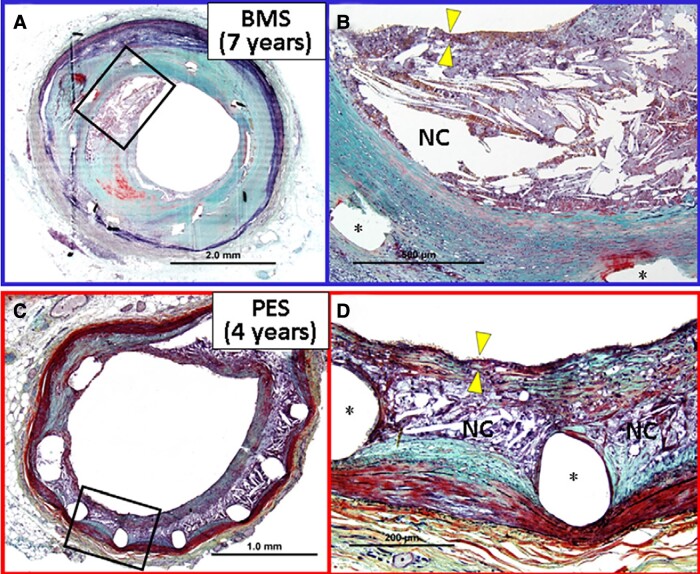
Histological findings of neo-atherosclerosis. (A) Cross-sectional histology of a bare-metal stent implanted in the coronary artery for 7 years ante mortem (×20). (B) High-power image of the box in A (×100). A large necrotic core containing cholesterol crystals is identified within the neointima. The fibrous cap overlying the necrotic core is infiltrated by numerous foamy macrophages and is markedly thinned (arrowheads point to thinnest portion), which resembles vulnerable plaque encountered in native coronary arteries. The asterisks represent metal struts. (C) Cross-sectional histology of a paclitaxel-eluting stent implanted in the coronary artery for 4 years ante mortem (×40). (D) High-power image of the box in C (×200). A relatively small necrotic core containing cholesterol crystals is formed around metal struts (asterisk). The fibrous cap is infiltrated by numerous foamy macrophages and is markedly thinned (yellow arrowheads point to the thinnest portion). NC, necrotic core; PES, paclitaxel-eluting stent. Reproduced with permission from Park et al.52
Neointimal hyperplasia is characterized by smooth muscle cell (SMC) proliferation embedded in an extracellular matrix rich in collagen.4 Interestingly, there are important differences between ISR after implantation of a BMS vs. a DES. The BMS-ISR is characterized by a homogeneous tissue with a high density of SMC, whereas the ISR-DES is more often hypocellular and proteoglycan rich.4 Also, the SMC phenotype is more frequently synthetic in ISR due to BMS, and contractile or intermediate in ISR following DES implantation.7 In contrast, neo-atherosclerosis is characterized by the accumulation of lipid-laden foamy macrophages within the neointima, with or without necrotic core formation and calcifications.5 Importantly, the in-stent neoatheroma can further progress to form a thin cap that can lead to in-stent plaque rupture and acute myocardial infarction,5 representing an accelerated form of atherosclerosis secondary to dysfunctional endothelial coverage of the stented vascular segment.5 Finally, in the former BMS era, ISR due to neo-atherosclerosis was mainly observed beyond 3 years, while it is observed at earlier time points in the current DES era.
Predisposing factors for in-stent restenosis
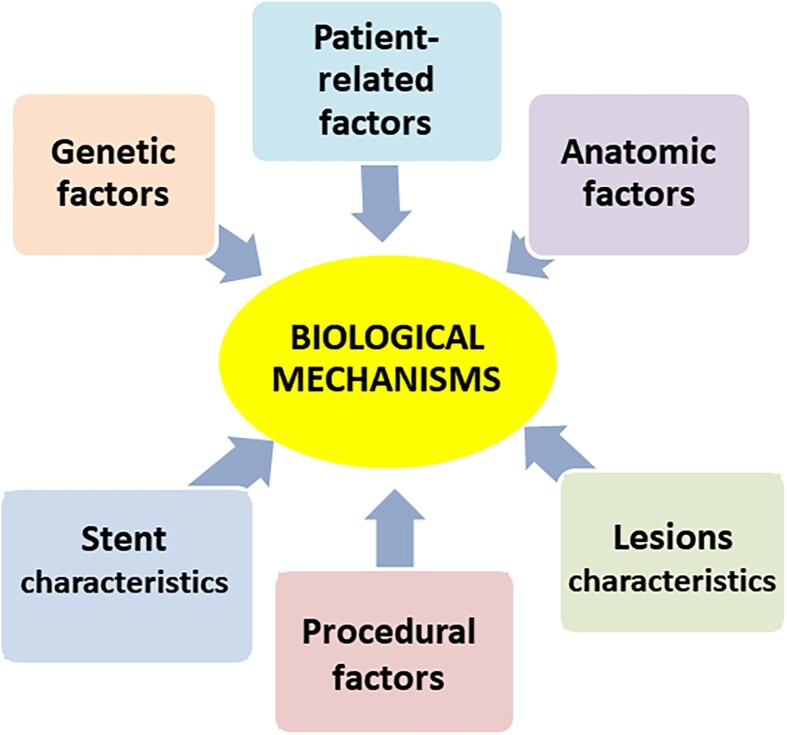
In-stent restenosis has been extensively investigated over the last two decades. It is now well documented that various mechanisms may predispose to its development, severity, and patterns, regardless of the type and efficacy of concomitant pharmacological treatment.6,8 Indeed, clinical and experimental investigations have shown that ISR can be associated with patient-related factors, genetic factors, anatomic factors, stent characteristics, lesion morphology, and procedural factors9 (Figure 3).
Figure 3.
The central role of biological mechanisms over possible underlying factors in the occurrence of in-stent restenosis. Reproduced with permission from Maleknia et al.53
Patient-related factors
The clinical predictors of ISR include diabetes mellitus, chronic kidney disease, older age, female sex, and higher body mass index, among others.9 In addition, traditional cardiovascular risk factors for atherosclerosis, including smoking, arterial hypertension, and dyslipidaemia, have been demonstrated to play a pathogenetic role in the formation of neointimal hyperplasia.10
Genetic factors
Genetic factors are currently thought to be associated with ISR independent of conventional clinical variables. Although more studies are needed to use genetic risk markers in clinical practice, growing evidence now exists that multiple phenomena related to ISR are genetically determined. Significant associations between polymorphisms in multiple genes and an increased risk of ISR have been reported. These include polymorphisms in the genes of the haemostatic system such as Platelet glycoprotein IIIa and the P2Y12 receptor.11 Interestingly, resistance to the drugs eluted from stents seems to occur in genetically predisposed patients as well.11
Anatomic factors
Anatomic factors determining ISR include vessel size and the location of coronary stenosis. Vessel size is a strong predictor of ISR after both BMS and DES implantation.12 Potential mechanisms underlying the poor outcomes associated with small vessel stenting include a smaller post-procedural minimal luminal area, a higher degree of vessel injury, and a higher metal strut density.12 Percutaneous coronary intervention at coronary bifurcations is also associated with higher rates of restenosis and target lesion revascularization compared with non-bifurcation lesions.13 Of importance, an appropriate technique (provisional vs. two-stent technique) is crucial to improve final stent geometry and vessel wall apposition, thus minimizing the risk of ISR.13
Stent characteristics
Stent-related factors influencing ISR include stent characteristics, such as type, strut thickness, and the occurrence of stent fracture.14 A plethora of different stent designs are available nowadays, but no single stent design incorporates all the characteristics of an ‘ideal stent’. For example, thinner stent struts are said to be associated with improved local blood rheology and less neointimal hyperplasia, although in specific subsets of conditions (i.e. chronic total occlusion), ultrathin-strut DESs (60 mm) have resulted in higher degrees of late lumen loss compared with thin-strut (81 mm) DES.15 A stent fracture is defined as a complete or partial separation of a stent that appears contiguous at the time of original implantation. This complication affects local drug delivery and causes a loss of platform support. Factors related to stent fracture include PCI of the right coronary artery, excessive vessel tortuosity or angulation, and longer or overlapping stents.15 Conversely, stents with larger diameters or an open-cell design appear to have a lower risk of fracture.13
Lesion morphology
Heavily calcified lesions may result in suboptimal stent expansion and wall apposition, thus explaining why severe coronary artery calcifications are associated with higher rates of target lesion revascularization.14 Residual uncovered atherosclerosis, as well as barotrauma at the stent edge, is associated with ISR. Residual plaque burden and lipid-rich plaque are also associated with edge restenosis after DES implantation.16 Other lesion factors include a lipid arc of >185° and a minimum stent area of 4.1 mm2.16
Procedural factors
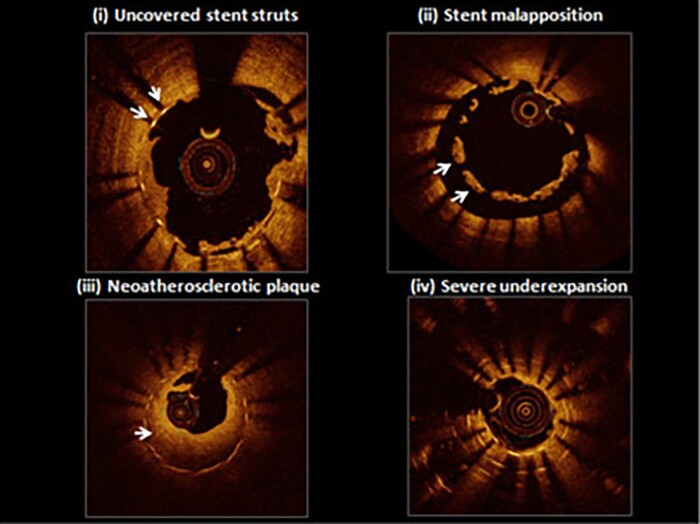
Optimal stent implantation is key to reducing the risk of ISR.17 Procedural factors predisposing to ISR include, among others, stent underexpansion, stent malapposition, and stent gap (Figure 4). Undersizing or poor lesion preparation during stent implantation are the most common causes of stent underexpansion, causing a smaller post-procedural minimal stent cross-sectional area, which, in turn, is associated with ISR.17 Similarly, over-dilatation has also been related to the possibility of increasing the frequency of ISR. Indeed, excessive stent post-dilatation enhances tissue proliferation in response to greater vessel injury, by altering the mechanical properties of the stent, disrupting the polymer coating, and increasing the distance between the stent struts.
Figure 4.
The joint effects of cytokines and growth factors in the pathogenesis of in-stent restenosis. Representative optical coherence tomography findings from patients presenting with stent thrombosis: (i) persistent uncovered stent struts late after implantation; (ii) marked stent malapposition in the target vessel, this may have been present at the time of implantation or acquired due to late positive remodelling; (iii) neo-atherosclerotic plaque formation: diffuse low-signal intensity with higher backscatter in deeper neointimal layers may indicate underlying lipid-rich atherosclerotic tissue; (iv) severe stent underexpansion at the site of overlap of multiple stent layers. Reproduced with permission from Byrne et al.9
Unlike underexpansion, stent malapposition refers to struts that are not apposed to the vessel wall and therefore form a virtual space between the struts and the arterial intima. Malapposition usually occurs when stents are undersized or in arteries with significant tortuosity and/or with variations in lumen diameter.13 Interestingly, acute malapposition is not associated with a higher risk of stent-related adverse events, while late malapposition has been associated with an increased risk of ISR. Finally, a stent gap—a discontinuous coverage of coronary lesions between two stents—leaves a zone of the coronary lesion not exposed to the antiproliferative effect of the eluted drug and the mechanical support of the strut, thus increasing the risk of ISR.13
Biological pathways
Regardless of the factors initiating ISR, there are common pathophysiological pathways of this complication, consisting of the multiple molecular phenomena triggered by mechanical PCI-related trauma.17 These biological pathways are the local and systemic responses that occur after denudation of the endothelial cells during balloon angioplasty and stenting, such as inflammation, hypersensitivity, and stem cell mobilization. The results of these processes may be either the healing or pathological processes, leading to the pathological substrates of ISR (neointimal hyperplasia and/or neo-atherosclerosis).
Vascular inflammation
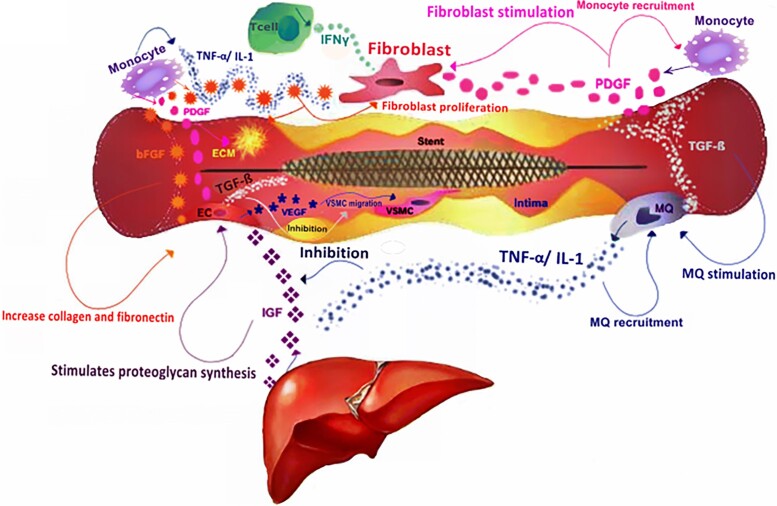
The role of inflammation in the development of ISR is clearly demonstrated by the evidence that autoimmune diseases, including inflammatory bowel diseases, rheumatoid arthritis, systemic lupus erythematosus, antiphospholipid-antibodies syndrome, and Hashimoto’s thyroiditis, are a risk factor for ISR.18 Indeed, vascular inflammation involves complex interactions between numerous cell types that release pro-inflammatory markers, cytokines, chemokines, and/or express cellular adhesion molecules (CAMs).19 Specifically, immediately following PCI, the surrounding endothelial cells are activated by pro-inflammatory cytokines, such as C-reactive protein, interleukin-1 beta (IL-1β), IL-6, transforming growth factor-beta (TGF-β), and tumour necrosis factor-alpha (TNF-α) secreted by monocytes (Figure 5).20 Inflammatory cytokines stimulate growth factors such as platelet-derived growth factor and fibroblast growth factor, which, in turn, trigger the activation of vascular SMCs and their migration to the intima, thus initiating restenosis.21
Figure 5.
The joint effects of cytokines and growth factors in the pathogenesis of in-stent restenosis. In the process of inflammation, the secretion of cytokines causes the invasion of inflammatory cells such as macrophages, monocytes, and T cells. Monocytes cause the proliferation of fibroblasts by secretion of platelet-derived growth factor. Also, platelet-derived growth factor causes more growth in monocytes. Macrophages with the secretion of inflammatory cytokines such as transforming growth factor-beta enhance the performance of platelet-derived growth factor, which increases vascular smooth muscle cells proliferation and their migration to the intima. On the other hand, the simultaneous secretion of tumour necrosis factor-alpha and interleukin 1 with basic fibroblast growth factor leads to stimulation of fibroblasts and endothelial cells. Endothelial cells through the secretion of vascular endothelial growth factor stimulate proliferation and migration of vascular smooth muscle cells to the intima, but in the presence of transforming growth factor-beta, their function is inhibited. On the other hand, in the presence of basic fibroblast growth factor, fibroblasts are affected by the interferon gamma, which increases the endothelial cell accumulation and provides conditions for inducing restenosis. Meanwhile, the release of TNF-α and interleukin 1 inhibits the biological function of the insulin-like growth factor, thus preventing the formation of the intima and reducing restenosis. bFGF, basic fibroblast growth factor; EC, endothelial cell; IL-1, interleukin 1; IFN-γ, interferon gamma; IGF, insulin-like growth factor; MQ, macrophage; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-beta; TNF-α, tumour necrosis factor-alpha; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell. Reproduced with permission from Maleknia et al.53
Considering the synergistic effects of inflammatory cytokines and growth factors in the pathogenesis of restenosis, these factors can also be used for prognostic purposes. C-reactive protein, which was originally used as a marker of inflammation in the BMS era,22 does not have any predictive role in the current DES era, as drugs eluted from stents halt the local inflammatory response that leads to ISR.23 In an attempt to identify alternative biomarkers of the risk of ISR, multiple studies have assessed the relation of ISR to matrix metalloproteinases (MMPs), plasminogen activator inhibitor-1, and the complement components C3a and C5a.24 Of them, a clear association with ISR has been demonstrated only with MMPs,25 which play fundamental roles in the migration of vascular SMC matrix remodelling, thus suggesting that they may predict a greater risk of ISR after DES implantation.
Hypersensitivity (allergic inflammation)
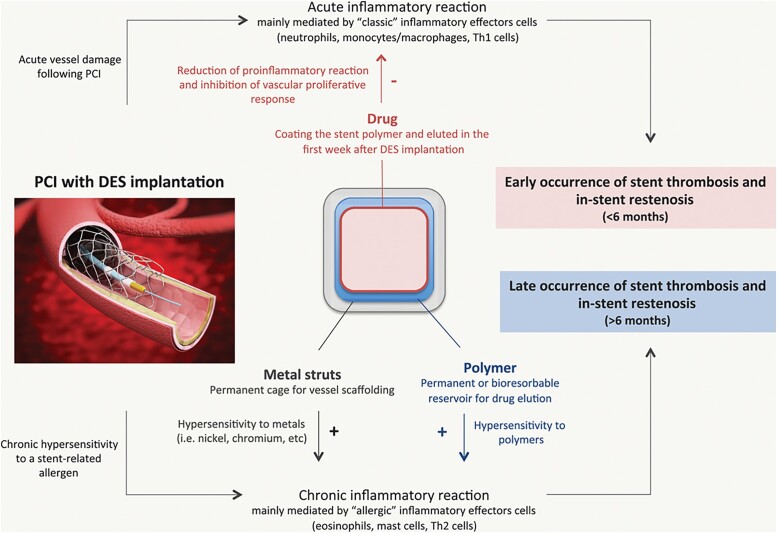
Besides classic inflammatory reactions, there is evidence suggesting that the effector cells of allergic inflammation may play a role in adverse reactions following coronary stent implantation.26 Initially described by Ehrlich,27 mast cells and eosinophils play a pivotal role in allergic inflammation. Evidence of the reciprocal modulation of functions between these two cell types through soluble mediators led to the definition of an ‘allergic effector unit’.28 Apart from the well-known systemic IgE-dependent pathway of allergic inflammatory activation, these cells are also endowed with a large repertoire of receptors, allowing them to respond to different IgE-independent local or systemic stimuli. Once activated, mast cells and eosinophils release a plethora of cytokines, growth factors, and vasoactive agents able to mediate tissue inflammation and remodelling.29
Metal stent struts and polymers may promote the local recruitment and activation of effector cells in allergic inflammation. In particular, IgE-independent phenomena, such as Type IV hypersensitivity or foreign body–induced activation, may be involved, resulting in delayed arterial healing with incomplete stent re-endothelialization and stent malapposition, conditions that may predispose to stent thrombosis (Figure 6).30 Accordingly, eosinophilic infiltrates surrounding stent struts have been described in ISR tissue of patients treated with BMSs, but rarely in post-balloon restenotic tissue.30 It is also worth noting that histopathological studies have shown that eosinophilic infiltrates are found more frequently with DES than with BMS, suggesting that allergy-mediated inflammation plays a greater role with ISR-DES than with ISR-BMS.28 Pathological evidence also supports the notion that hypersensitivity to the polymer is the most relevant mechanism, because in vivo studies have shown that polymers might produce hypersensitivity reactions and promote inflammation in coronary arteries.28
Figure 6.
The role of allergic inflammation in adverse reactions after stent implantation. DES, drug-eluting stents; PCI, percutaneous coronary intervention. Reproduced with permission from Niccoli et al. Circulation 2018;138:1736–1748.26
Endothelial progenitor cells
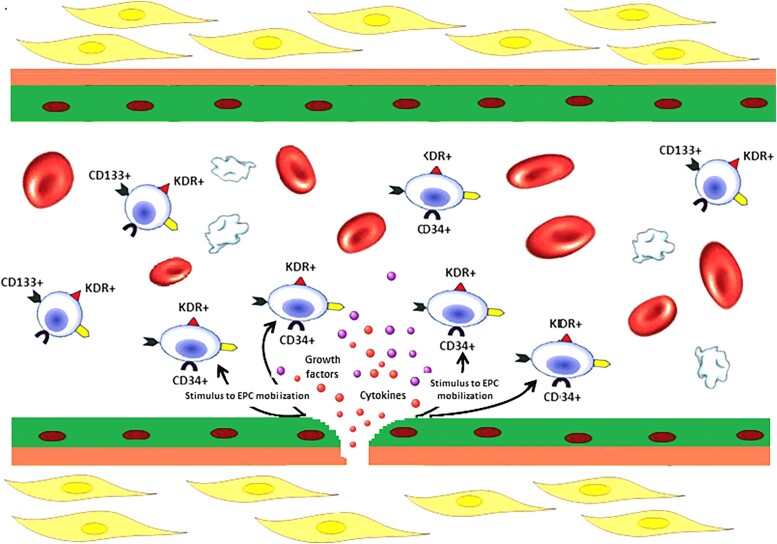
A subset of cells that play a major role in the occurrence of ISR is constituted by endothelial progenitor cells (EPCs).31 These, consisting of a small fraction (between 0.01 and 0.3%) of blood mononuclear cells, are present in peripheral blood at different stages of endothelial differentiation. In recent years, it has become evident that EPC-derived paracrine signals play a pivotal role in orchestrating the repair processes in damaged tissues.32 Endothelial progenitor cells are first mobilized into peripheral blood in response to chemo-attractants released by ischaemic or damaged tissues.33 Although the way this complex mixture of factors modulates the activities of target cells at the molecular level remains elusive, there is a general agreement that the paracrine signalling mediated by EPCs results in the production of an angiogenic microenvironment that stimulates proliferation in the nearby endothelium (Figure 7).34 After BMS implantation, the post-PCI increase of EPCs over baseline identified patients at higher risk of ISR with an augmented count of CD34+ cells being found as the best discriminating parameter.35 At variance with BMSs that do not exert any direct action on EPCs, DES may affect the number and function of EPCs.36 First-generation DESs were shown to reduce both late lumen loss and CD34+ cell mobilization and were therefore linked with re-endothelialization. Similarly, when second-generation DESs were used, the number of uncovered stent struts related significantly to the extent of mobilization and differentiation of EPCs.37 Importantly, a recent meta-analysis of the 5 studies including 651 patients that have so far assessed the relationship of EPC at the time of PCI with the subsequent occurrence of ISR found that a lower baseline EPC count was associated with a significantly greater occurrence of ISR [hazard ratio 1.33; 95% confidence interval (CI) 0.97–1.82, P = 0.045].38
Figure 7.
The paracrine activity of endothelial progenitor cells at the site of endothelial damage. When the endothelial layer is damaged, circulating endothelial progenitor cells are stimulated to act through paracrine mechanisms leading to the secretion of various cytokines and pro-angiogenic growth factors, such as vascular endothelial growth factor, stromal-derived factor-1, and nitirc oxide. The paracrine signalling mediated by endothelial progenitor cells results in the production of an angiogenic microenvironment that stimulates the nearby endothelium to proliferate. EPCs, endothelial progenitor cells; SDF-1, stromal-derived factor-1; VEGF, vascular endothelial growth factor. Reproduced with permission from Pelliccia et al.31
Prevention of in-stent restenosis: novel approaches
The elucidation of the molecular and cellular mechanisms of inflammation and cellular proliferation in vascular injury and repair has powered the development of the so-called EPC-coated stents. In the DES era, concern about the use of cytostatic or cytotoxic drugs that produce a long-lasting inflammatory response, delayed endothelization, and vasomotor dysfunction prompted the idea that a bioengineered DES with a luminal surface covered with an anti-CD34+ antibody able to capture EPCs might promote a ‘controlled’ healing.39 Upon stent placement, the anti-human CD34 antibodies would therefore attract circulating EPCs and promote rapid stent re-endothelization. Theoretically, the accelerated healing should translate into a decreased risk of stent thrombosis and restenosis, with the potential benefit to reduce the duration of dual antiplatelet therapy (DAPT).39 A representative EPC-capturing stent was the Genous™ stent (OrbusNeich, Ft. Lauderdale, FL, USA), which uses monoclonal antibodies against CD34+. Although preliminary findings showed the safety and efficacy of this novel stent,40 a trend towards higher rates of target vessel failure with this stent has been observed subsequently in the TRI-stent Adjudication Study-High risk of Restenosis study41 and in the Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth First-in-Man (HEALING) study and the HEALING II study.41,42
With this background, the EPC-capturing technology has been applied to a commercially available sirolimus-eluting stent to minimize the hyperproliferative reaction to the damaged vessel wall and suppress late loss.43 This led to the development of a specifically engineered device, the COMBO™ (OrbusNeich), which combines sirolimus elution from an abluminal biodegradable polymer matrix along with a covalently bound CD34 antibody layer, designed to control neointimal proliferation and promote vessel healing with accelerated stent strut tissue coverage.44 COMBO™ has been evaluated in four randomized controlled trials, i.e. the Randomized study to Evaluate the safety and effectiveness of an abluMinal sirolimus coatED bio-Engineered StEnt (REMEDEE)—comparing COMBO™ to the paclitaxel DES (Taxus Liberte™, Boston Scientific, Marlborough, MA, USA),45 the REMEDEE-OCT (optical coherence tomography) study,46 the Japan–USA Harmonized Assessment by Randomized, Multi-Center Study of OrbusNEich’s COMBO StEnt (HARMONEE)—comparing COMBO™ and XIENCE™ (Abbott Vascular, Santa Clara, CA, USA),47 and the SORT OUT X trial—comparing COMBO with the sirolimus-eluting Orsiro™ stent (Biotronik, Bülach, Switzerland).48 In this trial, 3146 patients were randomized to treatment with EPC-capturing or DES. At 12 months, the intention-to-treat analysis showed that rates of death, cardiac death, and myocardial infarction at 12 months did not differ significantly between the two-stent groups. However, the DES was superior to the EPC-capturing stent mainly because the latter was associated with an increased risk of target lesion revascularization when compared with the former. Differences in the stent technologies might well explain these results, as COMBO stents have a greater strut thickness (100 μm). Thus, the theoretical benefit of EPC-capture stents, which is currently documented only in terms of safety, will likely emerge when the new technology becomes available on DES with thin struts.
A systematic review analysed these 4 trials, including a total of 3961 patients.38 The meta-analysis did not detect any significant difference between EPC-coated DES and standard DES in the 1-year occurrence of cardiac death [relative risk (RR) 1.146; 95% CI 0.666–1.974, P = 0.98], but the EPC-capturing DES was associated with a significantly higher occurrence of target lesion revascularization when compared with DES (RR 1.727; 95% CI 1.199–2.487, P = 0.025). Similarly, there was a significantly higher occurrence of target vessel failure with EPC-capturing DES vs. standard DES (RR 1.591; 95% CI 1.213–2.088, P = 0.04). Similar findings have been reported recently by Nardin et al.49 who performed a large patient-level pooled analysis of subjects undergoing PCI with the COMBO stent participating in the randomized clinical trials performed so far or in observational investigations. In a total of 6753 patients, target lesion failure at 1 year occurred in 303 (4.6%) patients. The rates of cardiovascular death, myocardial infarction, and target lesion, revascularization were 1.3, 1.8, and 2.5%, respectively. The rate of definite/probable stent thrombosis was 0.73%, early ST (<1 month) was 0.48%, while late stent thrombosis (1–12 months) was 0.26%. On the basis of these results, the authors concluded that the low rates of unfavourable endpoints suggest that this stent technology may be a good alternative to other contemporary DES platforms.
In summary, available evidence suggests that EPC-capturing stents promote a rapid stent re-endothelization, which, in turn, has the potential to decrease the risk of stent thrombosis and allow the use of a shorter duration of DAPT. However, these benefits should be weighed against the possibility of increasing the risk of target lesion failure and target vessel revascularization at 12 months. The additional ongoing randomized trial (SORT OUT XI), which is comparing COMBO™ vs. BioMatrix Alpha™ stent in a population of 3140 patients (NCT03952273), is currently testing the hypothesis that the use of a newer thin-strut technology might improve the results of EPC-capture stents.
Conclusions
It is now well documented that different mechanisms predispose to the development, severity, and patterns of ISR. Although several factors, including clinical presentation, genetics, lesion morphology, stent characteristics, and procedural factors, are associated with ISR, recent evidence shows that the common pathophysiological pathways of this complication are constituted by the multiple molecular phenomena that are triggered by the mechanical trauma caused by PCI. These biological pathways are the local and systemic responses that occur after denudation of the endothelial cells during balloon angioplasty and stenting, such as inflammation, hypersensitivity, and stem cell mobilization. Unravelling the key molecular and signal pathways involved in the ISR process is crucial to identify appropriate strategies aimed at abolishing the ‘Achille’s heel’ of PCI. With this regard, research is currently focused on developing newer stents and novel pharmacological strategies.50 Ongoing studies are somewhat encouraging along these lines.
Contributor Information
Francesco Pelliccia, Department of Cardiovascular Sciences, University Sapienza, Viale del Policlinico 155, 00161 Rome, Italy.
Marco Zimarino, Department of Neuroscience, Imaging and Clinical Sciences, “G. d’Annunzio” University, Viale Abruzzo, 332, 66100 Chieti, Italy; Department of Cardiology, “SS. Annunziata Hospital”, ASL 2 Abruzzo, Via dei Vestini, 66100 Chieti, Italy.
Giampaolo Niccoli, Department of Cardiology, University of Parma, Piazzale S. Francesco, 3, 43121 Parma, Italy.
Doralisa Morrone, Department of Surgical, Medical and Molecular Pathology and of Critical Sciences, University of Pisa, Lungarno Antonio Pacinotti 43, 56126 Pisa, Italy.
Giuseppe De Luca, Division of Cardiology, AOU “Policlinico G. Martino”, Department of Clinical and Experimental Medicine, University of Messina, Via Consolare Valeria 1, 98124 Messina, Italy; Division of Cardiology, IRCCS Hospital Galeazzi-Sant'Ambrogio, Via Cristina Belgioioso 173, 20157 Milan, Italy.
Fabio Miraldi, Department of Cardiovascular Sciences, University Sapienza, Viale del Policlinico 155, 00161 Rome, Italy.
Raffaele De Caterina, Department of Surgical, Medical and Molecular Pathology and of Critical Sciences, University of Pisa, Lungarno Antonio Pacinotti 43, 56126 Pisa, Italy.
Lead author biography
 Francesco Pelliccia graduated from Sapienza University with a degree in medicine in 1985. He is currently working as an associate professor of cardiology at Sapienza University and as a consultant at Policlinico Umberto I, Rome, Italy. His major scientific interest lies in the pathophysiology of myocardial ischaemia in different settings, including coronary artery disease, non-obstructed coronary disease, Takotsubo syndrome, and cardiomyopathy.
Francesco Pelliccia graduated from Sapienza University with a degree in medicine in 1985. He is currently working as an associate professor of cardiology at Sapienza University and as a consultant at Policlinico Umberto I, Rome, Italy. His major scientific interest lies in the pathophysiology of myocardial ischaemia in different settings, including coronary artery disease, non-obstructed coronary disease, Takotsubo syndrome, and cardiomyopathy.
Data availability
No new data were generated in support of the article.
Funding
This research received no external funding.
References
- 1. Piccolo R, Giustino G, Mehran R, Windecker S. Stable coronary artery disease: revascularisation and invasive strategies. Lancet 2015;386:702–713. [DOI] [PubMed] [Google Scholar]
- 2. Madhavan MV, Kirtane AJ, Redfors B, Généreux P, Ben-Yehuda O, Palmerini T, Benedetto U, Biondi-Zoccai G, Smits PC, von Birgelen C, Mehran R, McAndrew T, Serruys PW, Leon MB, Pocock SJ, Stone GW. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol 2020;75:590–604. [DOI] [PubMed] [Google Scholar]
- 3. Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, Sharma SK, Pocock SJ, Dangas GD. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65:1298–1310. [DOI] [PubMed] [Google Scholar]
- 4. Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol 1999;10:499–506. [DOI] [PubMed] [Google Scholar]
- 5. Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015;36:2147–2159. [DOI] [PubMed] [Google Scholar]
- 6. Marazzi G, Wajngarten M, Vitale C, Patrizi R, Pelliccia F, Gebara O, Pierri H, Ramires JAF, Volterrani M, Fini M, Rosano GM. Effect of free fatty acid inhibition on silent and symptomatic myocardial ischemia in diabetic patients with coronary artery disease. Int J Cardiol 2007;120:79–84. [DOI] [PubMed] [Google Scholar]
- 7. Chieffo A, Foglieni C, Nodari RL, Briguori C, Sangiorgi G, Latib A, Montorfano M, Airoldi F, Michev I, Carlino M, Colombo A, Maseri A. Histopathology of clinical coronary restenosis in drug-eluting versus bare metal stents. Am J Cardiol 2009;104:1660–1667. [DOI] [PubMed] [Google Scholar]
- 8. Vitale C, Marazzi G, Pelliccia F, Volterrani M, Cerquetani E, Spoletini I, Mercuro G, Bonassi S, Dall’Armi V, Fini M, Rosano GMC. Trimetazidine improves exercise performance in patients with peripheral arterial disease. Pharmacol Res 2011;63:278–283. [DOI] [PubMed] [Google Scholar]
- 9. Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015;36:3320–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Zhang Q, Zhao K, Bian YJ, Liu Y, Xue YT. Risk factors for in-stent restenosis after coronary stent implantation in patients with coronary artery disease: a retrospective observational study. Medicine (Baltimore) 2022;101:e31707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azova M, Timizheva K, Ait Aissa A, Blagonravov M, Gigani O, Aghajanyan A, Tskhovrebova L. Gene polymorphisms of the renin-angiotensin-aldosterone system as risk factors for the development of in-stent restenosis in patients with stable coronary artery disease. Biomolecules 2021;11:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Habara S, Mitsudo K, Goto T, Kadota K, Fujii S, Yamamoto H, Kato H, Takenaka S, Fuku Y, Hosogi S, Hirono A, Yamamoto K, Tanaka H, Hasegawa D, Nakamura Y, Tasaka H, Otsuru S, Okamoto Y, Yamada C, Miyamoto M, Inoue K. The impact of lesion length and vessel size on outcomes after sirolimus-eluting stent implantation for in-stent restenosis. Heart 2008;94:1162–1165. [DOI] [PubMed] [Google Scholar]
- 13. Zimarino M, Briguori C, Amat-Santos IJ, Radico F, Barbato E, Chieffo A, Cirillo P, Costa RA, Erglis A, Gamra H, Gil RJ, Kanic V, Kedev SA, Maddestra N, Nakamura S, Pellicano M, Petrov I, Strozzi M, Tesorio T, Vukcevic V, De Caterina R, Stankovic G. Mid-term outcomes after percutaneous interventions in coronary bifurcations. Int J Cardiol 2019;283:78–83. [DOI] [PubMed] [Google Scholar]
- 14. Nakano H, Kataoka Y, Otsuka F, Nakashima T, Asaumi Y, Noguchi T, Asaumi Y, Noguchi T, Yasuda S. Refractory in-stent restenosis attributable to eruptive calcified nodule. JACC Case Rep 2020;2:1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leone A, Simonetti F, Avvedimento M, Angellotti D, Immobile Molaro M, Franzone A, Esposito G, Piccolo R. Ultrathin struts drug-eluting stents: a state-of-the-art review. J Pers Med 2022;12:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murai K, Kataoka Y, Nicholls SJ, Puri R, Nakaoku Y, Nishimura K, Kitahara S, Iwai T, Sawada K, Matama H, Honda S, Fujino M, Yoneda S, Takagi K, Nishihira K, Otsuka F, Asaumi Y, Tsujita K, Noguchi T. The residual lipid-rich coronary atheroma behind the implanted newer-generation drug-eluting stent and future stent-related event risks. Can J Cardiol 2022;38:1504–1515. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Mamuti W, Zhang F. Optimal interventional strategy for the treatment of coronary in-stent restenosis. J Thorac Dis 2015;7:1669–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pepe M, Napoli G, Carulli E, Moscarelli M, Forleo C, Nestola PL, Biondi-Zoccai G, Giordano A, Favale S. Autoimmune diseases in patients undergoing percutaneous coronary intervention: a risk factor for in-stent restenosis? Atherosclerosis 2021;333:24–31. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Jin X, Huang Y, Ran X, Luo D, Yang D, Jia D, Zhang K, Tong J, Deng X, Wang G. Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regen Biomater 2018;5:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Man RC, Sulaiman N, Ishak MF, Idrus RBH, Abdul Rahman MR, Yazid MD. The effects of pro-inflammatory and anti-inflammatory agents for the suppression of intimal hyperplasia: an evidence-based review. Int J Environ Res Public Health 2020;17:7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ran F, Liu C, Liu Z, Shang T, Zhou M, Qiao T. Preventive effects of basic fibroblast growth factor on vascular restenosis after balloon angioplasty. Exp Ther Med 2014;7:1193–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrante G, Niccoli G, Biasucci LM, Liuzzo G, Burzotta F, Galiuto L, Trani C, Rebuzzi AG, Crea F. Association between C-reactive protein and angiographic restenosis after bare metal stents: an updated and comprehensive meta-analysis of 2747 patients. Cardiovasc Revasc Med 2008;9:156–165. [DOI] [PubMed] [Google Scholar]
- 23. Yi M, Wu L, Ke X. Prognostic value of high-sensitivity C-reactive protein in in-stent restenosis: a meta-analysis of clinical trials. J Cardiovasc Dev Dis 2022;9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sillen M, Declerck PJ. A narrative review on plasminogen activator inhibitor-1 and its (patho)physiological role: to target or not to target? Int J Mol Sci 2021;22:2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu JP, Wang YZ, Li YK, Cheng Q, Zheng Z. Matrix metalloproteinase 9 level as an indicator for restenosis following cervical and intracranial angioplasty and stenting. Neural Regen Res 2015;10:631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niccoli G, Montone RA, Sabato V, Crea F. Role of allergic inflammatory cells in coronary artery disease. Circulation 2018;138:1736–1748. [DOI] [PubMed] [Google Scholar]
- 27. Ehrlich P. Beiträge zur Theorie und Praxis der histologischen Färbung [master’s thesis]. Leipzig University; 1878.
- 28. Gangwar RS, Friedman S, Seaf M, Levi-Schaffer F. Mast cells and eosinophils in allergy: close friends or just neighbors. Eur J Pharmacol 2016;778:77–83. [DOI] [PubMed] [Google Scholar]
- 29. Rittersma SZ, Meuwissen M, van der Loos CM, Koch KT, de Winter RJ, Piek JJ, van der Wal AC. Eosinophilic infiltration in restenotic tissue following coronary stent implantation. Atherosclerosis 2006;184:157–162. [DOI] [PubMed] [Google Scholar]
- 30. van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Ellis SG, Topol EJ. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation 1996;94:1690–1697. [DOI] [PubMed] [Google Scholar]
- 31. Pelliccia F, Zimarino M, De Luca G, Viceconte N, Tanzilli G, De Caterina R. Endothelial progenitor cells in coronary artery disease: from bench to bedside. Stem Cells Transl Med 2022;11:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelliccia F, Pasceri V, Meoni G, Pristipino C, Cianfrocca C, Li X, La Rocca S, Rosano G, Mercuro G, Richichi G. Numbers of endothelial progenitor cells in peripheral blood are similar in younger and older patients with coronary artery disease. Int J Cardiol 2009;133:277–279. [DOI] [PubMed] [Google Scholar]
- 33. Pelliccia F, Cianfrocca C, Rosano G, Mercuro G, Speciale G, Pasceri V. Role of endothelial progenitor cells in restenosis and progression of coronary atherosclerosis after percutaneous coronary intervention: a prospective study. JACC Cardiovasc Interv 2010;3:78–86. [DOI] [PubMed] [Google Scholar]
- 34. Fadini GP, Mehta A, Dhindsa DS, Bonora BM, Sreejit G, Nagareddy P, Quyyumi AA. Circulating stem cells and cardiovascular outcomes: from basic science to the clinic. Eur Heart J 2020;41:4271–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madonna R, De Caterina R. Circulating endothelial progenitor cells: do they live up to their name? Vascul Pharmacol 2015;67–69:2–5. [DOI] [PubMed] [Google Scholar]
- 36. Pelliccia F, Patti G, Rosano G, Greco C, Gaudio C. Efficacy and safety of eplerenone in the management of mild to moderate arterial hypertension: systematic review and meta-analysis. Int J Cardiol 2014;177:219–228. [DOI] [PubMed] [Google Scholar]
- 37. Pelliccia F, Pasceri V, Marazzi G, Rosano G, Greco C, Gaudio C. A pilot randomized study of ranolazine for reduction of myocardial damage during elective percutaneous coronary intervention. Am Heart J 2012;163:1019–1023. [DOI] [PubMed] [Google Scholar]
- 38. Pelliccia F, Pasceri V, Zimarino M, De Luca G, De Caterina R, Mehran R, Dangas G. Endothelial progenitor cells in coronary atherosclerosis and percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Revasc Med 2022;42:94–99. [DOI] [PubMed] [Google Scholar]
- 39. Co M, Tay E, Lee CH, Poh KK, Low A, Lim J, Lim IH, Lim YT, Tan HC. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J 2008;155:128–132. [DOI] [PubMed] [Google Scholar]
- 40. Klomp M, Beijk MA, Varma C, Koolen JJ, Teiger E, Richardt G, Bea F, van Geloven N, Verouden NJ, Chan YK, Woudstra P, Damman P, Tijssen JG, de Winter RJ. 1-Year outcome of TRIAS HR (TRI-stent adjudication study-high risk of restenosis) a multicenter, randomized trial comparing genous endothelial progenitor cell capturing stents with drug-eluting stents. JACC Cardiovasc Interv 2011;4:896–904. [DOI] [PubMed] [Google Scholar]
- 41. Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJB. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. J Am Coll Cardiol 2005;45:1574–1579. [DOI] [PubMed] [Google Scholar]
- 42. Duckers HJ, Silber S, de Winter R, den Heijer P, Rensing B, Rau M, de Winter R, Rau M, Mudra H, Silber S, Benit E, Verheye S, Wijns W, Serruys P. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the HEALING-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention 2007;3:67–75. [PubMed] [Google Scholar]
- 43. Ong AT, Aoki J, Kutryk MJ, Serruys PW. How to accelerate the endothelialization of stents. Arch Mal Coeur Vaiss 2005;98:123–126. [PubMed] [Google Scholar]
- 44. Granada JF, Inami S, Aboodi MS, Tellez A, Milewski K, Wallace-Bradley D, Parker S, Rowland S, Nakazawa G, Vorpahl M, Kolodgie FD, Kaluza GL, Leon MB, Virmani R. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv 2010;3:257–266. [DOI] [PubMed] [Google Scholar]
- 45. Haude M, Lee SW, Worthley SG, Silber S, Verheye S, Erbs S, Rosli MA, Botelho R, Meredith I, Sim KH, Stella PR, Tan H-C, Whitbourn R, Thambar S, Abizaid A, Koh TH, Den Heijer P, Parise H, Cristea E, Maehara A, Mehran R. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv 2013;6:334–433. [DOI] [PubMed] [Google Scholar]
- 46. Jaguszewski M, Aloysius R, Wang W, Bezerra HG, Hill J, De Winter RJ, Karjalainen PP, Verheye S, Wijns W, Lüscher TF, Joner M, Costa M, Landmesser U. The REMEDEE-OCT study: an evaluation of the bioengineered COMBO dual-therapy CD34 antibody-covered sirolimus-eluting coronary stent compared with a cobalt-chromium everolimus-eluting stent in patients with acute coronary syndromes: insights from optical coherence tomography imaging analysis. JACC Cardiovasc Interv 2017;10:489–499. [DOI] [PubMed] [Google Scholar]
- 47. Saito S, Krucoff MW, Nakamura S, Mehran R, Maehara A, Al-Khalidi HR, Rowland SM, Tasissa G, Morrell D, Joseph D, Okaniwa Y, Shibata Y, Bertolet BD, Rothenberg MD, Généreux P, Bezerra H, Kong DF. Japan-United States of America harmonized assessment by randomized multicentre study of OrbusNEich's combo StEnt (Japan-USA HARMONEE) study: primary results of the pivotal registration study of combined endothelial progenitor cell capture and drug-eluting stent in patients with ischaemic coronary disease and non-ST-elevation acute coronary syndrome. Eur Heart J 2018;39:2460–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jakobsen L, Christiansen EH, Freeman P, Kahlert J, Veien K, Maeng M, Raungaard B, Ellert J, Villadsen AB, Kristensen SD, Ahlehoff O, Christensen MK, Terkelsen CJ, Erik Bøtker H, Aaroe J, Thim T, Thuesen L, Aziz A, Eftekhari A, Jensen RV, Støttrup NB, Rasmussen JG, Junker A, Jensen SE, Hansen HS, Jensen LO. Randomized clinical comparison of the dual-therapy CD34 antibody-covered sirolimus-eluting combo stent with the sirolimus-eluting orsiro stent in patients treated with percutaneous coronary intervention: the SORT OUT X trial. Circulation 2021;143:2155–2165. [DOI] [PubMed] [Google Scholar]
- 49. Nardin M, Pivato CA, Cao D, Sartori S, Zhang Z, Vogel B, Nicolas J, Chiarito M, Qiu H, Chandrasekhar J, Spirito A, Abizaid A, Christiansen EH, Colombo A, de Winter RJ, Haude M, Jakobsen L, Jensen LO, Krucoff MW, Landmesser U, Saito S, Suryapranata H, De Luca G, Dangas G, Mehran R. The mega COMBO collaboration: an individual patient data pooled analysis of patients undergoing PCI with COMBO stent. Int J Cardiol 2023;370:149–155. [DOI] [PubMed] [Google Scholar]
- 50. Cesaro A, Gragnano F, Calabrò P, Moscarella E, Santelli F, Fimiani F, Patti G, Cavallari I, Antonucci E, Cirillo P, Pignatelli P, Palareti G, Pelliccia F, Bossone E, Pengo V, Gresele P, Marcucci R, Schiavo A, Vergara A, Pastori D, Menichelli D, Grossi G, Di Serafino L, Taglialatela V, del Pinto M, Gugliemini G. Prevalence and clinical implications of eligibility criteria for prolonged dual antithrombotic therapy in patients with PEGASUS and COMPASS phenotypes: insights from the START-ANTIPLATELET registry. Int J Cardiol 2021;345:7–13. [DOI] [PubMed] [Google Scholar]
- 51. Stefanadis C, Toutouzas K, Stefanadi E, Lazaris A, Patsouris E, Kipshidze N. Inhibition of plaque neovascularization and intimal hyperplasia by specific targeting vascular endothelial growth factor with bevacizumab-eluting stent: an experimental study. Atherosclerosis 2007;195:269–276. [DOI] [PubMed] [Google Scholar]
- 52. Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J Am Coll Cardiol 2012;59:2051–2057. [DOI] [PubMed] [Google Scholar]
- 53. Maleknia M, Ansari N, Haybar H, Maniati M, Saki N. Inflammatory growth factors and in-stent restenosis: effect of cytokines and growth factors. SN Compr Clin Med 2020;2:397–407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated in support of the article.