Abstract
Electrical conduction through cardiac muscle fibres separated from the main myocardial wall by layers of interposed adipose tissue are notoriously difficult to target by endocardial ablation alone. They are a recognised important cause for procedural failure due to the difficulties of delivering sufficient energy via the endocardial radiofrequency catheter to reach the outer epicardial layer without risking adverse events of the otherwise thin walled atria. Left atrial ablations for atrial fibrillation (AF) and tachycardia are commonly affected by the presence of several epicardial structures, with the septo-pulmonary bundle (SPB), Bachmann’s bundle, and the ligament of Marshall all posing substantial challenges for endocardial procedures. Delivery of a transmural lesion set is essential for sustained pulmonary vein isolation and for conduction block across linear atrial lines which in turn has been described to translate into a reduced AF/atrial tachycardia recurrence rate. To overcome the limitations of endocardial-only approaches, surgical ablation techniques for epicardial or combined hybrid endo-epicardial ablations have been described to successfully target these connections. Yet, these techniques confer an increase in procedure complexity, duration, cost, and morbidity. Alternatively, coronary venous system ethanol ablation has been successfully employed by sub-selecting the vein of Marshall to facilitate mitral isthmus line block, although this approach is naturally limited to this area by the coronary venous anatomy. Increased awareness of the pathophysiological relevance of these epicardial structures and their intracardiac conduction patterns in the era of high-resolution 3D electro-anatomical mapping technology has allowed greater understanding of their contribution to the persistence of AF as well as failure to achieve transmural block by traditional ablation approaches. This might translate into novel catheter ablation strategies with procedural success rates comparable to surgical ‘cut-and-sew’ techniques. This review aims to give an overview of percutaneous catheter ablation strategies to target the SPB, an important cause of failed block across the roof line and isolation of the left atrial posterior wall and/or the pulmonary veins. Existing and investigational technologies will be discussed and an outlook of future approaches provided.
Keywords: Atrial fibrillation, Percutaneous catheter ablation, Epicardial connections, Septo-pulmonary bundle, Ablation strategies
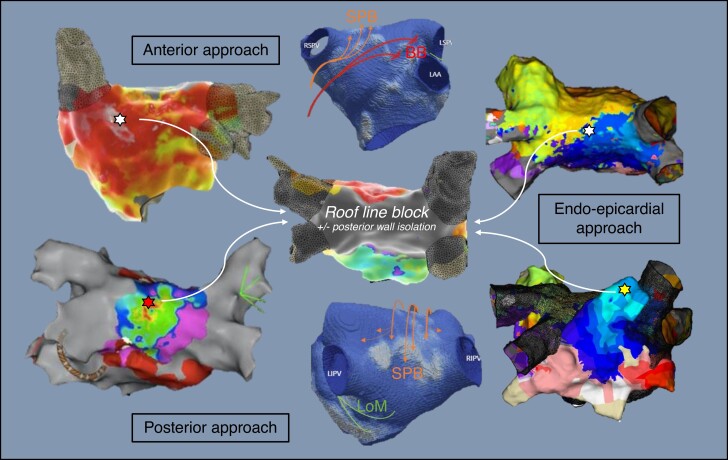
Graphical Abstract
Graphical Abstract.
How to ablate the septo-pulmonary bundle? Examples of electroanatomical activation maps and left atrial CT model with epicardial connections (Septopulmonary Bundle in orange, Bachmann's Bundle in red, Ligament of Marshall in green).
What’s new?
Increased awareness of the pathophysiological and procedural relevance of epicardial connections in the atria and high-density mapping of their intracardiac conduction pattern allow to design novel catheter ablation strategies to improve procedural success for complex atrial arrhythmias.
Endocardial mapping strategies for challenging roof line ablations combine differential pacing manoeuvres with rapid high-density mapping to allow identification of additional targets beyond the traditional linear lesion set. They can be categorised as ‘anterior–posterior’, ‘outside-in’, and ‘inside-out’ approaches.
Percutaneous endo-epicardial ablation is a recognised second line strategy to target persistent epicardial connections following exhausted endocardial options to achieve transmural bidirectional block across linear lesion sets.
Investigational strategies to achieve transmural lesion set include impedance modulation, bipolar radiofrequency ablation, and pulsed field ablation.
Introduction
In recent years, the argument for (early) rhythm control in atrial fibrillation (AF) has been strengthened with accumulating evidence demonstrating an improvement in prognosis if successful rhythm control can be achieved.1 Catheter ablation has been shown to be superior to antiarrhythmic drugs to maintain sinus rhythm.2,3 In persistent AF, delivery of additional linear lesions to achieve a posterior wall isolation is a commonly employed adjunctive approach to pulmonary vein isolation (PVI) to compartmentalise the atria, reduce critical myocardial mass to sustain AF,4 and exclude sources of focal drivers. This is supported by systematic reviews and meta-analysis suggesting significantly lower recurrence rates with deployment of linear lesion sets.5 In contrast, results from randomised studies6–9 have repeatedly failed to unequivocally demonstrate improved outcome with this strategy. The inability to create transmural lesions has been discussed as an important contributor for these disappointing results. Up to 38% of endocardial-only ablations fail to block left atrial linear lines, with the mitral lines followed by the roof line being the most challenging.10 In addition, failure to achieve block raises the concern of iatrogenic arrhythmogenic substrate pre-disposing to macro-re-entrant atrial tachycardias (ATs).11 In fact, linear lesion success relates to two functions, contiguity and transmurality. Durability relies on the permanence of these two.
Lesion contiguity: a problem solved?
Real-time visualisation of point-by-point lesions and their spatial relationship and proximity to each other has been facilitated by the high spatial accuracy of localisation and motion-tracking of sensor-enabled ablation catheters throughout the cardiac- and respiratory-cycle by 3D electro-anatomical mapping systems (EAMS). Computer tomography (CT)-phantom studies of spatial and point localisation accuracy for commercially available magnetic-based EAMS demonstrated a registration offset between virtual visualisation and reality of 1.62 ± 0.77 mm.12
In clinical studies, it has been found that recurrent electrical connections are, among other factors, more common at sites of high inter-lesion distance (>6 mm) implying discontinuity of the lesions set and pursuit of continuity may result in improved outcomes.13 Yet, lesion continuity is also dependent on the tissue depth it is assessed and true contiguity requires uninterrupted scar formation and electrical block from endo- to epicardium.14
Lesions transmurality: a surrogate for procedural success?
Achieving transmural block has been an important rationale behind offering combined endo-epicardial ablation procedures by means of stand-alone or hybrid surgical techniques.15,16 This approach is supported by evidence showing that this translates into improved AF outcome with a meta-analysis for hybrid AF procedures, reporting maintenance of sinus rhythm in 79% over 19 ± 25 months follow up for persistent and longstanding persistent AF.17 Yet, surgical or hybrid ablation are associated with higher periprocedural morbidity and infrastructure requirements, increased length of hospital stay, and—in the case of staged approaches—multiple procedures for the patient. Percutaneous endo-epicardial catheter ablation has been attempted18–20 to combat these short-falls, with encouraging results when applied in larger cohort studies.10,21,22 The only randomised trial for endo-epicardial vs. endocardial ablation targeted PVI and complex fractionated atrial electrogram (EGM) and failed to demonstrate an additive benefit of epicardial ablation; however, no linear ablation lesions were deployed.23
Experience of endo-epicardial techniques remains limited to very few tertiary arrhythmia centres, with a tendency towards long procedural durations with large cases series reporting an average of around 6 h.24 The exact role of these approaches still needs to be defined, as does the patient population in whom such procedures should be considered. Pending further research supporting a more aggressive endo-epicardial approach, improving techniques for endocardial-only strategies to achieve transmural block is just as crucial to minimise complexity, duration, and procedural cost.
Anatomy of the septo-pulmonary bundle as relevant for ablation strategies
Histological preparations25 and in vivo diffusion tensor imaging26 have alluded to the complex fibre architecture of the atria. This might assist in the development of mapping and ablation strategies to overcome the challenge of epicardial connections.
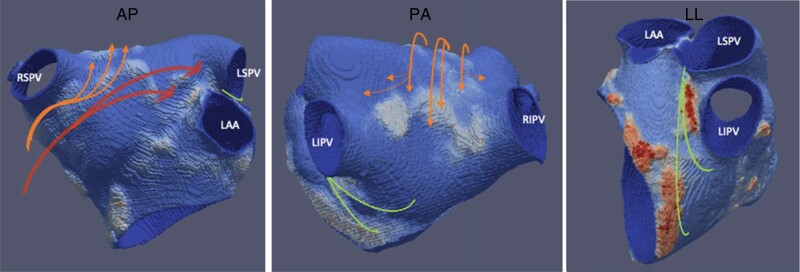
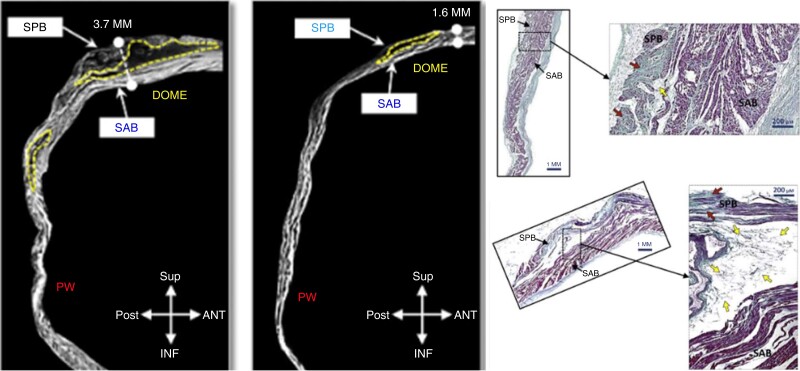
The anatomy and course of the septo-pulmonary bundle (SPB) is a particularly common cause of failure to block roof lines, and is therefore of great relevance to the interventional electrophysiologist. The SPB is composed of subepicardial fibres arising from the interatrial groove beneath the Bachmann’s bundle (BB), crossing anterior to the right superior pulmonary vein (RSPV) and fanning out over the dome of the left atrium (LA) before descending throughout the posterior wall. From the posterior wall, branches encircle the right and left pulmonary veins.27 This fan-like structure with multiple fibres explains the pathophysiological role which results in the inability to block some roof lines, and in some instances to achieve pulmonary vein (PV) isolation (see schematic representation in Figure 1). Histological preparations have described thicker, often bi-layered myocardium at the dome of the LA where the SPB overlays the septo-atrial bundle. Importantly, a recent study also highlighted the prevalent separation of these two layers by interposed adipose tissue, which may ‘protect’ the SPB from the thermal ablation energy (see Figure 2).28 Combined endo-epicardial AF mapping studies showed persistent conduction on the roof through epicardial layers even after extensive endocardial ablation,10 as well as following dedicated ablation protocols to assure appropriate energy delivery.29 In large case series, it was demonstrated that based on high-density mapping and EGM analysis, epicardial connections are the cause for failure to block the roof line in all cases and suggested that a floor line may be an effective alternative strategy to circumvent the problem.28 Although this strategy may overcome the challenge of creating a linear lesion set at the roof, it will not result in successful isolation of the posterior wall.
Figure 1.
CT wall thickness model of the LA with cropped pulmonary veins and LAA: orange arrows indicating course of the SPB originating in the interatrial groove, crossing over the dome and spreading out on the posterior wall. Red arrows indicating the Bachmann’s bundle as interatrial connection between the RA and LA inserting anterior to the LSPV. Green lines indicating course of vein of Marshall from the ridge between LAA/LSPV downward and inserting into the CS musculature and at times posterior wall. The septo-atrial bundle is formed by fibres running sub-endocardially from the interatrial raphe over the anterior wall to the lateral and superior (dome) parts of the LA and into the posterior wall (not shown here). CS, coronary sinus; CT, computer tomography; LA, left atrium; LAA, left atrial appendage; SPB, septo-pulmonary bundle.
Figure 2.
Left: microtomography slices sagittal view of LA of two explanted hearts: yellow dotted line marking interposed atrial fat at LA dome with substantial interindividual variability. Right: histological analysis: sample of the floor line region (top) shows only minimal fat tissue. Sample of the dome region with significant interposed fat separating the SAB from the SPB thought to be an important contributor to failed roof line ablations (adapted and reprinted from Pambrun et al.28 with permission of the author). LA, left atrium; SAB, septo-atrial bundle; SPB, septo-pulmonary bundle.
Epicardial connections have also been highlighted to play an important role in bi-ATs and may pose particular challenges as direct targeting of these interatrial connections has been associated with impaired atrial physiology following ablation.30 Equally, pervious surgical ablation or surgical atrial incisions can be associated with epicardial connections in nearly a third of the cases which may contribute to recurrent ATs and need to be taken into consideration when defining ablation strategies.31
Strategies to target the SPB by percutaneous radiofrequency ablation
There are three commonly employed linear lesion sets in the LA—floor line (joining the lower border of the inferior PVs), roof line (connecting the superior PVs, commonly at the most superior position of the LA, termed the ‘dome’), and mitral line (joining either the left superior or inferior PV or RSPV/roof line to the mitral annulus). For both roof and mitral lines, achieving durable block by endocardial ablation alone can be notoriously difficult. Various approaches have been described for performing mitral line ablations on the left lateral wall including combined endo-epicardial ablation with the latter being delivered over the distal part of the coronary sinus (CS). A growing body of evidence also supports adjunctive ethanol ablation over the vein of Marshall to improve acute, but importantly also long-term durability of mitral isthmus (MI) block.32,33
Options for roof line ablations are more limited with a lack of sufficiently large venous branches for accommodating an ablation catheter or let alone an anatomically consistent enough and accessible venous circulation for considering ethanol ablation. Even with ablation lesion sets of contiguous and optimised radiofrequency (RF) lesions with good stability and contact force, achieving high rates of acute block, recurrence rates of up to one-third are described.29
By means of high-density mapping as well as judicial use of differential pacing manoeuvrers, successful targets for roof line ablation can be identified. Alternatively, combined endo-epicardial approaches may represent a second strategy, if the former approaches fail.
Strategy 1: endocardial anterior–posterior approach with RA pacing
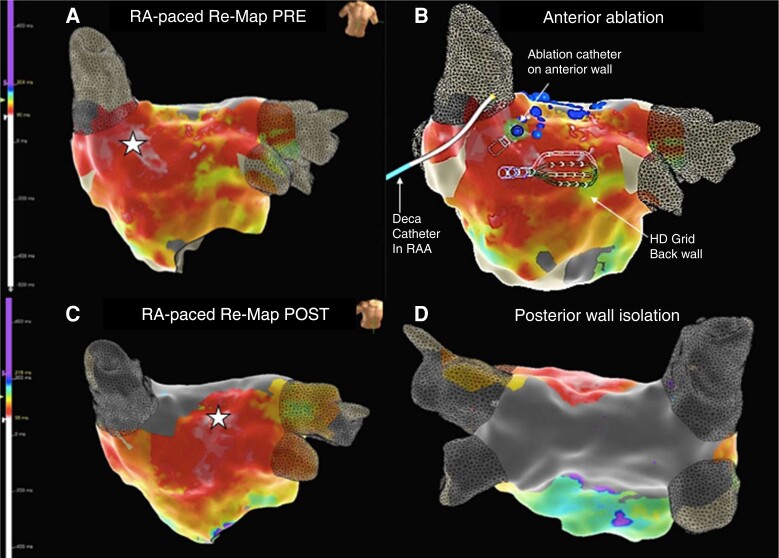
Setup: Triple femoral venous access should be acquired for insertion of a CS catheter, a multipolar mapping catheter, and the ablation catheter. An epicardial connection over the SPB can be suspected as cause of a failed roof line ablation based on typical activation patterns on posterior wall and EGM morphologies along the endocardial line with split potentials with far field (epicardial) and near field (endocardial) components. The CS catheter may then be repositioned within the right atrial appendage (RAA) and a remap of the LA with the multipolar catheter is acquired. During RAA pacing, the earliest activation on the anterior–superior surface of the left atrial map will indicate the insertion site of the SPB whereas the earliest activation towards the left superior pulmonary vein (LSPV)and left atrial appendage (LAA) mark the insertion site of the BB (see Figure 3). Ablation is then targeted to the earliest site of the SPB insertion anterior to the RSPV after excluding phrenic nerve capture via high output pacing at the target site. Sites of phrenic nerve capture should be manually tagged and avoided during subsequent ablation. Furthermore, prior to starting ablation, it is advisable to position the multipolar mapping catheter onto the posterior wall and ablate during ongoing RAA pacing to monitor for delay and isolation to gauge ablation success as shown in Figure 4. Little clinical evidence is available to formally guide power and duration ablation settings. Biophysical considerations of achieving deep lesions by longer duration with stable catheter and contact force >10 g seems reasonable to maximise the chance of transmural block, while closely and continuously monitoring for impedance changes. Following ablation, confirmation of intact interatrial conduction over the BB by a strategic remap is advisable to exclude adverse effects on atrial mechanics, or in rare cases inadvertent LAA isolation which has been associated with an increase in stroke risk.34
Figure 3.
Case vignette anterior–posterior approach with RAA pacing: 80-year-old patient attending for redo persistent AF ablation after preceding PVI two years earlier. Mapping with an Advisor™ HD Grid (Abbott Medical) and ablation with TactiFlex™ SE. Ablation of a continuous lesion set along the roof line with 50 W delayed conduction but failed to achieve block. Split double potentials on the endocardial roof line with earlier far field (epicardial) component followed by sharp local EGM (endocardial) and earliest activation in the middle of the box suggested an epicardial connection. Remap during RAA pacing: (A) anterior LA map during RAA pacing pre-ablation: white star indicates earliest activation anterior to the RSPV consistent with the SPB. (B) Catheter setup during the anterior posterior approach: decapolar catheter in the RAA on the right, HD grid on the left atrial posterior wall to monitor for block during ablation, and ablation catheter on the anterior wall at the earliest activation site as identified during RAA pacing to target the SPB. (C) Remapping of the left atrium during RAA pacing after ablation of the SPB insertion site shows a shift of the earliest activation (white star) towards the LSPV and LAA demonstrating now dominant conduction over the Bachmann’s bundle and no relevant interatrial delay (white = early, LAT colour scale on left of image). (D) Successful isolation of the left atrial posterior wall by ablation on the anterior wall (see Figure 4 for EGM traces during ablation). AF, atrial fibrillation; EGM, electrogram; LA, left atrium; LAA, left atrial appendage; LAT, local activation time; PVI, pulmonary vein isolation; SPB, septo-pulmonary bundle.
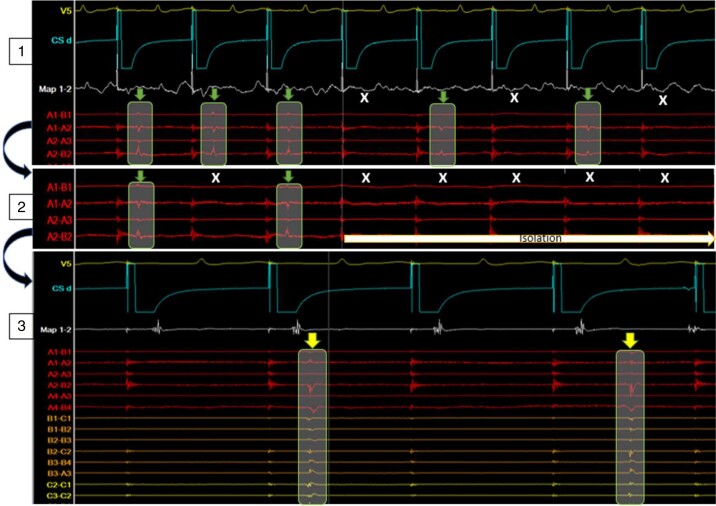
Figure 4.
Intracardiac EGM during anterior–posterior approach: HD grid on posterior wall, CS catheter pacing from RAA, and ablation catheter anterior to RSPV as indicated in Figure 3. Top (1) Ablation energy delivery at 12.18 p.m. causing 2:1 block (green arrows = conduction, white X = block) into the box as recorded by the HD grid. Middle (2) with continuation of energy delivery after eight beats of 2:1 conduction successful isolation of the posterior wall was achieved. Bottom (3) at 12:35 p.m., 17 min after isolation, ongoing independent activity within the box as recorded by the HD grid (yellow arrows). CS, coronary sinus; EGM, electrogram.
Strategy 2: endocardial outside-in and inside-out approach
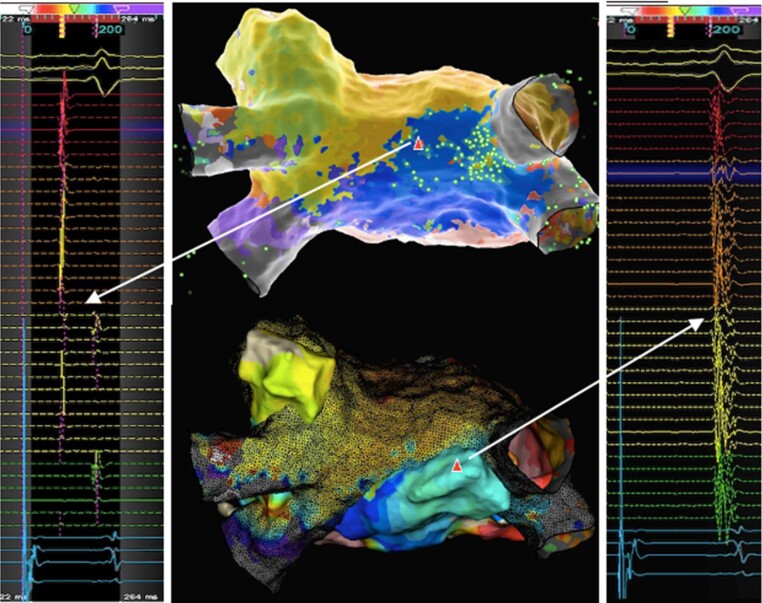
Setup: A similar baseline setup to the anterior–posterior approach involving triple femoral venous access for a CS catheter, a multipolar mapping catheter, and the ablation catheter as well as dual left atrial access is advisable. If the posterior box fails to isolate during the subsequent left atrial procedure with suspected breakthrough epicardially over the SPB based on EGM analysis and local activation timing along the floor and roof line, a high-density map of the posterior wall within the previously delivered linear lesions during CS or RAA pacing should be acquired. The earliest activation within the box will be marked and targeted. Due to concerns of ablating on the thin posterior wall, high power short duration settings to cause shallow lesions may be preferrable to standard ablation settings. If feasible the mapping catheter should be positioned in the box to record local EGMs and monitor for ablation effect.
If no isolation can be achieved with ablation at the earliest area of a carefully acquired high-density map, remapping to confirm appropriate target zone and/or assess for shift is recommended. Due to the often multiple widely spread and branched insertion sites of the SPB on the backwall, this is of relevant concern and multiple remaps (and further ablation) are not uncommon (see Figure 5). In challenging cases, an empiric horizontal line connecting the right and left posterior carina may at times be a pragmatic approach to achieve partial isolation. Yet, uncritical and excessive ablation lesions on the back wall should be avoided at all cost and carry substantial risk of thermal oesophageal injury. Oesophageal cooling has been suggested to mitigate this risk. Different methods have been proposed for this purpose which broadly may be considered ‘reactive’ (apply in response to an elevated oesophageal temperature) or ‘proactive’ cooling (pre-emptively cool prior to any risk of thermal injury).35 Reduced procedure time,36 fluoroscopy,37 and overall cost38 and no reduction in ablation efficacy, efficiency, and safety39 were some of the benefits highlighted for dedicated active cooling devices. In turn, a recent systematic review and meta-analysis found that oesophageal cooling did not reduce the overall risk of any oesophageal injury compared to control. Yet, it did suggest that it may shift the severity of oesophageal injuries to less severe injuries.40 As such, oesophageal cooling should be considered in patients under-going AF ablation particularly with expected ablation at the left atrial posterior wall.
Figure 5.
Case vignette with outside-in approach: 68-year-old patient with redo persistent AF ablation following previous cryo PVI ablation. Mapping with Pentaray NAV™ eco catheter confirmed isolated PV. Posterior box lesion set with the QDOT Micro™ ablation catheter using high power short duration settings in Q-mode + (90W, 4 s) for the floor line and Q-mode (45 W, target ablation index 500) for the roof line achieved first pass block on the floor line but only delay across roof line. Posterior wall LAT maps: (A) left atrial LAT map at outset during CS pacing with low to high activation. (B) Following posterior box lesion set with roof and floor line breakthrough left corner on roof line (white bold arrows). (C) Following consolidation lesions to the left corner earliest activation shifted more towards the middle of posterior wall (white bold arrows) suggestive of epicardial connection: blue EGM showing distinct far field (=epicardial) followed by sharp near field (=local endocardial) signal. Of note, the WOI was inappropriately short to account for the longer delay into the posterior wall and required adjusting. (D) Following ablation at posterior wall targeting earliest activation, a remap confirmed shift in earliest activation to a slightly higher leftward location (white bold arrow) highlighting the importance of remapping to prevent unnecessary long RF time on the posterior wall with the risk of increased complications; green EGM indicating typical EGM morphology with far field followed by near field component (note: colour scales adjusted in each map to highlight earliest area). AF, atrial fibrillation; CS, coronary sinus; EGM, electrogram; LAT, local activation time; PV, pulmonary vein; PVI, pulmonary vein isolation; RF, radiofrequency; WOI, window of interest.
Acknowledging this important limitation of the outside-in strategy, an inverse inside-out strategy may be considered. The latter involves pacing from a catheter positioned on the posterior wall and mapping outside of the box to identify the earliest activation anterior to the roof line. This has been successfully reported.41 In practice, however, this is often challenging due to interaction of the roving catheter with the catheter positioned on the backwall resulting in displacement, loss of capture, and/or change in pace location of the latter rendering the map less reliable.
Strategy 3: sequential unipolar endo-epicardial approach
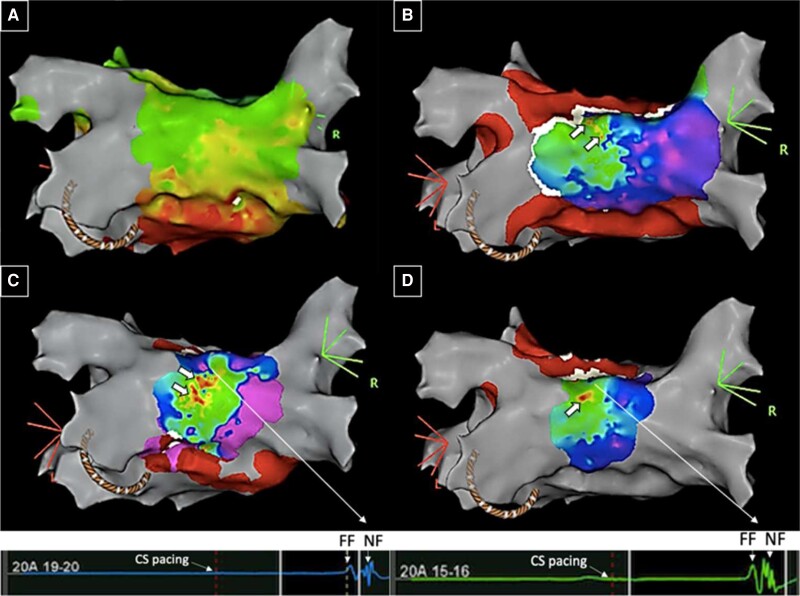
Setup: Combined endo-epicardial AF ablation procedures should preferentially be undertaken with general anaesthesia and under transoesophageal echocardiography monitoring. Antibiotic prophylaxis should be considered as per institutional practice as with any percutaneous epicardial intervention. Epicardial access should precede the left atrial accessin order to delay full heparinisation and minimise access related bleeding risk. A posterior steerable sheath position is required for catheter manipulation within the oblique and transverse pericardial sinuses. If no evidence of bleeding is present following successful epicardial puncture, the usual dual transseptal access to the LA can be acquired and therapeutic activated clotting time levels targeted. To facilitate interpretation of the combined maps, it is useful to acquire both endo- and epicardial maps during identical pacing site and rate. Substrate map acquisition during CS pacing is the first line approach in many centres, yet RAA pacing may facilitate identification of critical sites to target for ablation for epicardial connections. Given the interindividual variability in pericardial anatomy, careful exploration and assessment of the individual patients’ pericardial folds and course of the transverse and oblique sinus in regards to accessibility and overlap of endocardial segments is important. This subsequently guides the placement of ablation lesions endocardially to match the course of the accessible sinuses epicardially (see Figure 6 for exemplary endo- and epicardial electro-anatomical activation map).
Figure 6.
Case vignette endo-epicardial AF ablation: 43-year-old patient with symptomatic persistent atrial fibrillation (AF) with severely impaired left ventricular function secondary to uncontrolled rate and previous PVI and posterior box isolation. Mapping with an Advisor™ HD grid during CS pacing confirmed blocked floor but connected roof line. View on left atrial roof. Top and left: endocardial map with evidence of widely split double potentials (∼100 ms) on HD grid splines positioned along roof line. Note, earliest activation of posterior wall occurs at right superior corner at site of insertion of septo-pulmonary bundle (highlighted by “sparkles”, green bright dots). Bottom and right: Epicardial map (full colour) superimposed on endocardial map (black mesh) with evidence of long fractionated signals on opposing site to the earliest endocardial activation of the posterior box (adapted from Tonko et al.42). Ablation at the corresponding endo- and epicardial sites of suspected connection over the roof successfully isolated the posterior wall with no reconnection after a waiting time of 30 min. AF, atrial fibrillation; CS, coronary sinus; PVI, pulmonary vein isolation.
The initial linear roof line ablation lesion set should be placed endocardially on the dome between the two superior pulmonary veins. Subsequent remapping of the epicardial surface can identify remaining connections manifesting as prolonged EGMs spanning the gap between the endocardial double potentials. Targeting these connections from epicardially should be attempted with the endocardial mapping catheter simultaneously placed on the posterior wall to monitor for ablation effect. Ablation catheters with force vectors are preferred to confirm direction of ablation energy towards the endocardium. 45W irrigated ablation appears safe with continuous impedance monitoring. A negative pressure drain should be attached to the epicardial sheath to remove irrigation fluid to prevent an iatrogenic tamponade, and to closely monitor for bleeding during ablation.
Endo-epicardial AF/AT ablation is a recognised second line strategy to target persistent epicardial connections. Procedure duration and complexity increase compared to endocardial ablations. Operator skills for epicardial access and solid knowledge of pericardial anatomy and adjacent structures for safe catheter manipulation and energy delivery are required. If ablation lesions are delivered in the oblique sinus or within a posteriorly reaching fold of the transverse sinus, particular care needs to be given to direct RF energy delivery towards the endocardium away from the oesophagus. Oesophageal cooling methods as discussed above may mitigate the risk of thermal injury further. Even with experienced operators, epicardial procedures should be preferentially performed at centres with on-site cardiothoracic surgical cover in the event of acute complications requiring immediate intervention.
Investigational strategies to target the SPB
Investigational strategy 1: impedance modulation
The energy delivered to the tissue by a RF application defines the extent of tissues heating and necrosis (and therefore scar formation), which is substantially affected by the baseline impedance. Higher baseline impedances have been associated with less effective lesions in ablation-index guided PVI ablations.43 This is the biophysical basis which informed impedance modulation strategies to increase lesion dimensions and durability.44,45
A simple technique for impedance modulation is switching the traditional 0.9% to hypotonic 0.45% (half normal) saline (HNS) with a relatively higher impedance as the irrigant for open irrigated ablation catheters to enhance RF current delivery into the myocardium. This has been reported to result in deeper ablation lesion dimensions ex vivo and in vivo,46 but also associated with an increased risk of steam pops.47 For the mostly thin walled atria, only with the recognition of epicardial connections further research has been directed towards HNS in the atria. A randomised trial investigating its use in cavotricuspid isthmus (CTI) ablation for typical atrial flutter demonstrated a shorter time to achieving bidirectional block with no increase in complications.48 A double-blinded randomised clinical trial for first-time AF ablation showed that HNS was associated with shorter RF times with comparable procedure times, complication rates, and similar acute pulmonary vein reconnection.49 Beneficial effects on left atrial linear lesion durability are yet to be investigated.
Alternatively, modifying the location of the dispersive patch has been suggested, yet the latter did not show any meaningful effect on posterior left atrial ablation.50 Addition of a second dispersive patch to reduce baseline impedance has been proposed for ventricular ablation,51 but not investigated for atrial ablations.
Overall, despite a convincing biophysical-based rational and some supporting clinical evidence for the therapeutic value and safety of impedance modulation in interventional electrophysiology, controversies persist. Limitations in using baseline impedance and current for lesion quality have been referenced.52
Investigational strategy 2: bipolar RF ablation
For percutaneous RF bipolar ablation, one ablation catheter is connected as usual to the RF generator and the second ablation catheter to the indifferent electrode connection using a custom cable in that the distal pole of the second catheter acts as the indifferent electrode for the RF current. Based on the cable used, this might also allow EGM recording or catheter positioning within the mapping system.53 For atrial arrhythmias, application of bipolar ablation has been reported for MI related arrhythmias in case reports,54 septal related atrial flutters53 as well as for accessory pathways.55 Yet, to date there is no published clinical data for its use on the left atrial roof even though theoretically feasible in the context of a combined endo-epicardial ablation.
Investigational strategy 3: pulsed field ablation
Pulsed field ablation has increasingly gained popularity for PVI. It is presumed to provide a superior safety profile with less risk of collateral damage while maintaining efficiency and durability of the ablation.56,57 Its use for lesion sets beyond PVI to treat persistent AF and/or atrial re-entry tachycardias is less well investigated. Case series have demonstrated the feasibility of using a multi-spline PFA catheter to achieve posterior wall isolation and bidirectional block across linear lines.58,59 Case numbers remain low and difficulties with the mitral isthmus and the anterior lines have been highlighted.60 Available data do not yet allow to judge its final role and benefit for these types of lesion sets in general and the roof line in particular. Pre-clinical studies have demonstrated the feasibility and acceptable safety and durability for linear atrial lesions with focal pulsed field ablation catheters,61 which in humans so far has only been successfully employed on the CTI. An attempt to use the focal catheter on the roof and mitral line was found to be challenging.59
In summary, optimal PFA waveforms and dosing protocols are still under evaluation. For delivery of linear ablation lesion sets, multiple open questions remain, including in regard to lesion efficacy, durability, and safety. Also catheter design and integration in 3D mapping systems require further optimisation.
Outlook: imaging guided RF energy titration
Progress in cardiac imaging technology has opened up new opportunities to visualise the arrhythmogenic substrate. Left atrial wall thickness, fat distribution and interposition, as well as intraprocedural real-time lesion formation visualisation are all promising techniques which may allow for individualised ablation energy titration in the future. The spatial resolution of contrast enhanced cardiac CT allows to generate three-dimensional wall thickness models which can be co-registered with EAMS.62 Also CT-based quantification of peri-atrial or intramural fat has practical significance as it is a crucial factor in preventing ablation energy transmission through the wall to reach the opposing conducting layer.63
Real-time cardiac magnetic resonance (CMR) imaging guided ablation has been proposed for RF64 and cryoablation,65 but CMR compatible tools used during routine ablation procedures are rare and substantial logistical issues remain a major limitation of this approach. Intracardiac echocardiography is a more easily accessible modality which allows assessment of catheter location in relation to the myocardial wall. Echogenicity of the substrate may provide a rough estimate of tissues characteristics and thickness and strain-based imaging techniques have shown promising results for real-time ablation lesion visualisation.66
Conclusion
While the debate about the best ablation strategy and absolute impact on patient outcome and benefit may still be ongoing, gauging the effect of AF/flutter ablations is intrinsically linked to the technical and practical aspects of achieving lesion contiguity and transmurality to prevent confounding outcomes by (re)connections. While extensive left atrial ablation has traditionally raised concerns for increased adverse events (arrhythmogenic substrate creation, interatrial block, atrial electromechanical dysfunction, periprocedural complications), durable block of linear lesion sets, even if empiric, appear to have a beneficial effect in persistent and long-term persistent AF. Every reasonable effort to block these lines should therefore be undertaken.
Contemporary technology using high-density mapping and differential pacing as well as location-specific ablation energy settings can improve success rates of endocardial-only ablation of persistent epicardial connections. Impedance modulation may emerge as an important adjunctive tool, yet evidence to define its exact beneficial effect on lesion transmurality in the atria while not negatively affecting the procedural safety is still under investigations.
Endo-epicardial AF ablation has rightfully gained substantial momentum in recent years, yet important questions about the absolute benefit on hard cardiovascular outcome parameters, long-term outcome, as well as patient selection and cost-effectiveness remain.
Aggressive pursuit of transmurality necessarily has to be weighed against safety concerns in an often thin walled atria. Imaging guided ablation and real-time lesion formation visualisation may play a crucial role in the future for informed individualised ablation delivery.
Ethic statement
All patients gave written informed consent to the procedure and informed consent for publication.
Contributor Information
Johanna Bérénice Tonko, Institute for Cardiovascular Science, University College London, 5 University Street, WC1E 6JF London, UK; Department of Cardiology, Royal Sussex County Hospital, Brighton and Sussex University Hospitals NHS Foundation Trust, Eastern Rd, Brighton BN2 5BE, UK.
John Silberbauer, Department of Cardiology, Royal Sussex County Hospital, Brighton and Sussex University Hospitals NHS Foundation Trust, Eastern Rd, Brighton BN2 5BE, UK.
Ian Mann, Department of Cardiology, Royal Sussex County Hospital, Brighton and Sussex University Hospitals NHS Foundation Trust, Eastern Rd, Brighton BN2 5BE, UK.
Funding
None.
Data availability
Data available on request due to privacy/ethical restrictions.
References
- 1. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 2. Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah Ret al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 3. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale Aet al. ThermoCool AF trial investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 4. Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59–70. [DOI] [PubMed] [Google Scholar]
- 5. Kanitsoraphan C, Rattanawong P, Techorueangwiwat C, Kewcharoen J, Mekritthikrai R, Prasitlumkum Net al. The efficacy of posterior wall isolation in atrial fibrillation ablation: a systematic review and meta-analysis of randomized controlled trials. J Arrhythmias 2022;38:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 7. Lee JM, Shim J, Park J, Tae Yu H, Kim TH, Park JKet al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. J Am Coll Cardiol EP 2019;5:1253–61. [DOI] [PubMed] [Google Scholar]
- 8. Kim D, Yu HAT, Kim TH, Uhm JS, Joung B, Lee MHet al. Electrical posterior box isolation in repeat ablation for atrial fibrillation: a prospective randomized clinical study. JACC: Clin EP 2022;144:582–9. [DOI] [PubMed] [Google Scholar]
- 9. Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi Set al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation. The CAPLA randomized clinical trial. JAMA 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piorkowski C, Kronborg M, Hourdain J, Piorkowski J, Kirstein B, Neudeck Set al. Endo-/epicardial catheter ablation of atrial fibrillation. Feasibility, outcome and insights into arrhythmia mechanisms. Circ AE 2018;11:e005748. [DOI] [PubMed] [Google Scholar]
- 11. Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ AE 2010;3:243–8. [DOI] [PubMed] [Google Scholar]
- 12. Bourier F, Fahrig R, Wang P, Santangeli P, Kurzidim K, Strobel Net al. Accuracy assessment of catheter guidance technology in electrophysiology procedures. J Cardiovasc Electrophysiol 2013;25:74–83. [DOI] [PubMed] [Google Scholar]
- 13. El Haddad M, Taghji P, Phlips T, Wolf M, Demolder A, Choudhury Ret al. Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation. Circ Arrhythmias Electrophysiol 2017;10:e004867. [DOI] [PubMed] [Google Scholar]
- 14. Whitaker J, Fish J, Harrison J, Chubb H, Williams SE, Fastl Tet al. Lesion index-guided ablation facilitates continuous, transmural, and durable lesions in a porcine recovery model. Circ Arrhythmias Electrophysiol 2018;11:e005892. [DOI] [PubMed] [Google Scholar]
- 15. Gehi AK, Kiser AC, Mounsey PJ. Atrial fibrillation ablation by the epicardial approach. J Atr Fibrillation 2014;6:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tahir K, Kiser A, Caranasos T, Mounsey JP, Gehi A. Hybrid epicardial-endocardial approach to atrial fibrillation ablation. Curr Treat Options Cardiovasc Med 2018;20:25. [DOI] [PubMed] [Google Scholar]
- 17. Varzaly JA, Lau DH, Chapman D, Edwards J, Worthington M, Sanders Pet al. Hybrid ablation for atrial fibrillation: a systematic review and meta-analysis. JTCVS Open 2021;7:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy VY, Neuzil P, D’Avila A, Ruskin JN. Isolating the posterior left atrium and pulmonary veins with a ‘box’ lesion set: use of epicardial ablation to complete electrical isolation. J Cardiovasc Electrophysiol 2008;19:326–9. [DOI] [PubMed] [Google Scholar]
- 19. Pak HN, Hwang C, Lim HE, Kim JS, Kim YH. Hybrid epicardial and endocardial ablation of persistent or permanent atrial fibrillation: a new approach for difficult cases. J Cardiovasc Electrophysiol 2007;18:917–23. [DOI] [PubMed] [Google Scholar]
- 20. Berruezo A, Bisbal F, Fernandez-Armenta J, Calvo N, Cabrera JA, Sanchez-Quintana Det al. Transthoracic epicardial ablation of mitral isthmus for treatment of recurrent perimitral flutter. Heart Rhythm 2014;11:26–33. [DOI] [PubMed] [Google Scholar]
- 21. Jiang R, Buch E, Gima J, Upadhyay GA, Nayak HM, Beaser ADet al. Feasibility of percutaneous epicardial mapping and ablation for refractory atrial fibrillation: insights into substrate and lesions transmurality. Heart Rhythm 2019;16:1151–9. [DOI] [PubMed] [Google Scholar]
- 22. De Martino G, Compagnucii P, Mancusi C, Vassallo E, Calvanese C, Della Ratta Get al. Stepwise endo-/epicardial catheter ablation for atrial fibrillation: the mediterranea approach. J Cardiovasc Electrophysiol 2021;32:2107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee KN, Kim DY, Boo KY, Kim YG, Roh SY, Shim Jet al. Combined epicardial and endocardial approach for redo radiofrequency catheter ablation in patients with persistent atrial fibrillation: a randomized clinical trial. Europace 2022;24:1412–9. [DOI] [PubMed] [Google Scholar]
- 24. De Martino G, Compagnucci P, Mancusi C, Vassallo E, Calvanese C, Della Ratta Get al. Stepwise endo/epicardial catheter ablation for atrial fibrillation: the Mediterranea approach. Authorea 2021;32:2107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structures and fibres: morphologic bases of atrial conduction. Cardiovasc Res 2002;54:325–36. [DOI] [PubMed] [Google Scholar]
- 26. Pashakhanloo F, Herzka DA, Ashikaga H, Mori S, Gai N, Bluemke DAet al. Myofiber architecture of the human atria as revealed by submillimeter diffusion tensor imaging. Circ Arrhythmias Electrophysiol 2016;9:e004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia F, Enriquez A, Arroyo A, Supple G, Marchlinski F, Saenz L. Roof dependent atrial flutter with an epicardial component: role of the septo-pulmonary bundle. J Cardiovasc Electrophysiol 2019;30:1159–63. [DOI] [PubMed] [Google Scholar]
- 28. Pambrun T, Duchateau J, Delgove A, Denis A, Constantin M, Ramirez FDet al. Epicardial course of the septopulmonary bundle: anatomical considerations and clinical implications for roof line completion. Heart Rhythm 2021;18:349–57. [DOI] [PubMed] [Google Scholar]
- 29. Wolf M, El Haddad M, Fedida J, Taghji P, Van Beeumen K, Strisciuglio Tet al. Evaluation of left atrial linear ablation using contiguous and optimized radiofrequency lesions: the ALINE study. Europace 2018;20:f401–9. [DOI] [PubMed] [Google Scholar]
- 30. Lai Y, Guo Q, Sang C, Gao M, Huang L, Zuo Set al. Revisiting the characteristics and ablation strategy of biatrial tachycardias: a case series and systematic review. Europace 2023;25:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu W, Zhou D, Ding X, Yang G, Liu H, Wang Zet al. Arrhythmogenesis of surgical atrial incisions and lesions in maze procedure: insights from high resolution mapping of atrial tachycardias. Europace 2023;25:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valderrabano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RNet al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation. The VENUS randomized clinical trial. JAMA 2020;324:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakashima T, Pambrun T, Vlachos K, Goujeau C, Andre C, Krisai Pet al. Impact of vein of Marshall ethanol infusion on mitral isthmus block: efficacy and durability. Circ Arrhythmias Electrophysiol 2020;13:e008884. [DOI] [PubMed] [Google Scholar]
- 34. Rillig A, Tilz RR, Lin T, Fink T, Heeger CH, Arya Aet al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythmias Electrophysiol 2016;9:3003461. [DOI] [PubMed] [Google Scholar]
- 35. Leung L, Akhtar Z, Gallagher M. Letter to the editor: oesophageal cooling for protection during left atrial ablations. Europace 2023;25:euad153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joseph C, Sherman J, Ro A, Fisher WG, Nazari J, Metzl Met al. Procedural time reduction associated with active esophageal cooling during pulmonary vein isolation. J Interv Card Electrophysiol 2022;65:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zagrodzky J, Bailey S, Shah S, Kulstad E. Impact of active esophageal cooling on fluoroscopy usage during left atrial ablation. J Innov Card Rhythm Manage 2021;12:4749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joseph C, Cooper J, Sikka R, Zagrodzky J, Turer RW, McDonald SAet al. Improved hospital discharge and cost savings with esophageal cooling during left atrial ablation. J Med Econ 2023;26:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leung LW, Akhtar Z, Elbatran A, Bajpai A, Li A, Norman Met al. Effect of esophageal cooling on ablation lesion formation in the left atrium: insights from ablation index data in the IMPACT trial and clinical outcomes. J Cardiovasc Electrophysiol 2022;33:2546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamed M, Elseidy SA, Abdelazeem M, Morcos R, Abdallah A, Sammour Yet al. Role of oesophageal cooling in the prevention of oesophageal injury in atrial fibrillation catheter ablation: a systematic review and meta-analysis of randomized controlled trials. Europace 2023;25:euad080 (Online ahead of print. PMID: 37021812). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yokoyama Y, Yamamoto T, Takahashi T, Arase H, Ogasawara K, Kakutani Aet al. A case of successful radiofrequency ablation of an epicardial conduction breakthrough site probably via the septopulmonary bundle. Heart Rhythm Case Rep 2021;7:825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tonko J, Manoharan K, Amin R, Silberbauer J. Management of pericardial bleeding complications in percutaneous endoepicardial ablation for atrial fibrillation: a case for intra-pericardial tranexamic acid? Heart Rhythm Case Rep 2022;8:820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun Y, Xiao X, Yin X, Gao L, Yu X, Zhang Ret al. Impact of baseline impedance of pulmonary vein antrum on success of catheter ablation for paroxysmal atrial fibrillation guided by ablation index. BMC Cardiovasc Disord 2022;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barkagan M, Rottmann M, Leshem E, Shen C, Buxton AE, Anter E. Effect of baseline impedance on ablation lesion dimensions: a multi-modality concept validation from physics to clinical experience. Circ Arrhythmias Electrophysiol 2018;11:e006690. [DOI] [PubMed] [Google Scholar]
- 45. Bourier F, Ramirez FD, Martin CA, Vlachos K, Frontera A, Takigawa Met al. Impedance, power and current in radiofrequency ablation: insights from technical, ex vivo and clinical studies. J Cardiovasc Electrophys 2020;31:2836–45. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen DT, Nguyen K, Zheng L, Zheng L, Schuller J, Zipse Met al. Effect of environmental impedance surrounding a radiofrequency ablation catheter electrode on lesion characteristics. J Cardiovasc Electrophysiol 2017;28:564–9. [DOI] [PubMed] [Google Scholar]
- 47. Nguyen DT, Tzou W, Sandhu A, Gianni C, Anter E, Tung Ret al. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Clin. EP 2018;4:1176–85. [DOI] [PubMed] [Google Scholar]
- 48. Hong KL, Bakker D, Baley J, Babiolakis C, Bullen M, Xue Cet al. Half normal saline shortens the time to achieve bidirectional block in typical atrial flutter. J Am Coll Cardiol 2019;73:508. [Google Scholar]
- 49. Gianni C, Gallinghouse GJ, Al-Ahmad A, Horton RP, Bailey SM, Burkhardt JDet al. Half-normal saline versus normal saline for irrigation of open-irrigated radiofrequency catheter in atrial fibrillation ablation. J Cardiovasc Electrophys 2021;32:973–81. [DOI] [PubMed] [Google Scholar]
- 50. Irastorza R, Maher T, Barkagan M, Liubasuskas R, Berjano E, D’Avila A. Anterior vs posterior position of dispersive patch during radiofrequency catheter ablation: insights from in silico modelling. Europace 2023;25:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shapira-Daniels A, Barkagan M, Rottmann M, Sroubek J, Tugal D, Carlozzi MAet al. Modulating baseline impedance: an adjunctive technique for maximizing radiofrequency lesion dimensions in deep and intramural ventricular substrate. Circ Arrhythmias Electrophysiol 2020;12:e007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Irastorza RM, Maher T, Barkagan M, Liubasuskas R, Berjano E, D’Avila A. Limitations of baseline impedance, impedance drop and current for radiofrequency catheter ablation monitoring: insights from in silico modeling. J Cardiovasc Dev Dis 2022;9:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koruth JS, Dukkipati S, Miller MA, Neuzil P, Avila AD, Reddy VKet al. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm 2012;9:1932–41. [DOI] [PubMed] [Google Scholar]
- 54. Yamagata K, Wichterle D, Peichl P, Aldhoon B, Cihak R, Kautzner J. Bipolar radiofrequency catheter ablation for refractory accessory flutter: a case report. BMC Cardiovasc Disord 2015;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Futyma P, Kulakowski P. Bipolar ablation of high-risk postero-septal accessory pathway: back to the future. Heart Rhythm Case Rep 2020;6:166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet Het al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 57. Shtembari J, Shrestha DB, Pathak BD, Bishal D, Upadhaya Regmi B, Patel NKet al. Efficacy and safety of pulsed field ablation in atrial fibrillation: a systematic review. J Clin Med 2023;12:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gunawardene MA, Schaeffer BN, Jularic M, Eickholt C, Maurer T, Akbulak ROet al. Pulsed field ablation in patients with complex consecutive atrial tachycardia in conjunction with ultra-high density mapping: proof of concept. J Cardiovasc Electrophysiol 2022;33:2431–43. [DOI] [PubMed] [Google Scholar]
- 59. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami Ket al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 60. Reddy V, Anter E, Rackauskas G, Peichl P, Koruth JS, Petru Jet al. Lattice tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythmias Electrophysiol 2020;13:e008718. [DOI] [PubMed] [Google Scholar]
- 61. Koruth JS, Kuroki K, Kawamura I, Stoffregen WC, Dukkipati SR, Neuzil Pet al. Focal pulsed field ablation for pulmonary vein isolation and linear atrial lesions: a preclinical assessment of safety and durability. Circ Arrhythmias Electrophysiol 2020;13:e008716. [DOI] [PubMed] [Google Scholar]
- 62. Bishop M, Rajani R, Plank G, Gaddum N, Carr-White G, Wright Met al. Three dimension atrial wall thickness maps to inform catheter ablation procedures for atrial fibrillation. Europace 2015;18:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ciuffo L, Nguyen H, Diniz Marques M, Aronis KN, Sivasambu B, De Vasconcelos HDet al. Peri-atrial fat quality predicts atrial fibrillation ablation outcome. Circ Cardiovasc Imaging 2019;12:e008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chubb H, Williams SE, Whitaker J, Harrison JL, Razavi R, O’Neill M. Cardiac electrophysiology under MRI guidance: an emerging technology. Arrhythmias Electrophysiol Rev 2017;6:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lichter J, Kholmovski E, Coulombe N, Ghafoori E, Kamali R, MacLeod Ret al. Real-time MRI guided cryoablation of the pulmobarz veins with acute freeze-zone and chronic lesion assessment. Europace 2019;21:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sayseng V, Grondin J, Salgaonkar V, Grubb CS, Basij M, Mehrmohammadi Met al. Catheter ablation lesion visualization with intracardiac strain imaging in canines and humans. IEEE Trans Ultrason Ferroelectr Freq Control 2020;67:1800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.