Abstract
Glucocorticoids acting via the glucocorticoid receptors (GR) are key regulators of metabolism and the stress response. However, uncontrolled or excessive GR signaling adversely affects adipose tissue, including endocrine, immune, and metabolic functions. Inflammation of the adipose tissue promotes systemic metabolic dysfunction; however, the molecular mechanisms underlying the role of adipocyte GR in regulating genes associated with adipose tissue inflammation are poorly understood. We performed in vivo studies using adipocyte-specific GR knockout mice in conjunction with in vitro studies to understand the contribution of adipocyte GR in regulating adipose tissue immune homeostasis. Our findings show that adipocyte-specific GR signaling regulates adipokines at both mRNA and plasma levels and immune regulatory (Coch, Pdcd1, Cemip, and Cxcr2) mRNA gene expression, which affects myeloid immune cell presence in white adipose tissue. We found that, in adipocytes, GR directly influences Cxcr2. This chemokine receptor promotes immune cell migration, indirectly affecting Pdcd1 and Cemip gene expression in nonadipocyte or stromal cells. Our findings suggest that GR adipocyte signaling suppresses inflammatory signals, maintaining immune homeostasis. We also found that GR signaling in adipose tissue in response to stress is sexually dimorphic. Understanding the molecular relationship between GR signaling and adipose tissue inflammation could help develop potential targets to improve local and systemic inflammation, insulin sensitivity, and metabolic health.
Keywords: glucocorticoids, adipose tissue, glucocorticoid receptor, adipose tissue homeostasis
Glucocorticoids are steroid hormones secreted by the adrenal glands that play a critical role in maintaining homeostasis in several vital body systems, including the cardiovascular (1), immune (2), and metabolic systems (3-5), as well as in the physiological adaptation to stress (6). In times of energy demand, glucocorticoids, whether circulating or within tissues, activate pathways essential for both systemic energy homeostasis and redistribution in tissues such as the liver, skeletal muscle, and white adipose tissue (WAT) (5).
Due to their potent anti-inflammatory/immunosuppressive effects, synthetic glucocorticoids are widely used in clinical practice (7). However, prolonged exposure to high levels of glucocorticoids, whether from exogenous administration or endogenous overproduction (8, 9), is associated with an increased risk of adverse effects. Such adverse effects include immune (10), metabolic (11), and cardiovascular issues (12). Long-term elevation of glucocorticoid levels induces adipose tissue redistribution from peripheral to abdominal fat depot (13), resulting in weight gain, hyperglycemia, insulin resistance, and dyslipidemia (14), which makes adipose tissue a major site of metabolic dysfunction. Glucocorticoids exert their effects by binding to glucocorticoid receptors (GR; Nr3C1), members of the steroid hormone family of nuclear receptors widely expressed throughout the body. Adipocyte GR plays a significant role in regulating adipogenesis, lipolysis, lipogenesis, glucose uptake, insulin signaling, and the expression of various genes involved in insulin resistance and metabolism (6, 15-19).
Adipocyte GR may play a complex role in adipose tissue homeostasis and function. For example, deletion of GR in adipocyte leads to significant reductions in obesity associated with the consumption of a high-fat diet and, with age, decreased lipolysis and lipogenesis, decreased gluconeogenesis, decreased plasma levels of fatty acid metabolites, and increased insulin sensitivity (17, 18, 20). Others report only mild metabolic protection in the absence of adipocyte GR (21).
Adipose tissue contains immune cells that control the local inflammatory state and contribute to the regulation of systemic metabolism via cross-talk with adipocytes (22). Dysfunctional adipocytes disrupt the immune regulatory network by secreting proinflammatory adipokines that recruit immune cells in adipose tissue, thereby promoting adipose tissue inflammation (23). These immune cells subsequently produce proinflammatory cytokines that further aggravate the inflammatory response and contribute to insulin resistance, ultimately resulting in poor systemic metabolic outcomes (24). In this study, our aim was to investigate how adipocyte GR regulates the genes associated with adipose tissue inflammation, a critical factor contributing to systemic metabolic dysfunction. We used a mouse model lacking GR in adipocytes to investigate the effects of glucocorticoid receptor signaling on adipocyte morphology, adipokine expression, immune regulatory gene expression, and immune cell presence in adipose tissue, using both in vivo and in vitro approaches. Our data suggest that the modulation of GR signaling in adipose tissue could be a potential approach to prevent chronic low-grade adipose tissue inflammation and improve systemic health. These changes showed a degree of sexual dimorphism.
Materials and Methods
Animal Model
C57BL/6J GR flox/flox (GR Flox) mice (25) were bred with Adipoq-Cre mice, which expressed Cre recombinase under the direction of an adiponectin promoter to generate adipocyte-specific GR knockout (Adipo GRKO) mice, as previously described (26). Mice were maintained on a 12:12 light/dark cycle with free access to water and a regular chow diet in an environmentally controlled animal facility at Louisiana State University Health Sciences Center—Shreveport. The age group of the mice was either 5 months or 2 to 3 months depending on the experiment. The age ranges of the animals in the experiments were selected based on their availability. Mice between 2 and 5 months of age are considered adults (27). For baseline characterization and microarray analysis, 5-month-old male and female mice were used. Five-month-old male mice were used for adipose tissue fractionation and flow cytometry studies. For acute stress experiments, 2- to 3-month male and female mice were used. GR Flox and Adipo GRKO mice were sacrificed after isoflurane inhalation followed by cervical dislocation. All GR Flox mice served as littermate controls. White adipose tissue depots comprising of subcutaneous, mesenteric, retroperitoneal, and epididymal adipose tissue along with brown adipose tissue (BAT) and heart were collected and used for gene expression analysis. We focused our study on mesenteric adipose tissue, which is a type of visceral adipose tissue due to its potential role in the pathophysiology of inflammatory and metabolic diseases (28). Of the mesenteric adipose tissue collected, half was stored in a 4% formaldehyde solution for 48 hours and later processed for histological staining procedures. The other half was rapidly frozen in liquid nitrogen and stored at −80°C until further analysis. All animal studies were approved by Louisiana State University Health Sciences Center—Shreveport Animal Care and Use Committee.
Restraint Stress Model
Two- to three month-old male and female GR Flox and Adipo GRKO mice were randomly divided into nonstressed (control) and stressed groups (n = 4-6 per group/per genotype). Mice in the stressed group were removed from their cages and individually placed on a plastic board where the mouse's body was restrained using double-sided Velcro tapes. The mouse's tail was also secured to the board using nonadhesive tape to strengthen the restraint. Acute psychogenic stress sessions were started at the beginning of the animals’ nocturnal cycle (8:00 Am) during which the mice in the stressed group were exposed to an hour of restraint followed by 30 minutes of release when they were returned to their cages for free access to food and water. The periodic restraint followed by release was repeated 6 times for a total of 9 hours. Mice in the nonstressed group were left in their cages with no access to food and water as their stressed counterparts underwent the stress challenge. However, food and water were provided to the mice in both the stressed and nonstressed group during the release period. Blood samples and mesenteric adipose tissue were collected from the mice the next day following the stress challenge. Both stressed and nonstressed mice were perfused with phosphate buffered saline (PBS) prior to mesenteric adipose tissue collection.
Plasma Collection and Analysis of Plasma Hormones
Mice were briefly anesthetized using 2% isoflurane inhalation and the tail tips were snipped with a pair of sterile scissors. Blood samples were collected from the mouse tail vein using EDTA-coated capillary blood collection tubes (Microvette CB 300, Sarstedt) and centrifuged at 3500 RCF for 15 minutes at 4°C. The supernatant plasma samples were collected and stored at −80°C until assessment of plasma hormone levels. Plasma corticosterone (catalog no. K014-H5, RRID: AB_2877626, Arbor Assays), adiponectin (catalog no. ab108785, RRID: AB_2891131, Abcam), leptin (catalog no. MOB00B, RRID: AB_2943468, R&D Systems), and resistin (catalog no. MRSN00, RRID: AB_2943469, R&D Systems) were measured using commercially available ELISA kits. The corticosterone assay sensitivity and detection limits were 20.9 pg/mL and 17.5 pg/mL, respectively. The minimum detectable dose of adiponectin assay was 0.23 ng/mL, and the assay sensitivities for resistin and leptin assay were 8 pg/mL and 5.56 pg/mL, respectively. The intra- and inter-assay coefficients of variation for the corticosterone assay were 3.1% to 6.5% and 6.4% to 9.9%, for adiponectin assay were 7.5% and 10.8%, for leptin assay were 2% to 3.6% and 5.9% to 7.8%, and for resistin assay were 2.7% to 4% and 6.9% to 7.3%, respectively, as reported by the manufacturer. All samples were run in duplicate following the manufacturer's instructions.
Adipocyte Morphometry
The mean adipocyte size was determined with some modifications from previous methods (18, 29). Briefly, mesenteric adipose tissue embedded in paraffin was cut into 5 μm thick sections and stained with hematoxylin and eosin (H&E). Images were acquired using a light microscope (OMAX, USA). The cross-sectional area of adipocytes was measured in 4 to 5 fields per section covering the entire tissue surface from individual mice in each group at a magnification of 100 × using National Institutes of Health Image-J analysis software (https://imagej.nih.gov/ij/) (National Institutes of Health, Bethesda, MD, USA) with the Adiposoft plugin (https://imagej.net/plugins/adiposoft). In total, 1330 to 4700 adipocytes were assessed in each mouse. The H&E stained images were calibrated to microns per pixel, and the value was entered in Adiposoft plugin of ImageJ software, which then calculated the adipocyte area. The mean adipocyte area for all assessed adipocytes in individual mice was determined and compared between GR Flox and Adipo GRKO mice.
Microarray
RNA was extracted from mesenteric adipose tissue with QIAzol lysis reagent (catalog no. 79306, Qiagen) followed by purification with RNase Free Dnase (catalog no. 79254, Qiagen) treatment using an RNeasy Mini Kit (catalog no. 74134, Qiagen) according to the manufacturer's instructions. RNA integrity number was evaluated using an Agilent 2100 Bioanalyzer. Only samples with an RNA integrity number score > 7 were used in the experiments.
Gene expression analysis was conducted using Agilent Whole Mouse Genome 4 × 44 multiplex format oligo arrays (product no. 014868, Agilent Technologies) following the Agilent 1-color microarray-based gene expression analysis protocol. Starting with 500 ng total RNA, Cy3 labeled cRNA was produced according to the manufacturer's protocol. For each sample, 1.65 μg of Cy3 labeled cRNAs were fragmented and hybridized for 17 hours in a rotating hybridization oven. Slides were washed and then scanned with an Agilent scanner. Data were obtained using the Agilent Feature Extraction software (v12), using the 1-color defaults for all parameters. The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. Principal component analysis was performed on all samples and all probes to characterize the variability present in the data. In order to identify differentially expressed probes, ANOVA was used to determine if there was a statistical difference between the means of groups. Specifically, an ANOVA with an false discovery rate-corrected P-value of P < .05 was performed using OmicSoft Array Studio (Version 8.0) software.
The differentially expressed probes were further analyzed with Pathway Analysis (Ingenuity Systems). The microarray data will be available in the Gene Expression Omnibus repository at the National Center for Biotechnology Information (GEO: GSE241653).
Real-Time Quantitative PCR
Total RNA was extracted from tissues and cells using the Extracta Plus RNA kit (catalog no. 95214-050, Quantabio) following the manufacturer's instructions. The total RNA concentration was quantified using a NanoDrop (BioTek). mRNA gene expression levels were determined using the TaqMan probes and an iTaq Universal one-step kit (catalog no. 1725141, Bio-Rad) on Bio-Rad CFX96 Fast Real Time PCR systems. The probes, Adgre1 Mm00802529_m1, Adipoq Mm00456425, Cemip Mm00472921_m1, Coch Mm00483360_m1, Cxcr2 Mm99999117, Dnase1 Mm01342387_g1, Ighm Mm01718955_g1, Lep Mm00434759_m, Nr3c1 Mm00433832, Pdcd1 Mm01285676_m1, Ppib Mm00478295, and Retn Mm00445641_m1 were purchased from Thermo Fisher Scientific. The relative gene expression for all genes was analyzed using the 2−ΔΔCt method where duplicate Ct values for each sample were averaged and subtracted from those obtained from the housekeeping gene peptidyl-prolyl cis-trans isomerase B (Ppib) in all experiments.
Protein Extraction and Western Blot
Protein was extracted from heart, subcutaneous, mesenteric, retroperitoneal, and BAT in RIPA buffer (catalog no. 97063-270, Avantor) supplemented with a protease inhibitor cocktail (catalog no. A32963, Thermo Fisher Scientific). Protein was determined using a Pierce BCA protein assay kit (catalog no. 23225, Thermo Fisher Scientific). Protein lysates in aliquots of 20 μg per well were loaded, separated by 4% to 15% gradient SDS PAGE (catalog no. 4561086, Bio Rad), and transferred to a PVDF membrane (catalog no.1704156EDU, Bio-Rad). GR (catalog no.3660 RRID: AB_11179215, Cell Signaling Technology) and Actin (catalog no. 12620, RRID: AB_2797972, Cell Signaling Technology) were used as primary antibodies at a concentration of 1:1000. Secondary mouse anti-rabbit horse radish peroxidase antibody (catalog no. 211-032-171, RRID: AB_2339149, Jackson Immunoresearch) was used at a concentration of 1:5000. Protein bands were visualized by enhanced chemiluminescence detection (catalog No. 1705060S, Bio-Rad).
Adipocytes and Stromal Vascular Fraction Isolation
Epididymal adipose tissues from 2 mice were excised, pooled according to the genotype, washed with PBS, and finely minced. The adipose tissue dissociation kit (catalog no.130-105-808, Miltenyi Biotec) and gentleMACS Octo Dissociator (catalog no. 130-096-427, Miltenyi Biotec) were used for adipose tissue dissociation following the manufacturer's instructions. The digested tissue was filtered with a 100-μm nylon filter and centrifuged for 5 minutes at 1200 g at room temperature. Floating adipocytes were collected and washed twice with PBS at room temperature. The pelleted stromal vascular cells were washed with erythrocyte lysis buffer (catalog no. A1049201, Gibco) and resuspended in PBS. The adipocyte fraction and 100 μL of stromal vascular fraction (SVF) were used for RNA isolation. The remaining stromal vascular cells were used for flow cytometry.
Flow Cytometry and Data Acquisition
Single cell suspensions of stromal vascular cells (8 ×105-1 million) were seeded in FACS tubes (catalog no. 14-959-1A, Thermo Fisher Scientific) and incubated with Zombie Violet Live Dead stain (catalog no. 423113, BioLegend) at a concentration of 1:500 for 30 minutes at room temperature to confirm cell viability. The cells were treated with anti-CD 16/32 (catalog no. 156603, RRID: AB_2783137, BioLegend) at a concentration of 1:200 for 15 minutes followed by cell surface staining with the following antibodies for 30 minutes at 4οC: CD45 (1:400) (catalog no. 103138, RRID: AB_2563061, BioLegend), CD11b (1:2000) (catalog no.101217, RRID: 389305, BioLegend), and Ly6C (1:800) (catalog no. 128017, RRID: AB_1732093, BioLegend). Cells were fixed using freshly prepared 2% paraformaldehyde in PBS. The cells were further washed and resuspended in 200 μL FACS buffer and analyzed on an ACEA NovoCyte Quanteon flow cytometer (Biosciences). Fluorescence Minus One controls were used for each antibody marker to set the limit for the background signal of the excluded marker and to identify and gate for positive stained cell population. Cells gated on CD45 for total immune cells were subsequently gated on CD11b to identify the myeloid cell population. Furthermore, CD45 positive stained cells were then gated on CD11b and Ly6C to identify macrophages. Data analysis was performed using NovoExpress software.
3T3-L1 Mouse Embryonic Fibroblast Cell Culture
3T3-L1 mouse embryonic fibroblasts (BCRC catalog no. 60159, RRID: CVCL_0123) were purchased from the Animal Type Culture Collection (ATCC). Cells were maintained in preadipocyte expansion media containing 10% bovine calf serum (catalog no. 30-2030, ATCC), 1% penicillin-streptomycin (catalog no. 091670049, MP Biomedicals), and 90% Dulbecco's modified Eagle's medium (DMEM) (catalog no. 30-2002, ATCC) until 70% to 80% confluent. Following trypsinization, 8 × 104 cells were plated in 35-mm cell culture dishes. The cells were grown to 100% confluence and differentiated to adipocytes using a differentiation cocktail medium consisting of 90% DMEM, 10% fetal bovine serum (FBS) (catalog no. 30-2020, ATCC), 1% penicillin-streptomycin (catalog no. 091670049, MP Biomedicals), 1 μM dexamethasone (catalog no. API-04, G Biosciences), 1 μg/mL insulin (catalog no. I0516, Sigma), and 0.5 mM methylisobutylxanthine (catalog no. I5879, Sigma), for 48 hours. The differentiation medium was replaced with an equal volume of maintenance medium consisting of 90% DMEM, 10% FBS, and 1 μg/mL insulin (Sigma) (day 0) after 48 hours. The maintenance medium was changed every other day for 10 days, and all experiments were performed on the 10th day post-differentiation.
Differentiated 3T3-L1 cells were treated with 100 nM dexamethasone (Steraloids) for 0, 3, and 6 hours. For GR antagonist experiments, cells were first treated with 1 μg/mL RU 486 (catalog no. M8046, Sigma) for 2 hours followed by 100 nM dexamethasone for either 0 or 3 hours. For cycloheximide experiments, cells were treated with 10 μg/mL cycloheximide (catalog no. C7698, Sigma) for 1 hour followed by 100 nM dexamethasone for 0 and 3 hours.
Primary Adipocyte Cell Culture
Epididymal white preadipocytes were isolated and cultured as described previously (30). Briefly, 6- to 8-week-old male GR Flox and Adipo GRKO mice were euthanized by isoflurane inhalation followed by cervical dislocation. Four epididymal adipose tissue depots from 2 mice of the same genotype were pooled and minced with scissors in DMEM. The fragmented tissue was added to 5 mL of digestion buffer [ddH2O containing HEPES (100 mM), NaCl (123 mM), KCl (5 mM), CaCl2 (1.3 mM), glucose (5 mM), BSA (1.5% w/v), and collagenase type I (2 mg/mL) (catalog no.C6885, Sigma)] and shaken at 300 RPM at 37°C for 1 hour.
The digested solution was then passed through a 100-μm cell strainer and placed on ice for 20 minutes. The infranatant below the top mature adipocyte layer was collected, filtered through a 70-μm cell strainer, and centrifuged at 500 g for 10 minutes. The cell pellet was washed with DMEM, centrifuged, and then resuspended in preadipocyte expansion media and incubated at 37°C and 5% CO2. On the second day after confluency (day 0), the preadipocytes were differentiated into mature adipocytes and maintained using the same protocol used for the 3T3-L1 cell line. All experiments were performed on the 10th day after differentiation.
Immunofluorescence
Primary adipocytes differentiated from GR Flox and Adipo GRKO preadipocytes were washed with PBS three times and fixed with 4% paraformaldehyde for 30 minutes at room temperature. The cells were permeabilized using 0.1% Triton X-100, and nonspecific binding by antibodies was blocked using 0.1% BSA. The cells were incubated with the primary antibodies against adiponectin (catalog no. 22554, RRID: AB_447152, Abcam) and GR (catalog no. 3660, RRID: AB_11179215, Cell Signaling Technology) at a concentration of 1:500 overnight at 4οC. The cells were washed and incubated with a secondary antibody (catalog No. A28175, RRID: AB_2536161, Invitrogen) (Alexa Fluor 1:1000) for 1 hour the next day. Mounting medium containing DAPI stain (catalog no. H-1200-10, Vectashield) was used, and images were obtained on an EVOS M5000 confocal microscope using a 40 × objective.
Oil-Red-O Staining
Cells were washed with PBS and fixed in 10% formalin for 15 minutes followed by washes with distilled water and 60% isopropanol. Cells were incubated in 3 mg/mL Oil-Red-O solution (catalog no. A12989, Alfa Aesar) prepared in 100% isopropanol for 15 minutes. After washing with distilled water, images were captured using a light microscope with a 20 × objective (OMAX). Neutral lipids stained with Oil-Red-O were extracted in 100% isopropanol, and the absorbance was measured at 492 nm to quantify the amount of lipids accumulated by differentiated mature adipocytes.
Generation of Bone Marrow Derived Macrophages
Bone marrow derived macrophages were generated from 6- to 8-week-old GR Flox and Adipo GRKO mice using previously described protocols (31). Briefly, femurs were removed under sterile conditions and flushed with 5 mL DMEM (catalog no. 30-2002, ATCC) supplemented with 10% FBS (catalog no. 30-2020, ATCC) and 1% penicillin-streptomycin (catalog no. 091670049, MP Biomedicals) and centrifuged at 300 RCF for 5 minutes at 4°C. Erythrocytes were lysed using ACK lysis buffer (catalog no. A10492-01, Gibco) followed by a DMEM wash. The isolated cells were incubated in a sterile 100 mm Petri dish for 8 days in bone marrow derived macrophage differentiation medium: DMEM media supplemented with 5 ng/mL macrophage colony stimulating factor (catalog no. 315-02, Peprotech), 10% FBS (catalog no. 30-2020, ATCC), and 1% penicillin-streptomycin (catalog no. 091670049, MP Biomedicals), at 37°C and 5% CO2. Once cells reached 80% confluency, they were collected using 11 mM EDTA (pH 7.6).
Primary Adipocyte Treatment and Macrophage Migration Assay
Differentiated GR Flox and Adipo GRKO adipocytes were plated in the bottom chamber and were subjected to vehicle (control) or 100 nM dexamethasone treatment for 3 hours. Upon completion of treatment, the cells were washed with PBS and replaced with DMEM containing 10% FBS as a chemoattractant. Bone marrow derived macrophages (105 cells) were placed on the top chambers of cell culture inserts (24-wells) with 8 μm pore size (vWR) in serum-free DMEM media and incubated at 37°C and 5% CO2 for 24 hours. The inserts were washed with PBS, and any nonmigrated macrophages that remained on the upper surface of the insert were removed with a cotton swab. Migrated macrophages on the lower surface of the insert were fixed with 4% paraformaldehyde, stained with H&E and mounted on glass slides. The average number of migrated cells in 5 randomly chosen fields was counted for each treatment using a light microscope at 40 × magnification.
Statistics
Statistical analyses were performed using GraphPad Prism 9 Software (San Diego, CA), and the results are displayed as the mean ± standard deviation (SD). Comparisons between groups were carried out either by 2-way ANOVA followed by post hoc Tukey's multiple comparison test when 3 or more groups were compared or by a 2-tailed unpaired Student's t-test when 2 groups were compared. An alpha threshold of P < .05 was defined as statistically significant.
Results
Adipocyte-Specific GR Deletion Altered Plasma Adipokine Levels
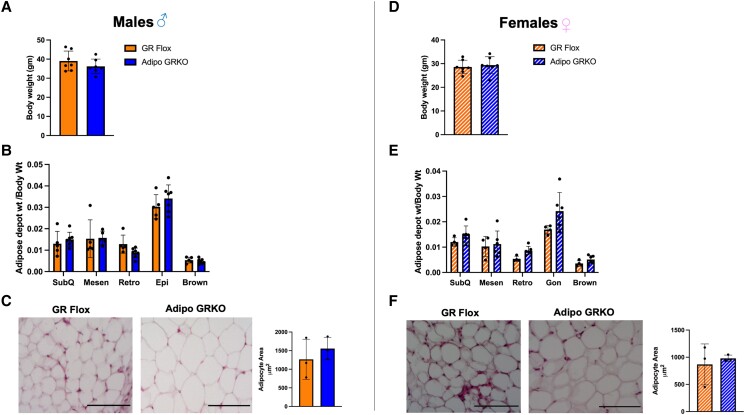
To determine the baseline characteristics we measured the body weight, adiposity, and adipocyte size of GR Flox and Adipo GRKO mice under a regular chow diet. Deletion of adipocyte GR did not affect the body weight (Fig. 1A and 1D) or the adiposity (Fig. 1B and 1E), as measured by the ratio of adipose tissue depot weight by body weight in both male and female mice. To test the impact of adipocyte GR deletion on adipose tissue morphology, we measured the adipocyte area in mesenteric adipose tissue of both GR Flox and Adipo GRKO mice. Our results revealed no significant difference in mean adipocyte area between the genotypes in both male and female mice. (Fig. 1C and 1F).
Figure 1.
Deletion of adipocyte GR did not affect the body weight, adiposity, or adipocyte size. (A), (D) Body weight of male (solid bars) and female (striped bars) GR Flox and Adipo GRKO mice (n = 7 mice/genotype). (B), (E) Ratio of different adipose tissue depot weight to body weight was calculated as a measure of adiposity in GR Flox and Adipo GRKO male and female mice (n = 4-6 mice/genotype). (C), (F) Representative hematoxylin and eosin stained image of GR Flox and Adipo GRKO male and female mesenteric adipose tissue. Average adipocyte area was calculated using ImageJ software (n = 3 mice/genotype). Scale bar: 10 μm. All values represent mean ± SD. Data were statistically analyzed using unpaired Student's t-test.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox; GRKO, GR knockout.
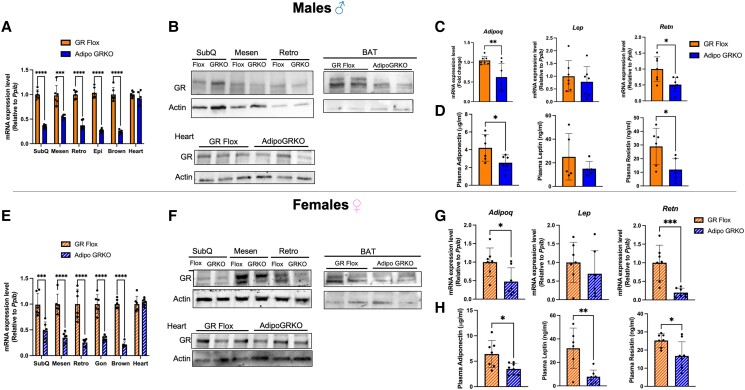
To confirm the successful deletion of GR from adipose tissue, we measured GR mRNA levels in different depots of WAT such as subcutaneous, mesenteric, retroperitoneal, epididymal or gonadal, BAT, and heart. Our results demonstrated that Adipo GRKO mice had significantly decreased GR mRNA expression in all WAT and BAT depots examined compared to GR Flox mice while normal expression was observed in nonadipose tissue such as heart in both males and females [males: subcutaneous t(10) = 15.65, P < .0001; mesenteric t(10) = 5.143, P = .0004; retroperitoneal t(10) = 12.38, P < .0001; epididymal t(10) = 15.9, P < .0001; BAT t(10) = 12.71, P < .0001; heart t(10) = 1.295, P = .224; females: subcutaneous t(10) = 4.417, P = .001; mesenteric t(10) = 7.058, P < .0001; retroperitoneal t(10) = 6.106, P < .0001; gonadal t(10) = 8.458, P < .0001; BAT t(10) = 18.07, P <.0001; heart t(10) = 0.613, P = .553] (Fig. 2A and 2E). No statistically significant differences were found in GR expression levels between male and female mice [Supplementary Fig. S1 (32)]. The residual GR expression in the adipose tissue of Adipo GRKO is likely from nonadipose cells, collectively termed SVF cells, since adipose tissue is a heterogeneous organ. Protein level analyses of WAT and BAT confirmed the deletion of GR in adipocytes in both male and female mice (Fig. 2B and 2F). Similar GR levels were detected in the hearts of GR Flox and Adipo GRKO male and female mice (Fig. 2B and 2F).
Figure 2.
Characterization of adipocyte-specific GR knockout mice. (A), (E) GR mRNA expression relative to Ppib was assessed by quantitative PCR in SubQ, Mesen, Retro, Epi, BAT, and heart in male GR Flox and Adipo GRKO mice as well as female GR Flox and Adipo GRKO (n = 6 mice/genotype). (B), (F) Representative Western blot of GR expression in total white adipose tissue (SubQ, Mesen, and Retro), BAT, and heart protein extracts of GR Flox and Adipo GRKO male and female mice (n = 3 mice/genotype). (C), (G) Quantitative PCR assessment of mRNA expression of adiponectin (Adipoq), leptin (Lep), and resistin (Retn) relative to Ppib in mesenteric adipose tissue of GR Flox and Adipo GRKO male and female mice (n = 6 mice/genotype). (D), (H) Plasma adiponectin, leptin, and resistin levels were measured by enzyme linked immunoassay in GR Flox and Adipo GRKO male and female mice (n = 5-8 mice/genotype). All values represent mean ± SD. Data were statistically analyzed using unpaired Student's t-test. * P < .05, ** P < .01, *** P < .001, **** P < .0001.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; BAT, brown adipose tissue; Epi, epididymal; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox; Mesen, mesenteric; Retro, retroperitoneal; SubQ, subcutaneous.
To evaluate the effect of adipocyte GR deletion on adipokines, we determined the mRNA expression of adiponectin, leptin, and resistin in mesenteric adipose tissue (Fig. 2C and 2G) as well as measured the plasma levels of adipokines (Fig. 2D and 2H) in both males and females. We found that adiponectin and resistin mRNA levels were significantly decreased in Adipo GRKO mice compared to GR Flox mice in both male and female mice [male: adiponectin, t(12) = 3.124, P = .008; resistin, t(12) = 2.940, P = .012; female: adiponectin, t(12) = 2.617, P = .0225; resistin, t(12) = 4.354, P = .0009]. There were no differences in leptin mRNA levels between the genotypes [males: t(12) = 0.6733, P = .5135; females t(12) = 0.9774, P = .347]. Plasma adiponectin and resistin protein levels measured by ELISA were also significantly reduced in both male and female Adipo GRKO mice [male: adiponectin, t(10) = 2.362, P = .039; resistin, t(10) = 2.655, P = .0241; female: adiponectin, t(13) = 2.833, P = .014; resistin, t(13) = 2.626, P = .021]. We also found a significant reduction in plasma leptin protein levels in female Adipo GRKO mice compared to GR Flox [t(11) = 3.518, P = .0048], but no difference was observed in plasma leptin protein levels in males [t(8) = 1.092, P = .306].
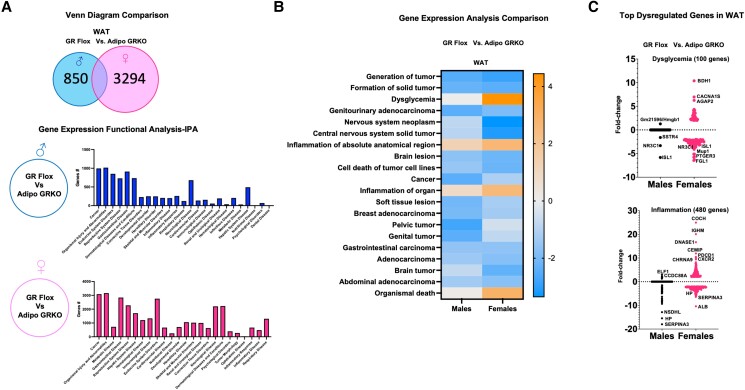
Deletion of Adipocyte GR Caused Widespread Alteration in Gene Expression in Mesenteric Adipose Tissue
To assess the impact of adipocyte GR deletion on genome-wide gene expression, we performed microarray analysis on mRNA extracted from mesenteric adipose tissue of GR Flox and Adipo GRKO mice. Microarray analysis revealed several differentially expressed genes in both male and female Adipo GRKO mice compared to GR Flox mice (Fig. 3A). We compared the number of altered genes in male and female GR Flox mice with Adipo GRKO mice using QIAGEN Ingenuity Pathway Analysis. The analysis identified 850 genes specifically altered in male and 3294 genes specifically altered in female Adipo GRKO mice (Fig. 3A). Ingenuity Pathway Analysis of the gene expression data also predicted that deletion of adipocyte GR would alter the expression of genes associated with several diseases and disorders in the mesenteric adipose tissue of both male and female Adipo GRKO mice. The top 20 diseases associated with our data set included cancer, endocrine system disorders, and inflammatory diseases, among other disorders (Fig. 3A). The comparison analysis of gene expression differences between male and female GR Flox and Adipo GRKO mice showed changes in gene networks related to biological processes such as dysglycemia and inflammation (Fig. 3B). One hundred genes were linked to dysglycemia (Fig. 3C), while 480 genes were linked to inflammation (Fig. 3C). The majority of these alterations were observed in females rather than males. Inflammatory genes with a fold-change greater than 2 included C-X-C motif chemokine receptor 2 (CXCR2), programmed cell death protein 1 (PDCD1), cell migration inducing hyaluronidase 1 (CEMIP), deoxyribonuclease 1 (DNASE1), immunoglobulin heavy constant mu (IGHM), and Cochlin (COCH), which were predicted to have a greater than 10-fold increase in expression in female Adipo GRKO mice (Fig. 3C). Genes displaying a greater than 2-fold change in the adipose tissue of male and female Adipo GRKO mice were cholinergic receptor nicotinic alpha 9 (CHRNA9), E74 ETS transcription factor 1 (ELF1), and coiled-coil domain containing 88A (CCDC88A) (Fig. 3C). Genes such as haptoglobin (HP), serpin family A member 3 (SERPINA3), albumin (ALB), and NAD(P) dependent steroid dehydrogenase like (NSDHL) were predicted to decrease by greater than a 2-fold change in both male and female Adipo GRKO mice (Fig. 3C).
Figure 3.
Deletion of adipocyte GR significantly alters visceral white adipose tissue gene expression. (A) Venn diagram comparison between mesenteric adipose tissue of GR Flox and Adipo GRKO mice representing the number of differentially expressed genes in males and females. Functional clustering using the bioinformatics tool Ingenuity Pathway Analysis revealed several differentially regulated genes implicated in cancer, metabolic, and inflammatory diseases in both males and females. (B) Heat map generated from the microarray data comparing the gene expression analyzed from GR Flox and Adipo GRKO mice between males and females. Upregulated and downregulated genes associated with several pathologies, including cancer, dysglycemia, and inflammation, were associated with adipocyte GR deletion. (C) Pathologies linked to metabolic dysfunction such as dysglycemia and inflammation were among the top upregulated genes. Microarray analysis revealed enrichment of multiple dysglycemia and inflammation associated genes with a greater than 2-fold change in male and female Adipo GRKO mice.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox.
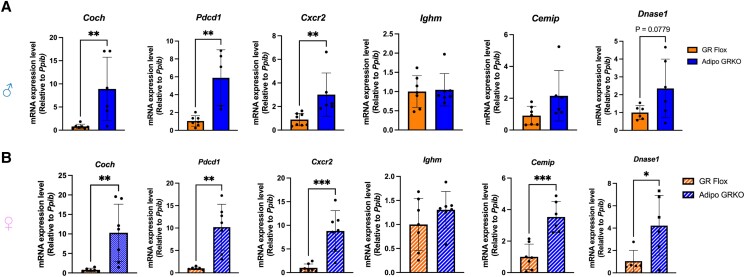
Immune Regulatory Genes Were Significantly Increased in the Mesenteric Adipose Tissue of Adipo GRKO Mice
To confirm the microarray results, mRNA from mesenteric adipose tissue of both male and female GR Flox and Adipo GRKO mice were assessed for the expression of the most significantly upregulated genes in our array (Fig. 4). Significant increases in mRNA expression were found for Coch [males: t(12) = 3.193, P = .007; females: t(11) = 3.152, P = .009], Pdcd1 [males: t(10) = 4.075, P = .002; females: t(11) = 4.367, P = .001], and Cxcr2 [males: t(12) = 3.135, P = .0086; females: t(12) = 4.677, P = .0005] in mesenteric adipose tissue of Adipo GRKO mice (Fig. 4A and 4B). The mRNA expression of Ighm [males: t(12) = 0.2132, P = .834; females: t(12) = 1.222, P = .245] and males Cemip [males: t(12) = 1.485, P = .163] were comparable between the 2 genotypes (Fig. 4A and 4B). However, the mRNA expression of Cemip [t(11) = 5.078, P = .0004] and Dnase1 [t(9)= 2.482, P = .034] was significantly higher in the female Adipo GRKO mice. Although not statistically significant, Dnase1 mRNA expression exhibited a trend toward higher expression in Adipo GRKO male mice [males: t(10) = 1.964, P = .077] (Fig. 4A and 4B).
Figure 4.
Validation of microarray data. Quantitative PCR was performed to analyze Cochlin (Coch), programmed cell death protein 1 (Pdcd1), C-X-C chemokine receptor 2 (Cxcr2), immunoglobulin heavy chain mu (Ighm), cell migration inducing hyaluronidase 1 (Cemip), and deoxyribonuclease1 (Dnase1) mRNA gene expression in GR Flox and Adipo GRKO male (A) and female (B) mice (n = 5-8 mice/genotype). The mRNA gene expression level was determined using the 2−ΔΔCt method with cyclophilin B (Ppib) as the reference gene. All values represent mean ± SD. Data were analyzed using Student's t-test. * P < .05, ** P < .01, *** P < .001.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox.
Deletion of Adipocyte GR Increased Cxcr2 Gene Expression in Adipocytes and Cemip and Pdcd1 Expression in Nonadipocyte Fraction of Adipo GRKO Mice
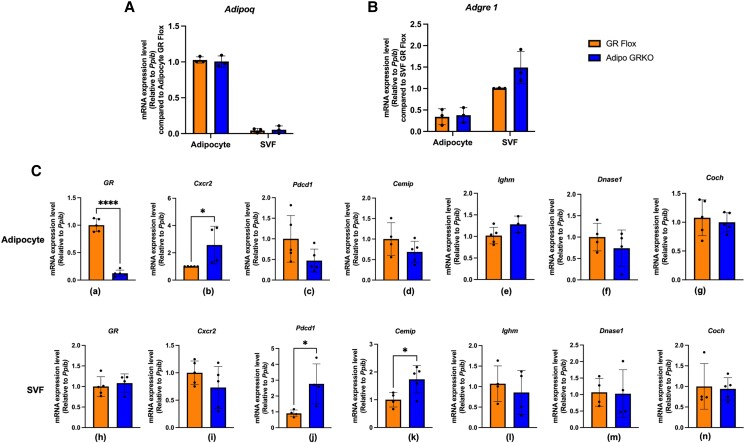
To further explore the source of immune regulatory gene expression in adipose tissue, we used enzymatic digestion to dissociate the tissue into adipocyte and nonadipocyte fractions. The nonadipocyte fraction, also called the SVF, is composed of immune, endothelial, and stem cells (33). For all subsequent experiments, we used male epididymal adipose tissue (1) because it can be readily localized, (2) because adipose tissue dissociation protocols using epididymal adipose tissue for flow cytometry studies (34) and primary adipocyte culture (35) are widely available in the published literature, and (3) to avoid accidentally collecting uterine tissue while isolating female gonadal fat. To confirm the efficiency of this separation, we evaluated the expression of Adipoq and Adgre1, which are markers of adipocytes and macrophages, respectively (Fig. 5). Using the adipocyte fraction of GR Flox as a control, we observed that Adipoq expression in the SVF was reduced by 98.34% in GR Flox and by 97.25% in Adipo GRKO. No significant difference in Adipoq expression was observed between the genotypes in the adipocyte fraction (P = .9569) (Fig. 5A). Compared to the SVF of GR Flox, Adgre1 expression in the adipocyte fraction was reduced by 66% in GR Flox and by 63% in Adipo GRKO, indicating successful separation of the 2 fractions. No significant difference in Adgre1 expression was observed between the genotypes in the SVF fraction (P = .115) (Fig. 5B). In the adipocyte fraction, GR expression was significantly reduced in Adipo GRKO (P < .0001) confirming the adipocyte-specific deletion of GR [Fig 5C (a)]. However, Cxcr2 mRNA expression was significantly increased in Adipo GRKO mice compared to GR Flox (P = .036) [Fig 5C (b)]. Additionally, Pdcd1, Cemip, Ighm, Dnase1, and Coch mRNA gene expression did not differ significantly between the genotypes [Fig. 5C (c)-(g)]. In the SVF, mRNA gene expression of GR, Cxcr2, Ighm, Dnase1, and Coch were similar between the genotypes [Fig. 5C (h)-(i), (l)-(n)]. However, both Pdcd1(P = .0263) and Cemip (P = .0316) mRNA gene expression were significantly higher in the Adipo GRKO mice compared to GR Flox [Fig. 5C (j)-(k)].
Figure 5.
Adipose tissue fractionation revealed that both adipocytes and stromal vascular fraction cells express immune regulatory genes. (A) Expression of the Adipoq gene, which codes for the adipocyte marker adiponectin, was quantified in adipocytes and the SVF fraction using adipocyte GR Flox mice as the control. (B) Expression of the Adgre1 gene, which codes for the macrophage marker F4/80, was quantified in adipocyte and SVF fraction using SVF GR Flox as the control. (C)-(I) GR, Cxcr2, Pdcd1, Cemip, Ighm, Dnase1, and Coch mRNA gene expression measured in adipocytes and the SVF fraction compared to the respective fractions of GR Flox mice (n = 3-5 mice/genotype). All values represent mean ± SD. Data were combined from 3 or more independent experiments and statistically analyzed using unpaired Student's t-test. *, P < .05 ****, P < .0001.
Abbreviations: GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox; SVF, stromal vascular fraction.
Increased Expression of Immune Regulatory Genes is Associated With Increased Presence of Myeloid Immune Cells in Adipo GRKO
We had observed an increase in Coch, Cxcr2, and Pdcd1 mRNA expression in the mesenteric adipose tissue of Adipo GRKO. We then sought to determine the source of Cxcr2 protein expression in both the adipocytes and SVF cells of GR Flox and Adipo GRKO mice by flow cytometry. To our surprise, Cxcr2 expression was not detected in either the adipocyte or SVF cells despite being known to be expressed by both neutrophils and macrophages, which are abundant in the adipose tissue (36, 37). We tested whether collagenase treatment, a necessary step for adipose tissue dissociation, led to Cxcr2 protein cleavage by using spleen samples that were either treated with collagenase or left untreated. We found that collagenase treatment was responsible for Cxcr2 protein cleavage, which explained why we were unable to detect Cxcr2 protein expression in both the adipocyte and SVF cells by flow cytometry [Supplementary Fig. S2 (32)]. Using quantitative PCR techniques, we observed an increased Cxcr2 mRNA expression in adipocytes of Adipo GRKO mice [Fig. 5 C (b)].
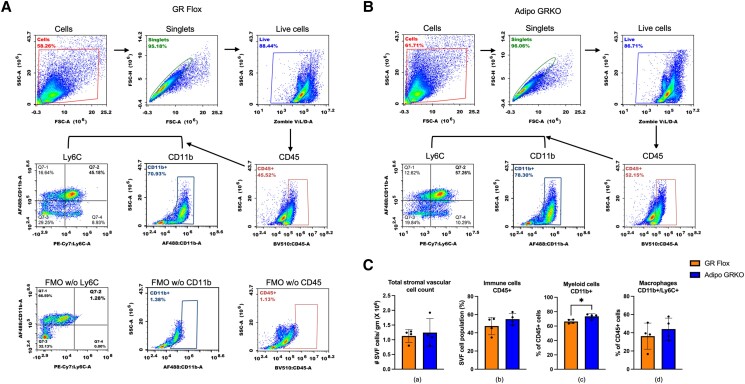
Coch encodes the protein product cochlin that is associated with regulation of innate immunity (38). Since in the mesenteric adipose tissue of Adipo GRKO mice we had observed an increase in Coch gene expression, we focused on quantifying immune cells in the SVF of epididymal adipose tissue in both GR Flox and Adipo GRKO mice using flow cytometry. Prior to cell staining, we found that the number of SVF cells per gram was similar between the 2 genotypes [t(6) = 0.4386, P = .6763] (Fig. 6A).
Figure 6.
Gating strategy for flow cytometry and quantification of immune cells from epididymal SVF cells. Total SVF cells were first gated on a forward scatter/side scatter plot and then gated for the selection of singlets. Singlets were further gated for live cells followed by CD45 gating for total immune cells. These were then gated for myeloid cells (CD45+/CD11b+) and macrophages (CD45+/Cd11b+/Ly6C+). Fluorescence Minus One (FMO) controls were used for each antibody marker to set the limit for the background signal of the excluded marker and to gate for a positive stained cell population. (A), (B) Representative gating strategy for GR Flox and Adipo GRKO mice. (C) Quantitative analysis of SVF cells prior to cell staining, immune cells, myeloid cells, and macrophages. Epididymal adipose tissues were pooled from 2 mice per genotype for each experiment. n = 4 independent experiments. All values represent mean ± SD. Data were analyzed by Student's t-test. * P < .05.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox; SVF, stromal vascular fraction.
Our results showed that the percentage of CD45 + immune cells and CD45+/Cd11b+/Ly6C + macrophages were not significantly altered in Adipo GRKO mice compared to GR Flox mice [Fig. 6C (b), (d)] [CD45 + percentage: t(6) = 1.337, P = .2296; macrophage percentage: t(6) = 0.8272, P = .4398]. However, the percentage of the myeloid cell population was significantly higher in Adipo GRKO mice compared to GR Flox mice [Fig. 6C (c)] [myeloid cell percentage: t(6) = 3.158, P = .0196].
GR Stimulation Represses Cxcr2 Gene Expression In Vitro
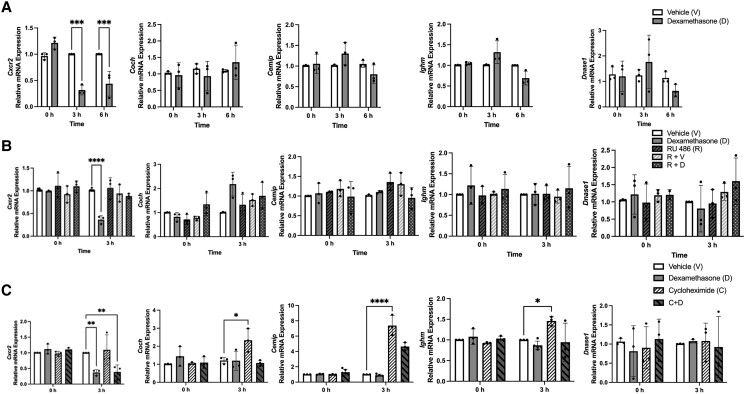
To investigate the role of adipocyte GR signaling in modulating immune regulatory gene expression, we treated differentiated 3T3-L1 adipocytes with vehicle or 100 nM of the synthetic GR agonist dexamethasone (Dex) for 3 and 6 hours. Dex treatment significantly decreased Cxcr2 mRNA expression at both the 3- and 6-hour time points (3 hours: V vs D, P < .001; 6 hours: V vs D, P < .001) but had no effect on the mRNA expression of Coch, Cemip, Ighm, or Dnase1 at either time point (Coch: 3 hours: V vs D, P = .953; 6 hours V vs D, P = .9123; Cemip: 3 hours: V vs D, P = .3939; 6 hours V vs D, P = .5385; Ighm: 3 hours: V vs D, P = .1102; 6 hours V vs D, P = .1106; and Dnase1: 3 hours: V vs D, P = .824; 6 hours V vs D, P = .847) (Fig. 7A). To determine the extent to which GR agonism regulated gene expression, we pretreated the adipocytes with vehicle or 1 μM RU486 for 2 hours to antagonize GR. We then added 100 nM Dex alone or in combination with 1 μM RU486 for 3 hours. Dex treatment alone decreased Cxcr2 mRNA expression (P < .0001) but had no effect on Coch, Cemip, Ighm, or Dnase1 mRNA expression (Coch: P = .483; Cemip: P > .999; Ighm: P = .998; and Dnase1: P > .999) (Fig. 7B). RU486 treatment alone or in combination with vehicle did not alter Cxcr2, Coch, Cemip, Ighm, or Dnase1 mRNA expression [(RU 486 alone: Cxcr2: P = .995; Coch: P > .999; Cemip: P = .6814; Ighm: P > .999; and Dnase1: P > .999) and RU 486 combined with vehicle (Cxcr2: P = .992; Coch: P > .999; Cemip: P = .6145; Ighm: P > .999; and Dnase1: P = .999)] (Fig. 7B). However, treatment with combined RU486 and Dex abolished the repression of Cxcr2 mRNA expression in differentiated adipocytes (P = .7529) (Fig. 7B). Treatment with combined RU 486 and Dex had no effect on the expression of other examined genes (Coch: P = .445; Cemip: P > .99; Ighm: P = .993; and Dnase1: P = .816). To determine the mechanism responsible for Cxcr2 repression by GR, differentiated adipocytes were treated with 10 μg/mL cycloheximide for 1 hour, followed by vehicle, 100 nM Dex treatment, or combined cycloheximide and 100 nM Dex treatment. We observed a significant decrease in Cxcr2 expression in the adipocytes treated with combined cycloheximide and Dex (P = .0029) but not in the mRNA expression of the Coch, Cemip, Ighm, or Dnase1 genes (Fig. 7C).
Figure 7.
Cxcr2 is directly repressed by the glucocorticoid receptor in differentiated 3T3-L1 adipocytes. (A) 3T3-L1 cells were treated with vehicle (control) (white bars) or 100 nM Dex, a synthetic GR agonist, for 3 and 6 hours (grey bars). Gene expression for Cxcr2, Coch, Cemip, Ighm, and Dnase1 were assessed by qPCR. (B) 3T3-L1 adipocytes were pretreated with vehicle (control) (white bars), 100 nM Dex (grey bars), 1 µM RU 486 (slanted black striped bars), 1 µM RU 486 and vehicle (slanted white striped bars) or both 1 µM RU 486 and 100 nM Dex (white dots on black bars). mRNA expression levels were measured by qPCR. (C) 3T3-L1 cells were pretreated for 1 hour with vehicle (control) (white bars), 100 nM Dex (grey bars), 10 µg/mL cycloheximide (slanted black stripes on white bars), or combined cycloheximide followed by 100 nM Dex for 3 hours (slanted black stripes on grey bars). mRNA levels of Cxcr2, Coch, Cemip, Ighm, and Dnase1 were measured using qPCR. All values represent mean ± SD. Data were combined from 3 independent experiments (in duplicate for each experiment) and statistically analyzed using 2-way ANOVA. P-values were based on Tukey's multiple comparison test. * P < .05, ** P < .01, *** P < .001, **** P < .0001.
Abbreviations: GR, glucocorticoid receptors; qPCR, quantitative PCR.
GR Stimulation Did Not Alter Cxcr2 mRNA Expression in Primary Adipocytes Derived From Adipo GRKO Mice
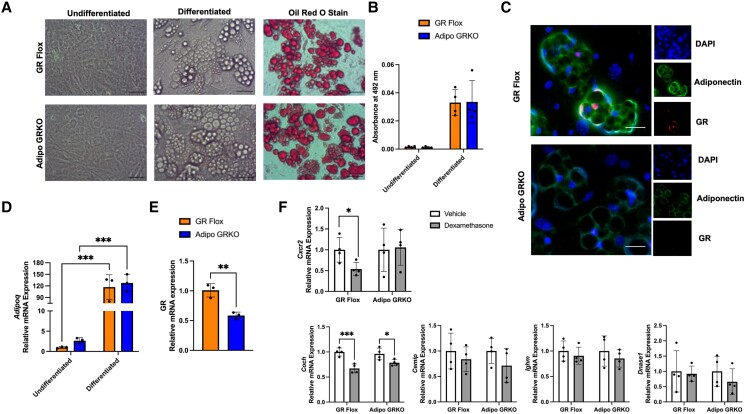
Primary adipocytes were cultured from epididymal adipose depots of GR Flox and Adipo GRKO mice. Differentiated mature adipocytes were identified by the presence of lipid droplets in the cytoplasm (Fig. 8A). Positive Oil-Red-O staining confirmed the presence of lipids accumulated by both GR Flox and Adipo GRKO differentiated adipocytes (Fig. 8B). Adiponectin proteins were confirmed by immunofluorescence staining in both GR Flox and Adipo GRKO adipocytes (Fig. 8C). Differentiated GR Flox adipocytes revealed positive GR staining (Fig. 8C). The positive staining for adiponectin and GR appeared less intense in adipocytes from Adipo GRKO (Fig. 8C). Differentiated primary adipocytes showed significantly increased Adipoq mRNA expression compared to undifferentiated GR Flox (P = .0004) and Adipo GRKO (P = .0005) primary adipocytes. However, Adipoq mRNA expression was similar between differentiated GR Flox and Adipo GRKO primary adipocytes (P = .907), which was similar to the results obtained from fractionated in vivo adipocytes (Fig. 8D). As expected, GR mRNA expression was significantly decreased in differentiated primary adipocytes derived from Adipo GRKO mice compared to primary adipocytes from differentiated GR Flox (P = .0022) (Fig. 8E). To determine the effect of GR agonism on gene expression in primary adipocytes, we treated differentiated GR Flox and Adipo GRKO primary adipocytes with either vehicle or 100 nM Dex for 3 hours. Dex treatment elicited a significant decrease in Cxcr2 mRNA expression in Dex-treated GR Flox primary adipocytes (P = .046) (Fig. 8F), but no difference in Dex-treated Adipo GRKO primary adipocytes (P = .9653). Coch mRNA expression was significantly decreased in Dex-treated GR Flox (P < .0001) and Adipo GRKO (P = .0323) primary adipocytes. Dex treatment did not affect the mRNA expression of Cemip, Ighm, or Dnase1 in either GR Flox or Adipo GRKO primary adipocytes [(GR Flox: Cemip: P = .2868; Ighm: P = .316; and Dnase1: P = .9035) and Adipo GRKO (Cemip: P = .115; Ighm: P = .5419; and Dnase1: P = .9984)] (Fig. 8F). Pdcd1 gene expression was not detected in either primary or 3T3-L1 adipocytes.
Figure 8.
Primary adipocytes differentiated from GR flox and Adipo GRKO mice showing significantly reduced GR mRNA expression and the effect of GR stimulation on immunoregulatory genes. (A) Representative images of undifferentiated, differentiated lipid laden, and Oil-Red-O-stained lipid droplets in primary GR Flox and Adipo GRKO adipocytes on day 10. Scale bar: 5 μm. (B) Stained lipid droplets were quantified using the extracted Oil-Red-O stain and absorbance was measured at 492 nm. (C) Representative immunofluorescence staining of adiponectin and GR proteins in differentiated GR Flox and Adipo GRKO adipocytes. (D) Adipoq mRNA expression as measured by qPCR in undifferentiated and differentiated GR Flox and Adipo GRKO adipocytes on day 10. (E) GR mRNA expression as measured by qPCR in differentiated GR Flox and Adipo GRKO adipocytes on day 10. (F) Primary GR Flox and Adipo GRKO adipocytes were treated with vehicle (control) or 100 nM dexamethasone (grey bars) for 3 hours. The mRNA gene expression of Cxcr2, Cemip, Coch, Ighm, and Dnase1 was measured by qPCR. All values represent mean ± SD. (D)-(F) Data from 3 independent experiments (in duplicate for each experiment) were combined and statistically analyzed using either 2-way ANOVA with Tukey's multiple comparisons (D) or Student's t-test [E-F). * P < 0.05, ** P < 0.01, ***P < 0.001.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox; qPCR, quantitative PCR.
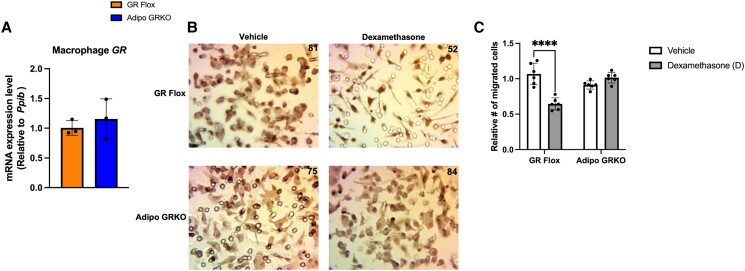
Macrophage Migration Toward Dex-treated GR Flox Adipocytes But Not Toward Adipo GRKO Adipocytes Was Significantly Reduced
To test whether adipocyte GR activation could influence immune cell migration, we co-cultured macrophages and adipocytes that were either treated with vehicle or 100 nM of Dex for 3 hours. Macrophages derived from GR Flox and Adipo GRKO mice expressed similar GR mRNA levels as expected (Fig. 9A). Macrophages that migrated through the pores to the lower side of the insert membrane were fixed, stained, and counted at 40 × magnification. The migration of macrophages toward Dex-treated GR Flox adipocytes was significantly reduced compared to vehicle-treated GR Flox adipocytes (P < .0001) (Fig. 9C). Interestingly, macrophage migration toward Adipo GRKO adipocytes treated with Dex was unaltered compared to macrophage migration toward vehicle-treated Adipo GRKO adipocytes (P = .3750).
Figure 9.
Dexamethasone treatment inhibits macrophage migration toward GR flox but not toward adipo GRKO adipocytes. (A) GR mRNA expression in bone marrow derived macrophages from GR Flox and Adipo GRKO mice. (B) Macrophages that migrated through the pores to the lower side of the insert membrane were fixed, stained, and counted at 40 × magnification. The number on each panel indicates the cell count. Five images were taken for each group and the representative images are shown here. Images were taken with a light microscope (magnification 400x). (C) Adipocytes were treated with vehicle (white bars) or 100 nM dexamethasone (grey bars) prior to adding DMEM with 10% fetal bovine serum in a transwell migration assay. Data are representative of 3 independent experiments (in duplicate for each experiment). Statistics were calculated either using Student's t-test (A) or 2-way ANOVA (C). P-values were based on Tukey's multiple comparison test. **** P < 0.001. All values represent mean ± SD.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox.
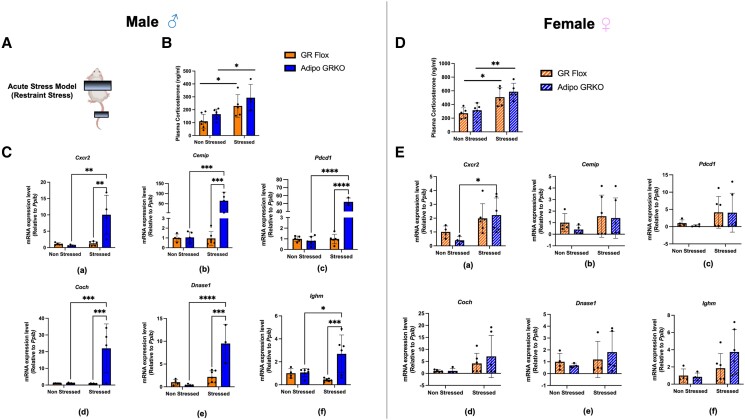
Acute Stress Significantly Increased the Immune Regulatory Gene Expression and Immune Cell Presence in Adipose Tissue
Restraint stress was used to activate the stress signaling pathway as previously described (39). We used this model to determine the effect of adipocyte GR on modulating immune regulatory gene expression in mesenteric adipose tissue of 2- to 3-month-old male and female mice under stress. We observed significantly higher plasma corticosterone levels in stressed compared to nonstressed GR Flox and Adipo GRKO mice in both males and females (Fig. 10B and 10D). By perfusing the mice with PBS, our objective was to obtain clean mesenteric adipose tissue to eliminate the contribution of circulating immune cells that also express immune genes, although we acknowledge tissue resident immune cells may not have been completely removed by PBS wash. In theory, removal of circulating immune cells from the adipose tissue could be a model to study the adipocyte-specific contribution of GR in the regulation of immune gene expression under stress conditions. Under nonstressed conditions, mesenteric adipose tissue of male and female GR Flox and Adipo GRKO mice expressed similar mRNA levels of Cxcr2, Cemip, Pdcd1, Coch, Dnase1, and Ighm, which differed from our previous observation in 5-month-old Adipo GRKO mice that expressed significantly higher mRNA levels (Fig. 10C and 10E).Under stress conditions, mesenteric adipose tissue of male Adipo GRKO mice expressed significantly higher mRNA gene expression levels of Cxcr2 (P = .0018), Cemip (P = .0006), Pdcd1 (P < .0001), Coch (P = .0003), Dnase1 (P = .0003), and Ighm (P = .0010) compared to stressed GR Flox mice. Similarly, stressed Adipo GRKO mice showed significantly increased expression of Cxcr2 (P = .0015), Cemip (P = .0008), Pdcd1 (P < .0001), Coch (P = .0003), Dnase1 (P < .0001), and Ighm (P = .0232), compared to nonstressed Adipo GRKO mice (Fig. 10C). However, no alteration in Cxcr2, Pdcd1, Ighm, Coch, Cemip, or Dnase1 mRNA gene expression levels was observed in stressed GR Flox mice compared to nonstressed GR Flox mice [Cxcr2 (P = .996), Cemip (P > .999), Pdcd1 (P > .999), Coch (P > .999), Dnase1 (P = .7405), and Ighm (P = .7218)] (Fig. 10C). Mesenteric adipose tissue of female mice under stress conditions showed high variability in the expression of all the examined immune regulatory genes. Only Cxcr2 gene expression was significantly increased in the stressed Adipo GRKO mice compared to nonstressed Adipo GRKO mice (P = .0386). Cemip, Pdcd1, Coch, Dnase1, and Ighm gene expression were statistically not significant between the stressed and the nonstressed groups [Fig. 10E (b)-(f)].
Figure 10.
Effect of exposure to acute stress on immune regulatory genes in mesenteric adipose tissue. (A) Model of psychogenic stress used to induce acute stress. (B), (D) Plasma corticosterone levels of nonstressed and stressed GR Flox and Adipo GRKO male and female mice, respectively. (C), (E) Quantitative PCR assessment of mRNA expression of Cxcr2, Cemip, Pdcd1, Coch, Dnase1, and Ighm, gene expression levels in nonstressed and stressed GR Flox and Adipo GRKO male and female mice (n = 4-6 mice/genotype/group) . All values represent mean ± SD. Statistics were calculated using 2-way ANOVA. P-values were based on Tukey's multiple comparison test. *P < .05, ** P < .01, *** P < .001, **** P < 0.0001.
Abbreviations: Adipo GRKO, adipocyte-specific GR knockout; GR, glucocorticoid receptors; GR Flox, C57BL/6J GR flox/flox.
Discussion
The goal of this study was to investigate the role of adipocyte GR in regulating adipocyte morphology, adipokine expression, and immune regulatory genes in the context of adipose tissue homeostasis. Our studies primarily concentrated on visceral WAT due to its significant impact on metabolic health and disease (23, 40). We paid particular attention to mesenteric fat because of its heightened metabolic activity and reported role in adipose tissue inflammation (28).
Our findings suggest that adipocyte-specific GR deletion is associated with altered adipokine levels, increased expression of immune regulatory genes, and increased myeloid cell presence in visceral adipose tissue. Adipocyte GR regulates adipokine gene expression levels, influencing bidirectional cross-talk with nonadipocyte cells involved in adipose tissue homeostasis, including immune cells. Our findings indicate that GR in adipose tissue directly modulates Cxcr2 gene expression in adipocytes and influences immune regulatory genes in nonadipocytes or stromal cells in vivo. Adipocyte-specific GR-mediated modulation of immune regulatory gene expression may represent a crucial pathway linking stress signaling with adipose tissue inflammation. Our results expand on previous work (21) by demonstrating a potential molecular mechanism by which adipocyte GR regulates adipose tissue inflammation and maintains the balance of resident immune cells to preserve adipose tissue homeostasis. Deletion of adipocyte-specific GR was associated with decreased levels of adiponectin and resistin mRNA and circulating plasma protein levels. We found that mesenteric adipose tissue isolated from Adipo GRKO male and female mice expressed significantly reduced levels of both adiponectin Adipoq and resistin Retn mRNA expression compared to GR Flox mice. However, in epididymal adipose tissue, we observed no difference in Adipoq mRNA expression similar to previous studies (20, 21, 29) suggesting depot-specific differences in Adipoq gene expression. Several factors could have contributed to the difference in mesenteric adipose tissue mRNA expression including (1) cell population heterogeneity in mesenteric adipose tissue compared to other depots (41), (2) altered regulation of adipogenic transcription factors (42-44), and (3) adipose tissue inflammation modulating adiponectin expression (42, 45-47). Compared to controls, resistin mRNA levels were not changed in Adipo GRKO mice after Dex treatment, suggesting a direct link between GR signaling and resistin levels (17). At the systemic level, our data showed significantly decreased plasma levels of adiponectin and resistin in both male and female Adipo GRKO mice. Although leptin mRNA levels showed no significant differences between the 2 genotypes, we found a statistically significant reduction in plasma leptin protein levels in female Adipo GRKO mice when compared to littermate controls. One possible explanation for these findings is the sex-specific effects of GR deletion in adipocytes. These effects may involve interactions between GR and estrogen receptors, affecting leptin expression. Previous studies have shown that estrogen can regulate leptin levels (48). Moreover, data from our lab have demonstrated that GR can influence estrogen gene regulation (49). Therefore, it is possible that, in the absence of GR, the effects of estrogen on leptin gene expression and protein levels are altered, leading to significant decreases in its circulating levels. Future research focusing on the impact of adipose tissue GR on the effects of sex hormones on adipokine production, feeding behavior, and energy expenditure is needed to better understand the sex-specific hormonal cues that influence these physiological processes.
Our microarray analysis of mRNA from mesenteric adipose tissue revealed an abundance of genes in Adipo GRKO mice including Coch, Cxcr2, and Pdcd1, which are linked to inflammatory diseases. We found that Coch and Cxcr2 mRNA gene expression was significantly increased in both male and female Adipo GRKO mice. However, Cemip mRNA levels were increased only in females. Additionally, Pdcd1, which is essential to attenuate inflammation, was also significantly increased in both male and female Adipo GRKO mice. The results obtained from our work and previous work (18) suggest a potential role for adipocyte GR in inhibiting proinflammatory cytokine signaling upon GR activation.
To determine the contribution of adipocytes or nonadipocytes to the expression of the immunomodulatory genes, we separated adipose tissue by enzymatic digestion. While both adipocytes and SVF cells expressed the chemokine receptor Cxcr2, a significant increase in Cxcr2 expression was observed only in the adipocytes of Adipo GRKO mice. Previous in vitro (50) and in vivo (51) studies have suggested that Cxcr2 is involved in adipocyte growth and development. Adipocyte-specific Cxcr2 has also been shown to play a role in modulating macrophage infiltration using adipocyte-specific Cxcr2 knockout mice (52). In our in vivo model of adipocyte GR deletion, we found evidence of Cxcr2 expression in adipocytes. The chemokine receptor Cxcr2 expressed by nonimmune cells could also contribute to the immune environment within tissues to maintain homeostasis.
Using 3T3-L1 cells we found a significant decrease in Cxcr2 gene expression upon Dex treatment. We found that Cxcr2 repression in adipocytes was mediated by GR since combined Dex treatment with the GR antagonist RU486 abrogated the repression. Additionally, we found the repression of Cxcr2 by GR involves a direct molecular mechanism, since Cxcr2 repression occurred upon combined treatment with a protein synthesis inhibitor and Dex. Our results obtained from primary adipocytes further suggest that adipocyte GR stimulation decreased Cxcr2 gene expression in GR Flox but did not alter Cxcr2 gene expression in Adipo GRKO adipocytes. These results demonstrate that adipocyte GR represses Cxcr2 expression by a direct molecular mechanism. GR regulation of the chemokine receptor Cxcr2 expressed by adipocytes could therefore contribute to the immune environment within adipose tissues to maintain homeostasis.
Interestingly, increased Cemip and Pdcd1 mRNA expression was observed only in nonadipocyte cells after GR deletion in adipocytes. This could suggest that both paracrine and endocrine mediated cross-talk between mature adipocytes and progenitor cells such as fibroblasts and mesenchymal stromal cells (53, 54) was altered in Adipo GRKO mice. Altered communication between adipocyte and progenitor cells could affect adipogenesis and tissue remodeling (55). Furthermore, emerging studies have highlighted the role of Cemip expression in inflammatory and neoplastic diseases affecting adipose-rich tissues (56, 57), although the physiological role of Cemip in this context is still not fully understood.
The Pdcd1 receptor, which is known to be expressed by B-cells, T cells, natural killer cells, and dendritic cells, is mainly involved in the inhibition of immune responses (58-60). Altered paracrine, endocrine, or cytokine mediators in Adipo GRKO mice could signal the recruitment of Pdcd1 expressing immune cells to the adipose tissue to suppress cellular responses and maintain the immune balance (61-63). Our results suggest potential activation of immune inhibitory receptors in the immune cells of SVF to dampen adipose tissue inflammation and maintain immune homeostasis to prevent immune-mediated tissue damage.
Using whole mesenteric adipose tissue, we observed an increase in Coch gene expression, which encodes cochlin—an extracellular matrix protein that is known to physically bind and interact with collagen VII and is associated with the regulation of innate immunity (38, 64). However, upon fractionation of epididymal adipose tissue, we did not find significant differences in Coch expression in either the adipocytes or the SVF of Adipo GRKO mice. Coch gene product cochlin also colocalizes with collagen type II (65, 66). It is possible that decreased levels of collagen due to collagenase treatment may have decreased cochlin, but the mechanism of how Coch gene expression may have been affected needs further research. This is a limitation of the research technique used to separate the adipose tissue into adipocyte and SVF fractions. While Coch and GR stimulation has been reported in lung fibroblasts (67), the finding of Coch gene expression in adipose tissue in our study and others (68) suggests a role in adipose tissue immune homeostasis.
In our study, increased immune regulatory gene expression was also accompanied by an increased presence of myeloid cells in adipose tissue of Adipo GRKO mice. While we did not find a significant increase in either the total immune cell presence or macrophages, we speculate that the number of other myeloid cells, such as dendritic cells, natural killer cells, or myeloid-derived immune cell types, could be increased in the adipose tissue of Adipo GRKO mice compared to controls. Several studies have shown that myeloid cells are the main targets of glucocorticoids in their actions to limit sustained inflammation and restore homeostasis (69, 70). Glucocorticoids stimulate myeloid cells in multiple ways, including downregulation of proinflammatory cytokines such as IL-6 and IL-12 and shifting the immune cell polarization to resolve inflammation (71, 72). Endogenous glucocorticoids secreted during inflammation induce a shift toward an anti-inflammatory-activated phenotype such as the induction of anti-inflammatory cytokine IL-10 production by monocytes (73). An increased myeloid cell presence in Adipo GRKO mice may indicate a potential compensatory mechanism to maintain immune homeostasis in adipose tissue.
Using 3T3-L1 cells we found no decrease in Coch, Cemip, Dnase1, or Ighm gene expression upon Dex treatment or with combined Dex and RU 486 or with cycloheximide and Dex. These results suggest other mechanisms are involved in repressing the aforementioned genes. Coch, Cemip, Dnase1, and Ighm genes are most abundantly expressed by the extracellular matrix proteins and immune cells of the SVF in adipose tissue. This may explain why we were unable to observe detectable changes upon Dex treatment in in vitro adipocytes.
We also found that Coch, Cemip, and Ighm mRNA expression was greatly increased by cycloheximide treatment alone compared to treatment with vehicle. Previous research by Newton and colleagues (74) reported that cycloheximide increased nuclear factor kappa-light-chain-enhancer of activated B cells and primary response genes, involved in innate and adaptive immune responses, glucose metabolism, and oncogenic transformation, among other biological processes (75). We did not detect Pdcd-1 expression in in vitro primary or 3T3-L1 adipocytes.
We also observed that Dex treatment inhibited Coch gene expression in mature adipocytes differentiated from both GR Flox and Adipo GRKO mice. Decreased Coch gene expression in both GR Flox and Adipo GRKO mice suggests the presence of other cell types in the primary culture that were sensitive to the effects of GR stimulation. Although the functional relevance of Coch gene expression change cannot be inferred from our study, the Coch gene product cochlin has been implicated in the regulation of immune system and extracellular matrix function, making Coch an interesting gene target for further studies involving adipose tissue function.
Restraint stress can dysregulate the immune system, increasing the risk of infectious diseases and tissue damage, and can subsequently worsen disease progression (76-78). Under nonstressed conditions, 2-3 month-old GR Flox and Adipo GRKO mice expressed similar mRNA levels of Cxcr2, Pdcd1, Ighm, Coch, and Cemip, which differed from our previous observation in 5-month-old Adipo GRKO mice that expressed significantly higher mRNA levels, due potentially to age-related GR signaling. Our study showed that, following acute psychological stress in male mice, adipocyte-specific GR deletion led to significantly increased expression of immunoregulatory genes such as Cxcr2, Pdcd1, Ighm, Coch, Cemip, and Dnase1, indicating that adipocyte GR play a crucial role in immunosuppression in adipose tissue during exposure to stress. In contrast to males, stressed female mice displayed high variability in the mRNA expression of immune-regulatory genes. This was observed under stress conditions in GR Flox and Adipo GRKO mice, and no statistically significant differences were detected in the expression of any target genes. As previously discussed, one potential explanation for this variability could be the interaction between GR and sex hormone signaling. Future research that focuses on the sex-specific effects of stress on adipose tissue may offer new insights into how systemic stress exposure leads to changes in gene expression within adipose tissue, potentially contributing to metabolic impairments and disease.
In conclusion, our findings suggest that the sex-specific modulation of GR signaling in adipocytes could be a potential approach to suppress inflammatory signals and prevent chronic low-grade adipose tissue inflammation that could contribute to both local and systemic diseases. Additionally, the Adipo GRKO mouse model could be used to study the association between stress signaling, peri-organ adipose tissue inflammation, and associated tissue damage in the context of impaired immune homeostasis in adipose tissue to study both local and systemic disease progression. Understanding the mechanism by which adipocyte GR modulate immune cell infiltration in adipose tissue could be used to develop potential targets to improve systemic inflammatory state, insulin sensitivity and metabolic health.
Acknowledgments
We thank Dr. Weinan Zhou from the University of Illinois—Urbana Champaign for technical advice on culturing primary adipocytes. We thank Nathaniel D. Glassy for practical suggestions on writing the manuscript. We acknowledge the assistance of David Custis from the Flow Cytometry Core Facility for running our flow samples and Drs. Minsup Lee and Randa Eshaq for technical guidance on immunofluorescence imaging at LSU Health Sciences Center—Shreveport. We also acknowledge Dr. Kevin Gerrish from the Molecular Genomics Core at the National Institute of Environmental Health Sciences.
Contributor Information
Shripa Amatya, Department of Molecular and Cellular Physiology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA; Center for Cardiovascular Diseases and Sciences and Center for Redox Biology and Cardiovascular Disease, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Dylan Tietje-Mckinney, Department of Molecular and Cellular Physiology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Schaefer Mueller, Department of Molecular and Cellular Physiology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Maria G Petrillo, Department of Health and Human Services, Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA.
Matthew D Woolard, Center for Cardiovascular Diseases and Sciences and Center for Redox Biology and Cardiovascular Disease, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA; Department of Microbiology and Immunology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Sushma Bharrhan, Department of Microbiology and Immunology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Anthony Wayne Orr, Center for Cardiovascular Diseases and Sciences and Center for Redox Biology and Cardiovascular Disease, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA; Department of Pathology and Translational Pathobiology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Christopher G Kevil, Center for Cardiovascular Diseases and Sciences and Center for Redox Biology and Cardiovascular Disease, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA; Department of Pathology and Translational Pathobiology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
John A Cidlowski, Department of Health and Human Services, Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA.
Diana Cruz-Topete, Department of Molecular and Cellular Physiology, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA; Center for Cardiovascular Diseases and Sciences and Center for Redox Biology and Cardiovascular Disease, Louisiana State University Health Sciences Center—Shreveport, Shreveport, LA 71103, USA.
Funding
This research was supported by the Department of Molecular and Cellular Physiology at Louisiana State University Health Sciences Center—Shreveport (S.A. and D.C.-T.); National Heart, Lung, and Blood Institute Grant 5K01HL144882-03 (D.C.-T.), Center for Redox Biology and Cardiovascular Disease grant 3P20GM121307-03S1 (C.G.K., A.W.O., D.C.-T.), National Institutes of Health grants HL133497 and HL141155 (A.W.O.), Center for Applied Immunology and Pathological Processes grant 1P20GM134974 (M.D.W.), and the Intramural Research Program of the National Institutes of Health (J.A.C.).
Disclosures
The authors have no conflicts of interest and nothing to disclose.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Burford NG, Webster NA, Cruz-Topete D. Hypothalamic-Pituitary-Adrenal axis modulation of glucocorticoids in the cardiovascular system. Int J Mol Sci. 2017;18(10):2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243‐251. [DOI] [PubMed] [Google Scholar]
- 3. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions*. Endocr Rev. 2000;21(1):55‐89. [DOI] [PubMed] [Google Scholar]
- 4. Timmermans S, Souffriau J, Libert C. A general Introduction to glucocorticoid biology. Front Immunol. 2019;10:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swarbrick M, Zhou H, Seibel M. Mechanisms in endocrinology: local and systemic effects of glucocorticoids on metabolism: new lessons from animal models. Eur J Endocrinol. 2021;185(5):R113‐R129. [DOI] [PubMed] [Google Scholar]
- 6. de Guia RM. Stress, glucocorticoid signaling pathway, and metabolic disorders. Diabetes Metab Syndr. 2020;14(5):1273‐1280. [DOI] [PubMed] [Google Scholar]
- 7. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferraù F, Korbonits M. Metabolic comorbidities in Cushing's Syndrome. Eur J Endocrinol. 2015;173(4):M133‐M157. [DOI] [PubMed] [Google Scholar]
- 9. Noppe G, van den Akker ELT, de Rijke YB, Koper JW, Jaddoe VW, van Rossum EFC. Long-term glucocorticoid concentrations as a risk factor for childhood obesity and adverse body-fat distribution. Int J Obes. 2016;40(10):1503‐1509. [DOI] [PubMed] [Google Scholar]
- 10. Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The immune system in Cushing's Syndrome. Trends Endocrinol Metab. 2020;31(9):655‐669. [DOI] [PubMed] [Google Scholar]
- 11. Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022;18(9):540‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mebrahtu TF, Morgan AW, West RM, Stewart PM, Pujades-Rodriguez M. Oral glucocorticoids and incidence of hypertension in people with chronic inflammatory diseases: a population-based cohort study. CMAJ. 2020;192(12):E295‐E301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva F dF, Komino ACM, Andreotti S, et al. Dexamethasone-Induced adipose tissue redistribution and metabolic changes: is gene expression the main factor? An animal model of chronic hypercortisolism. Biomedicines. 2022;10(9):2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fardet L, Fève B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74(15):1731‐1745. [DOI] [PubMed] [Google Scholar]
- 15. Gathercole LL, Bujalska IJ, Stewart PM, Tomlinson JW. Glucocorticoid modulation of insulin signaling in human subcutaneous adipose tissue. J Clin Endocrinol Metab. 2007;92(11):4332‐4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee MJ, Fried SK. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int J Obes. 2014;38(9):1228‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen Y, Roh HC, Kumari M, Rosen ED. Adipocyte glucocorticoid receptor is important in lipolysis and insulin resistance due to exogenous steroids, but not insulin resistance caused by high fat feeding. Mol Metab. 2017;6(10):1150‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalle H, Garcia M, Antoine B, et al. Adipocyte glucocorticoid receptor deficiency promotes adipose tissue expandability and improves the metabolic profile under corticosterone exposure. Diabetes. 2018;68(2):305‐317. [DOI] [PubMed] [Google Scholar]
- 19. Do TTH, Marie G, Héloïse D, et al. Glucocorticoid-induced insulin resistance is related to macrophage visceral adipose tissue infiltration. J Steroid Biochem Mol Biol. 2019;185:150‐162. [DOI] [PubMed] [Google Scholar]
- 20. Mueller KM, Hartmann K, Kaltenecker D, et al. Adipocyte glucocorticoid receptor deficiency attenuates aging- and HFD-induced obesity and impairs the feeding-fasting transition. Diabetes. 2017;66(2):272‐286. [DOI] [PubMed] [Google Scholar]
- 21. Desarzens S, Faresse N. Adipocyte glucocorticoid receptor has a minor contribution in adipose tissue growth. J Endocrinol. 2016;230(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 22. Macdougall CE, Wood EG, Loschko J, et al. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 2018;27(3):588‐601.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol-Cell Physiol. 2021;320(3):C375‐C391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oakley RH, Ren R, Cruz-Topete D, et al. Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc Natl Acad Sci. 2013;110(42):17035‐17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radulescu CI, Cerar V, Haslehurst P, Kopanitsa M, Barnes SJ. The aging mouse brain: cognition, connectivity and calcium. Cell Calcium. 2021;94:102358. [DOI] [PubMed] [Google Scholar]
- 28. Bilski J, Mazur-Bialy A, Wojcik D, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9(12):780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Kloet AD, Krause EG, Solomon MB, et al. Adipocyte glucocorticoid receptors mediate fat-to-brain signaling. Psychoneuroendocrinology. 2015;56:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao W, Kong X, Yang Q. Isolation, primary culture, and differentiation of preadipocytes from mouse brown adipose tissue. Methods Mol Biol Clifton NJ. 2017;1566:3‐8. [DOI] [PubMed] [Google Scholar]
- 31. Schilke RM, Blackburn CMR, Rao S, Krzywanski DM, Finck BN, Woolard MD. Macrophage-Associated lipin-1 promotes β-oxidation in response to proresolving stimuli. ImmunoHorizons. 2020;4(10):659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amatya S, Tietje-Mckinney D, Mueller S, et al. Data from: Adipocyte glucocorticoid receptor inhibits immune regulatory genes to maintain immune cell homeostasis in adipose tissue. Deposited 14 September2023. 10.60688/lsuhs.24139464 [DOI] [PMC free article] [PubMed]
- 33. Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity. 2015;23(3):512‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hearnden R, Sandhar B, Vyas V, Longhi MP. Isolation of stromal vascular fraction cell suspensions from mouse and human adipose tissues for downstream applications. STAR Protoc. 2021;2(2):100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruan H, Zarnowski MJ, Cushman SW, Lodish HF. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. J Biol Chem. 2003;278(48):47585‐47593. [DOI] [PubMed] [Google Scholar]
- 36. Ferrante AW Jr. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15(s3):34‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rigamonti E, Fontaine C, Lefebvre B, et al. Induction of CXCR2 receptor by peroxisome proliferator-activated receptor γ in human macrophages. Arterioscler Thromb Vasc Biol. 2008;28(5):932‐939. [DOI] [PubMed] [Google Scholar]
- 38. Py BF, Gonzalez SF, Long K, et al. Cochlin produced by follicular dendritic cells promotes antibacterial innate immunity. Immunity. 2013;38(5):1063‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paré WP, Glavin GB. Restraint stress in biomedical research: A review. Neurosci Biobehav Rev. 1986;10(3):339‐370. [DOI] [PubMed] [Google Scholar]
- 40. Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129(10):3990‐4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, physiological and functional diversity of adipose tissue. Cell Metab. 2018;27(1):68‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. da Silva Rosa SC, Liu M, Sweeney G. Adiponectin synthesis, secretion and extravasation from circulation to interstitial space. Physiology. 2021;36(3):134‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2009;425(1):41‐52. [DOI] [PubMed] [Google Scholar]
- 44. Ringold GM, Chapman AB, Knight DM, Torti FM. Hormonal control of adipogenesis. Ann N Y Acad Sci. 1986;478(1):109‐119. [DOI] [PubMed] [Google Scholar]
- 45. Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46(12):1594‐1603. [DOI] [PubMed] [Google Scholar]
- 46. Sukumaran S, DuBois DC, Jusko WJ, Almon RR. Glucocorticoid effects on adiponectin expression. Vitam Horm. 2012;90:163‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6(2):87‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fungfuang W, Terada M, Komatsu N, Moon C, Saito TR. Effects of estrogen on food intake, serum leptin levels and leptin mRNA expression in adipose tissue of female rats. Lab Anim Res. 2013;29(3):168‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dhaibar HA, Carroll NG, Amatya S, et al. Glucocorticoid inhibition of estrogen regulation of the serotonin receptor 2B in cardiomyocytes exacerbates cell death in hypoxia/reoxygenation injury. J Am Heart Assoc. 2021;10(17):e015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kabir SM, Lee ES, Son DS. Chemokine network during adipogenesis in 3T3-L1 cells. Adipocyte. 2014;3(2):97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dyer DP, Nebot JB, Kelly CJ, Medina-Ruiz L, Schuette F, Graham GJ. The chemokine receptor CXCR2 contributes to murine adipocyte development. J Leukoc Biol. 2019;105(3):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choe D, Lee ES, Beeghly-Fadiel A, et al. High-Fat diet-induced obese effects of adipocyte-specific CXCR2 conditional knockout in the peritoneal tumor microenvironment of ovarian cancer. Cancers (Basel). 2021;13(19):5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmaus A, Rothley M, Schreiber C, et al. Sulfated hyaluronic acid inhibits the hyaluronidase CEMIP and regulates the HA metabolism, proliferation and differentiation of fibroblasts. Matrix Biol. 2022;109:173‐191. [DOI] [PubMed] [Google Scholar]
- 54. Spataro S, Guerra C, Cavalli A, et al. CEMIP (HYBID, KIAA1199): structure, function and expression in health and disease. FEBS J. 2023;290(16):3946‐3962. [DOI] [PubMed] [Google Scholar]
- 55. Haylett WL, Ferris WF. Adipocyte-progenitor cell communication that influences adipogenesis. Cell Mol Life Sci CMLS. 2020;77(1):115‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dong X, Yang Y, Yuan Q, Hou J, Wu G. High expression of CEMIP correlates poor prognosis and the tumur microenvironment in breast cancer as a promisingly prognostic biomarker. Front Genet. 2021;12:768140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Banach A, Jiang YP, Roth E, Kuscu C, Cao J, Lin RZ. CEMIP upregulates BiP to promote breast cancer cell survival in hypoxia. Oncotarget. 2019;10(42):4307‐4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fanoni D, Tavecchio S, Recalcati S, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011;134(2):157‐160. [DOI] [PubMed] [Google Scholar]
- 59. Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015‐3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393‐5399. [DOI] [PubMed] [Google Scholar]
- 61. Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021;12(9):2735‐2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435(7042):598‐604. [DOI] [PubMed] [Google Scholar]
- 63. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775‐787. [DOI] [PubMed] [Google Scholar]
- 64. Nyström A, Bornert O, Kühl T, et al. Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa. Proc Natl Acad Sci U S A. 2018;115(4):E705‐E714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Robertson NG, O’Malley JT, Ong CA, et al. Cochlin in normal middle ear and abnormal middle ear deposits in DFNA9 and CochG88E/G88EMice. J Assoc Res Otolaryngol. 2014;15(6):961‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mizuta K, Ikezono T, Iwasaki S, et al. Ultrastructural co-localization of cochlin and type II collagen in the rat semicircular canal. Neurosci Lett. 2008;434(1):104‐107. [DOI] [PubMed] [Google Scholar]
- 67. Seow BKL, McDougall ARA, Short KL, Wallace MJ, Hooper SB, Cole TJ. Identification of betamethasone-regulated target genes and cell pathways in fetal rat lung mesenchymal fibroblasts. Endocrinology. 2019;160(8):1868‐1884. [DOI] [PubMed] [Google Scholar]
- 68. Ugi S, Maeda S, Kawamura Y, et al. CCDC3 Is specifically upregulated in omental adipose tissue in subjects with abdominal obesity. Obesity. 2014;22(4):1070‐1077. [DOI] [PubMed] [Google Scholar]
- 69. Quatrini L, Ugolini S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell Mol Immunol. 2021;18(2):269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yona S, Gordon S. Inflammation: glucocorticoids turn the monocyte switch. Immunol Cell Biol. 2007;85(2):81‐82. [DOI] [PubMed] [Google Scholar]
- 71. Ma W, Gee K, Lim W, et al. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J Immunol Baltim Md 1950. 2004;172(1):318‐330. [DOI] [PubMed] [Google Scholar]
- 72. Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL 6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990;20(11):2439‐2443. [DOI] [PubMed] [Google Scholar]
- 73. Mozo L, Suárez A, Gutiérrez C. Glucocorticoids up-regulate constitutive interleukin-10 production by human monocytes. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2004;34(3):406‐412. [DOI] [PubMed] [Google Scholar]
- 74. Newton R, Adcock IM, Barnes PJ. Superinduction of NF-κB by actinomycin D and cycloheximide in epithelial cells. Biochem Biophys Res Commun. 1996;218(2):518‐523. [DOI] [PubMed] [Google Scholar]
- 75. Fowler T, Sen R, Roy AL. Regulation of primary response genes. Mol Cell. 2011;44(3):348‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li Y, Jiang W, Li ZZ, et al. Repetitive restraint stress changes spleen immune cell subsets through glucocorticoid receptor or β-adrenergic receptor in a stage dependent manner. Biochem Biophys Res Commun. 2018;495(1):1108‐1114. [DOI] [PubMed] [Google Scholar]
- 77. Shimba A, Ikuta K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol. 2020;42(6):669‐680. [DOI] [PubMed] [Google Scholar]
- 78. Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol. 1998;82(1):40‐46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.