Abstract
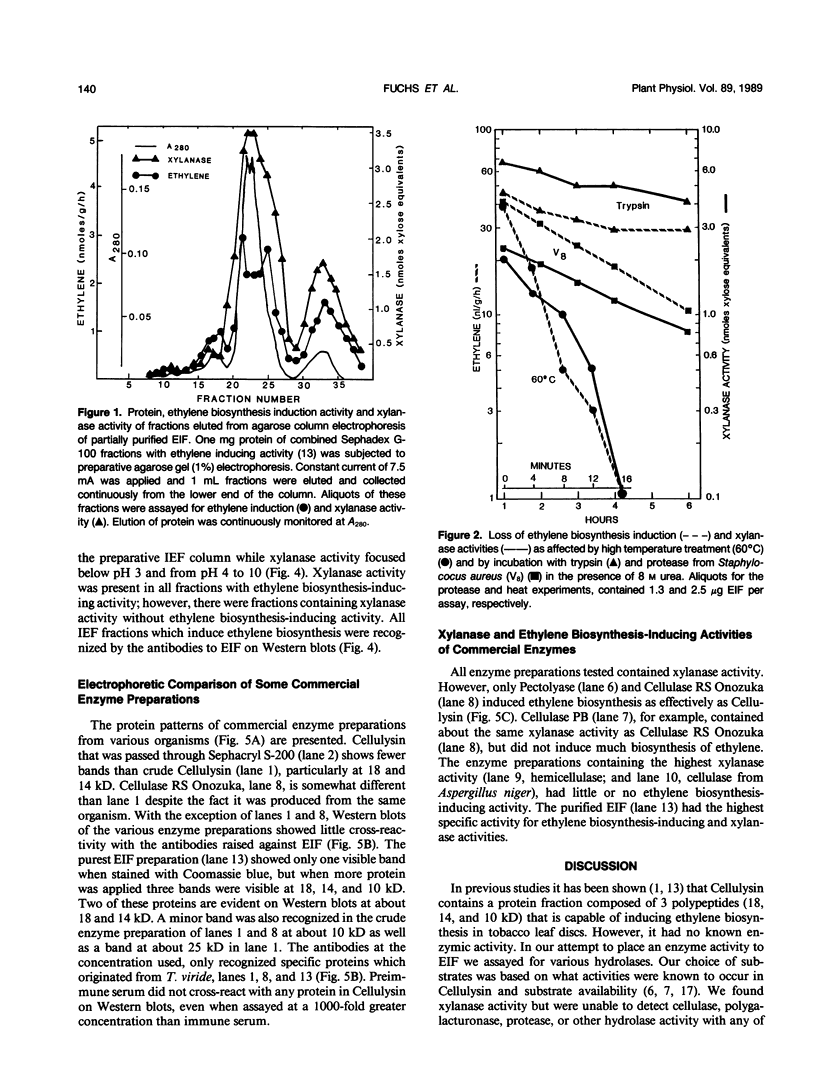
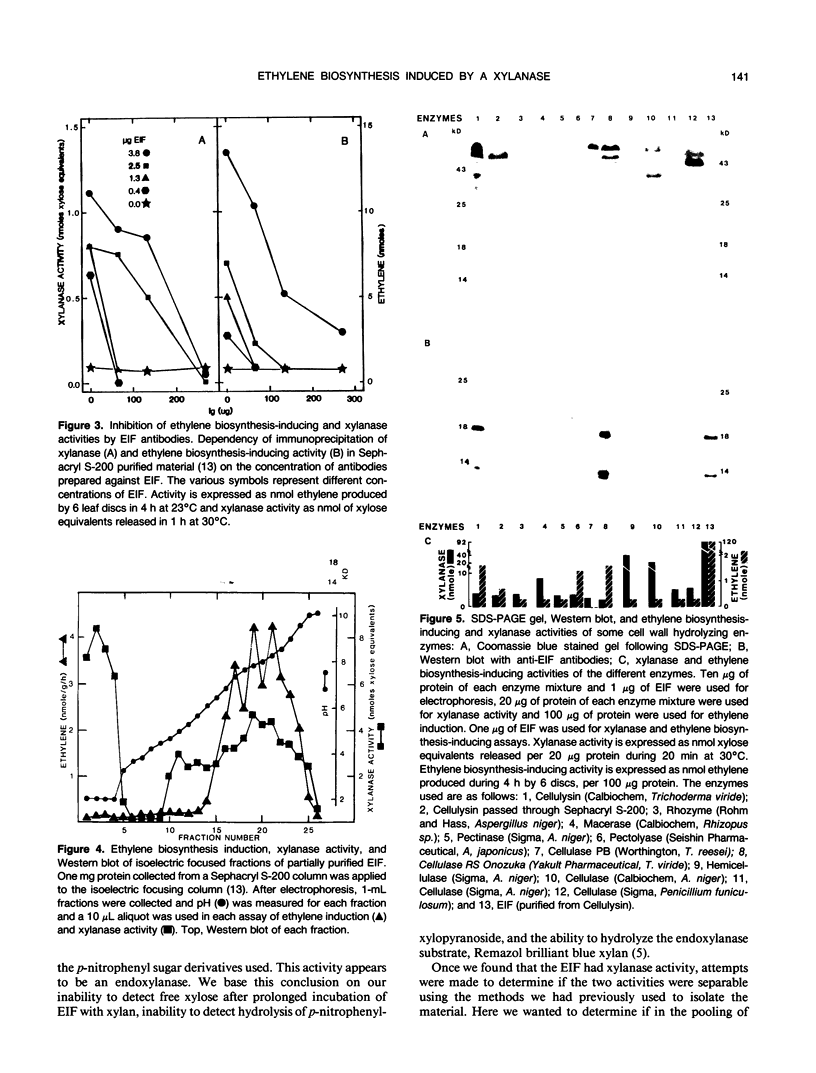
The proteinaceous ethylene biosynthesis-inducing factor (EIF) that was purified from Cellulysin was also shown to contain a xylanase activity. In all nondenaturing protein separation methods employed (Sephacryl S-200 chromatography, and preparative isoelectric focusing and agarose electrophoresis), xylanase activity copurified with the ethylene biosynthesis-inducing activity. Treatment with heat (60°C) or proteases in 8 molar urea inhibited both ethylene-inducing and xylanase activities. Antibodies raised against purified EIF, which contains three polypeptides of 18, 14, and 10 kilodaltons, immunoprecipitated both ethylene biosynthesis-inducing and xylanase activities. The purified EIF contained no detectable cellulase, polygalacturonase, or protease activity. Other hydrolytic activities as estimated by using p-nitrophenyl derivatives of several sugars as substrates also were not detected. Different commercially available hydrolytic enzyme preparations were tested for both ethylene biosynthesis-inducing and xylanase activities. All enzymes tested contained xylanase activity, but only a few induced ethylene biosynthesis. Western blots of proteins separated by SDS-PAGE, using antibodies prepared against the non-denatured purified EIF, revealed two major bands of about 18 and 14 kilodaltons in EIF. These antibodies seem to be specific for these proteins from Trichoderma viride, because there was little cross-reactivity with the other proteins in Cellulysin and other commercial enzyme preparations. Based on these data, we suggest that EIF contains a specific xylanase activity which is involved in inducing ethylene biosynthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Lieberman M., Stewart R. N. Ethylene production by apple protoplasts. Plant Physiol. 1979 May;63(5):931–935. doi: 10.1104/pp.63.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Mattoo A. K., Lieberman M. Induction of ethylene biosynthesis in tobacco leaf discs by cell wall disesting enzymes. Biochem Biophys Res Commun. 1982 Jul 30;107(2):588–596. doi: 10.1016/0006-291x(82)91532-7. [DOI] [PubMed] [Google Scholar]
- Biely P., Markovic O., Mislovicová D. Sensitive detection of endo-1,4-beta-glucanases and endo-1,4-beta-xylanases in gels. Anal Biochem. 1985 Jan;144(1):147–151. doi: 10.1016/0003-2697(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Biely P., Mislovicová D., Toman R. Soluble chromogenic substrates for the assay of endo-1,4-beta-xylanases and endo-1,4-beta-glucanases. Anal Biochem. 1985 Jan;144(1):142–146. doi: 10.1016/0003-2697(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalutz E., Mattoo A. K., Solomos T., Anderson J. D. Enhancement by ethylene of cellulysin-induced ethylene production by tobacco leaf discs. Plant Physiol. 1984 Jan;74(1):99–103. doi: 10.1104/pp.74.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Helgeson J. P. An Extracellular Protein from Phytophthora parasitica var nicotianae Is Associated with Stress Metabolite Accumulation in Tobacco Callus. Plant Physiol. 1987 Nov;85(3):733–740. doi: 10.1104/pp.85.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Anderson J. D. Purification and characterization of ethylene inducing proteins from cellulysin. Plant Physiol. 1987 Jul;84(3):732–736. doi: 10.1104/pp.84.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Greve L. C. Cell Wall Metabolism in Ripening Fruit : III. Purification of an Endo-beta-1,4-xylanase that Degrades a Structural Polysaccharide of Pear Fruit Cell Walls. Plant Physiol. 1983 Jul;72(3):668–673. doi: 10.1104/pp.72.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Tong C. B., Labavitch J. M., Yang S. F. The induction of ethylene production from pear cell culture by cell wall fragments. Plant Physiol. 1986 Jul;81(3):929–930. doi: 10.1104/pp.81.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]