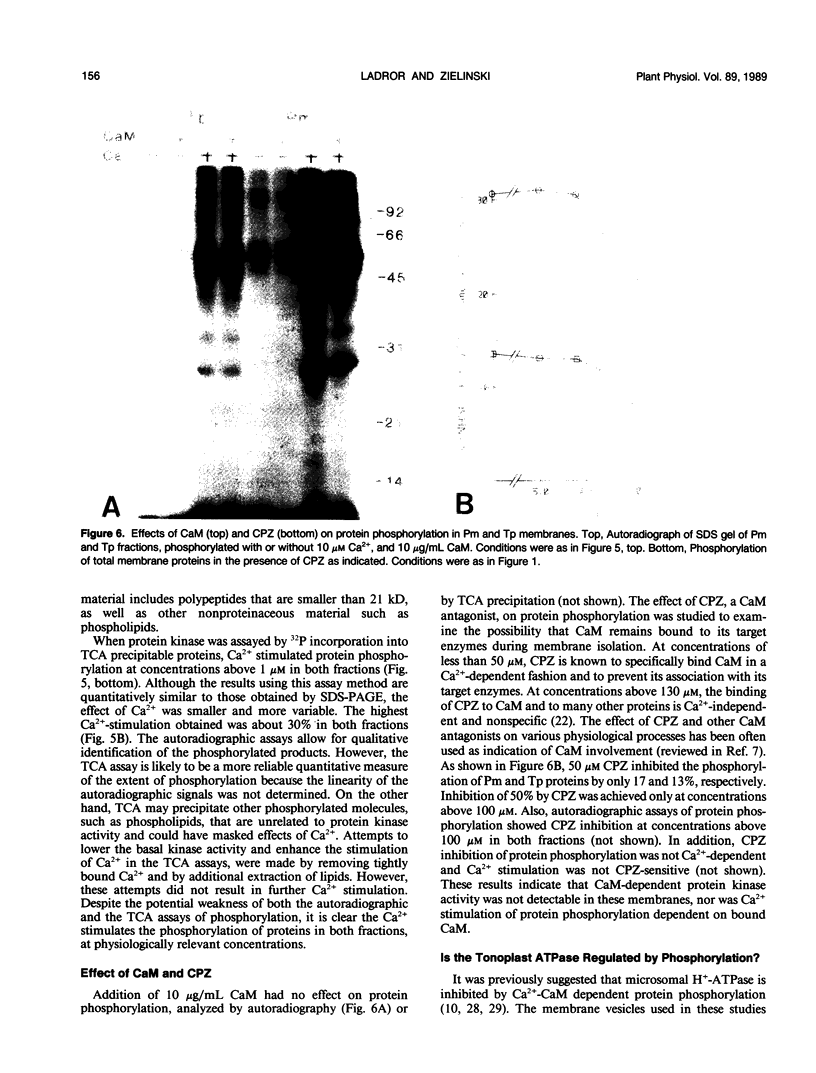
Abstract
Protein kinase and phosphatase activities were studied in plasmalemma and tonoplast membrane fractions from corn (Zea mays L.) roots in order to test the hypothesis that the tonoplast H+-ATPase is regulated by intrinsic protein phosphorylation (G Zocchi, SA Rogers, JB Hanson 1983 Plant Sci Lett 31: 215-221), and to facilitate future purification of kinase activities from these membranes. Kinase activity in the plasmalemma was about three-fold higher than in the tonoplast, and displayed Michaelis Menten-type behavior with a Km value for MgATP2− of about 50 micromolar. Both activities were optimal at 3 millimolar free Mg2+ and had pH optima at 6.6 and 7.0 for the plasmalemma and tonoplast, respectively. Kinase activities in both fractions were stimulated by 1 micromolar free Ca2+, but calmodulin had no stimulatory effect, and chlorpromazine was inhibitory only at high concentrations. The pattern of phosphopeptides on SDS polyacrylamide gel electrophoresis was similar in both fractions except for one band of 50 kilodaltons that was present only in the tonoplast. A partially purified H+-ATPase fraction was prepared from tonoplast membranes, incubated under conditions optimal for protein phosphorylation. The three polypeptides (of 67, 57, and 36 kilodaltons), enriched in this fraction, did not become phosphorylated, suggesting that this protein is not regulated by endogenous protein phosphorylation. Protein phosphatase activity was detected only in the plasmalemma fraction. These results indicate that a regulatory cycle of protein phosphorylation and dephosphorylation may operate in the plasmalemma. The activity in the tonoplast appears to originate from plasmalemma contamination.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Polya G. M. Purification and properties of a high specific activity protein kinase from wheat germ. Plant Physiol. 1983 Mar;71(3):489–495. doi: 10.1104/pp.71.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin P. P., Key J. L. Histone Kinase from Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES. Plant Physiol. 1980 Sep;66(3):360–367. doi: 10.1104/pp.66.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. P. Phosphoprotein Phosphatase of Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES . Plant Physiol. 1980 Sep;66(3):368–374. doi: 10.1104/pp.66.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Characterization of the subunit structure of the maize tonoplast ATPase. Immunological and inhibitor binding studies. J Biol Chem. 1986 Sep 25;261(27):12850–12855. [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Key J. L. 2,4-Dichlorophenoxyacetic Acid-enhanced Phosphorylation of Soybean Nuclear Proteins. Plant Physiol. 1978 Feb;61(2):190–198. doi: 10.1104/pp.61.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Davies J. R. Resolution and properties of a protein kinase catalyzing the phosphorylation of a wheat germ cytokinin-binding protein. Plant Physiol. 1983 Mar;71(3):482–488. doi: 10.1104/pp.71.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium-promoted protein phosphorylation in plants. Science. 1984 Jan 13;223(4632):167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- Yan T. F., Tao M. Purification and characterization of a wheat germ protein kinase. J Biol Chem. 1982 Jun 25;257(12):7037–7043. [PubMed] [Google Scholar]





