Abstract
Background and Objectives
Research on olfaction and brain neuropathology may help understand brain regions associated with normal olfaction and dementia pathophysiology. To identify early regional brain structures affected in poor olfaction, we examined cross-sectional associations of microstructural integrity of the brain with olfaction in the Atherosclerosis Risk in Communities Neurocognitive Study.
Methods
Participants were selected from a prospective cohort study of community-dwelling adults; selection criteria included the following: evidence of cognitive impairment, participation in a previous MRI study, and a random sample of cognitively normal participants. Microstructural integrity was measured by 2 diffusion tensor imaging (DTI) measures, fractional anisotropy (FA) and mean diffusivity (MD), and olfaction by a 12-item odor identification test at the same visit. Higher FA and MD values indicate better and worse microstructural integrity, respectively, and higher odor identification scores indicate better olfaction. We used brain region–specific linear regression models to examine associations between DTI measures and olfaction, adjusting for potential confounders.
Results
Among 1,418 participants (mean age 76 ± 5 years, 41% male, 21% Black race, 59% with normal cognition), the mean olfaction score was 9 ± 2.3. Relevant to olfaction, higher MD in the medial temporal lobe (MTL) regions, namely the hippocampus (β −0.79 [95% CI −0.94 to −0.65] units lower olfaction score per 1 SD higher MD), amygdala, entorhinal area, and some white matter (WM) tracts connecting to these regions, was associated with olfaction. We also observed associations with MD and WM FA in multiple atlas regions that were not previously implicated in olfaction. The associations between MD and olfaction were particularly stronger in the MTL regions among individuals with mild cognitive impairment (MCI) compared with those with normal cognition (e.g., βhippocampus −0.75 [95% CI −1.02 to −0.49] and −0.44 [95% CI −0.63 to −0.26] for MCI and normal cognition, respectively, p interaction = 0.004).
Discussion
Neuronal microstructural integrity in multiple brain regions, particularly the MTL (the regions known to be affected in early Alzheimer disease), is associated with odor identification ability. Differential associations in the MTL regions among cognitively normal individuals compared with those with MCI may reflect the earlier vs later effects of the dementia pathogenesis. It is likely that some of the associated regions may not have any functional relevance to olfaction.
Introduction
Olfactory impairment, or loss of sense of smell, is a common condition, especially in older adults (with a prevalence of approximately 25% in individuals aged 53 years or older and up to 63% in those aged 80 years or older).1 It can result from gradual or sudden damage to peripheral or central olfactory structures. Olfactory impairment is also an early manifestation of neurodegenerative diseases including Alzheimer disease (AD) and Parkinson disease (PD).2,3 Olfactory structures have been suggested to be one of the earliest brain structures to develop AD-related tauopathy and PD-related α-synucleinopathy, years before the onset of clinical symptoms.2,3
With accumulating evidence on the associations of olfaction with AD and PD risk,4-6 olfaction has been suggested to have potential to serve as a noninvasive, yet nonspecific, marker of early brain changes related to these diseases.2,3 A few cross-sectional studies have also linked olfactory impairment with adverse neuropathologic outcomes including diminished primary and secondary olfactory cortices, tau pathology, and elevated brain amyloid accumulation in individuals with normal cognition,7-10 although inconsistently.11 However, additional clarity on where olfactory impairment lies in the pathophysiologic continuum of AD and other dementias is warranted. Furthermore, we also lack adequate evidence of the association of olfaction with possible brain effects other than the central olfactory structures.
Diffusion tensor imaging (DTI) is an MRI technique that uses water molecule diffusion properties to inform about the integrity of tissue microstructures.12,13 DTI has been widely used for studying white matter (WM) of the brain, although less often for gray matter (GM) microstructure.13 Due to its sensitivity to subtle cellular alterations, DTI of the brain has been suggested to detect early neurodegenerative microstructural changes, before the emergence of more conspicuous pathology and cognitive decline.14-17 Prior studies examining the neuroanatomical correlates of olfaction have mainly focused on brain volume measures (which are late macrostructural changes occurring downstream of neurodegenerative processes),9,18 thus warranting research on olfaction and DTI markers. In addition to helping understand brain regions associated with normal olfaction physiology, research on microstructural changes in the brain and olfaction, both early subclinical manifestations of neurodegeneration, can provide further clarity on the early pathophysiology of dementia and PD. However, there have been only a few epidemiologic studies on brain microstructure and olfaction, nearly all based on small clinical samples (n < 100), and 1 population-based study of 265 participants.19-21
Building on a prior study that examined the neuroanatomic correlates of olfaction using voxel-based morphometry and regional brain volumes,18 in this study, we examined cross-sectional associations between microstructural integrity of brain tissue, including WM and GM, and olfaction using data from the community-based Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study (NCS). Based on the prior understanding, we hypothesize that better microstructural integrity of the brain, specifically in some temporal and frontal regions, is associated with better olfaction. In the previous ARIC study, olfactory impairment was associated with smaller amygdala volume in individuals with normal cognition and smaller medial temporal lobe (MTL) region volumes (those regions known to be the primary targets of AD pathogenic processes) in individuals with mild cognitive impairment (MCI), potentially reflecting the earlier and later effects of the dementia pathogenesis.18 Therefore, we additionally evaluated whether the patterns of microstructural changes related to olfaction are distinct among individuals with MCI, a dementia precursor, compared with those among individuals with normal cognition.
Methods
Study Population
The ARIC study is an ongoing prospective cohort study of 15,792 community-dwelling adults (aged 45–64 years at enrollment [visit 1] in 1987–1989) from 4 US communities: Forsyth County, NC; Jackson, MS; Washington County, MD; and suburbs of Minneapolis, Minnesota.22 At the fifth in-person examination (visit 5, 2011–2013), all surviving ARIC participants were invited to participate in the ARIC NCS. The ARIC NCS performed comprehensive neuropsychological assessments including olfactory assessment in all ARIC participants attending visit 5 (n = 6,538) but selected only a subset of participants for MRI. Participants were selected for MRI if they had evidence of cognitive impairment at their visit 5 examination or had previously participated in the ARIC Brain MRI study in 2004–2006. In addition, a random sample of the remaining cognitively normal individuals was selected. So, this analysis is a cross-sectional investigation that included the participants who received a brain MRI scan and underwent odor identification testing at ARIC visit 5, as a part of the ARIC NCS. Of the 6,538 participants who attended the visit 5 examination, 1,978 were selected for MRI examination. Participants without DTI measures (n = 27) and olfactory assessment (n = 84) were excluded. Furthermore, participants of race other than Black or White (n = 6, due to small numbers), Black participants from the Minnesota and Maryland centers (n = 9, due to race/center aliasing), and those with missing covariates selected for confounding adjustment (n = 434) were excluded, leaving 1,418 individuals in the final analytic sample (eFigure 1, links.lww.com/WNL/D27). We did not exclude participants with dementia (n = 67) from the primary analyses but performed a sensitivity analysis excluding them.
Olfaction
We used the 12-item Sniffin' Sticks odor identification test to measure olfaction.23 Participants were presented with an odorant in a felt-tip pen and asked to identify the correct odorant from 4 possible answer choices in a forced-choice format. Each correctly identified odorant was given 1 point, with a total possible score of 12. We used continuous olfaction score for our primary analyses. We also conducted a sensitivity analysis creating a categorical outcome, anosmia, defined as olfaction score ≤6.23
Neuroimaging
The brain imaging protocol is described in detail elsewhere.24 In brief, brain MRI scans were performed at 3T and processed by the ARIC MRI Reading Center at the Mayo Clinic (Rochester, MN). Estimated total intracranial volume was measured on sagittal T1-weighted 3D volumetric magnetization-prepared rapid acquisition gradient echo (with 1.2 × 1.0 × 1.0 mm resolution in the x, y, and z directions, respectively) using Freesurfer version 5.1, and white matter hyperintensity (WMH) volume was measured on axial T2 fluid-attenuated inversion recovery sequences (with 0.9 × 0.9 × 5.0 mm resolution in the x, y, and z directions, respectively).
Axial DTI sequences were used to obtain region of interest (ROI)–wise diffusion measurements, fractional anisotropy (FA) and mean diffusivity (MD). MD (expressed in mm2/s) provides the overall magnitude of diffusion. FA measures the extent to which water molecule diffusion is constrained in one direction relative to other directions. FA is unitless, with values ranging from 0 (perfectly isotropic diffusion) to 1 (anisotropic with all molecules diffusing in the same direction). Higher MD and lower FA values generally indicate lower microstructural integrity. FA and MD were used to evaluate WM microstructural integrity, and MD was used to evaluate GM integrity. Unlike WM tracts, GM is not expected to contribute detectible FA signal.
We used 2 brain atlases for brain parcellation—the Lobar-22 atlas (based on the inhouse STAND400 template, eFigure 2, links.lww.com/WNL/D27), which delineated the brain into lobar and deep GM and WM regions, and the Johns Hopkins University (JHU) single-subject “Eve” atlas that provided more fine-grained ROIs (primarily WM tracts and a few GM structures).25,26 This allowed us to comprehensively examine the associations of olfaction with broader regions not covered by the JHU atlas and to identify specific, more granular WM tracts. Details on DTI including preprocessing steps are described in eAppendix 1 (links.lww.com/WNL/D25) and elsewhere.24
Other Covariates
Information on time-invariant covariates (sex, center race [Mississippi-Black, Minnesota-White, Maryland-White, North Carolina-Black, and North Carolina-White], and APOE ε4 status) and education (<high school, high school or equivalent, and >high school) was obtained at enrollment. Race was self-reported. Information on age, smoking status (current, former, and never), body mass index (BMI, kg/m2), serum total cholesterol (mg/L), diabetes (defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, self-reported physician-diagnosed diabetes, or antidiabetic medication use), blood pressure (BP) status (hypertension [defined as systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg or antihypertensive medication use]), prevalent stroke, coronary heart disease, myocardial infarction, head injury, and global cognition factor score was obtained during the visit 5 examination. Global cognition factor scores were derived from 10 cognitive tests administered at the visit 5 examination using a latent variable approach; the cognitive tests and methods are described elsewhere.27 Participants' cognitive status (normal cognition, MCI, and dementia) was determined based on in-person cognitive evaluations, informant interviews, surveillance of hospitalizations, and expert adjudication for in-person examinations.28
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by institutional review boards at all participating study centers, and all participants provided informed consent.
Statistical Analysis
DTI measures from right and left brain ROIs were combined to form single ROI measures. In addition to examining specific atlas ROI microstructural integrity measures (FA and MD), we also considered broader region-specific or pathway-specific composite measures. Specifically, we aggregated DTI information of relevant atlas ROIs (i.e., associated structural components) based on topography/anatomy into a composite measure for a broader “brain ROI” (e.g., the cingulate gyrus and the hippocampal cingulum JHU atlas ROIs were aggregated into the “cingulum” brain ROI in the JHU atlas). Likewise, we aggregated DTI information of the atlas ROIs that were implicated in prior studies of olfaction (i.e., literature based) to form an “a priori olfaction pathway ROI” (all atlas, brain, and pathway ROIs are summarized in eTables 1A and 1B, links.lww.com/WNL/D26).9,29,30 Last, we combined the atlas ROIs that showed associations between microstructural integrity measures and olfaction in our analysis (data-driven approach) with the literature-based a priori olfaction pathway ROI to form a “combined olfaction pathway ROI.” More details on this and DTI measure computation are available in supplemental methods (eAppendix 2, links.lww.com/WNL/D25).
We used separate multivariable linear regression models to examine cross-sectional associations between ROI-specific microstructural integrity measures (independent variable) and olfaction test score (dependent variable). We adjusted for age, sex, center race, education, smoking status, alcohol drinking status, prevalent heart disease, BP status, diabetes, total cholesterol, BMI, stroke, head injury, APOE ε4 carrier status, and estimated total intracranial volume in our primary model (referred to as primary covariates hereafter). We present the results as the difference in olfaction scores associated with 1 SD higher FA or MD. To examine the influence of global cognition on the associations, we performed regression analysis additionally adjusting for global cognition factor scores. Furthermore, we estimated associations separately for individuals with normal cognition and individuals with MCI to evaluate whether the associations differed between these groups and tested for the interaction between cognitive status (MCI/normal) and DTI measures using the likelihood ratio test. We excluded dementia from this stratified analysis because of small numbers (n = 67). To facilitate the interpretation of our findings, we compared the magnitude of associations between DTI measures and olfaction with the magnitude of the association between age and olfaction; the age association was examined using a separate model with age as the independent variable adjusting for sex, education, and center race.
We conducted several sensitivity analyses: (1) excluding individuals with dementia from the primary analysis (n = 67); (2) adjusting for small vessel disease (SVD) markers, including WMH volume, cerebral infarcts, and microbleeds, in addition to primary covariates; (3) incorporating sampling weights to account for the sampling approach used to select participants for MRI; (4) limiting adjustment covariates to a minimal set of covariates for primary analyses (i.e., age, sex, center race, education, and estimated total intracranial volume, n = 1,840); (5) using negative binomial regression models for olfaction (to examine robustness of our findings toward non-normality); and (6) using modified Poisson regression models to examine the association with anosmia prevalence. Last, in addition to raw/nominal p values, we also report p values corrected for the Benjamini-Hochberg false discovery rate (FDR) and the Bonferroni family-wise error rate (FWER) (with family error rates of 5% for each atlas) and we compare interpretations. We used STATA/SE version 16.0 (StataCorp LLC, College Station, TX) for statistical analyses.
Data Availability
The ARIC study data used for this analysis are available to qualified investigators on request. Details on data availability and study protocols can be accessed at the ARIC website (sites.cscc.unc.edu/aric/).
Results
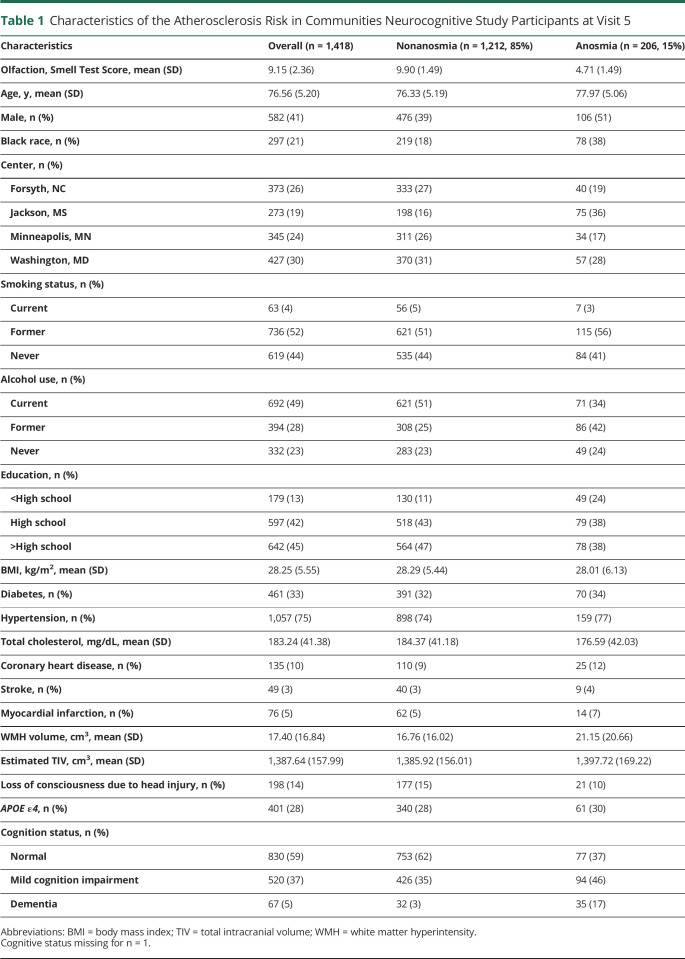
Among 1,418 participants (mean age = 76 ± 5 years, 41% men, 21% Black participants, and 87% with at least high school education), the mean olfaction score was 9 ± 2.3 (25th, 50th, and 75th percentiles = 8, 10, and 11, respectively) and 15% (n = 206) had anosmia (Table 1). There were 59% (n = 830) with normal cognition and 37% (n = 520) with MCI.
Table 1.
Characteristics of the Atherosclerosis Risk in Communities Neurocognitive Study Participants at Visit 5
| Characteristics | Overall (n = 1,418) | Nonanosmia (n = 1,212, 85%) | Anosmia (n = 206, 15%) |
| Olfaction, Smell Test Score, mean (SD) | 9.15 (2.36) | 9.90 (1.49) | 4.71 (1.49) |
| Age, y, mean (SD) | 76.56 (5.20) | 76.33 (5.19) | 77.97 (5.06) |
| Male, n (%) | 582 (41) | 476 (39) | 106 (51) |
| Black race, n (%) | 297 (21) | 219 (18) | 78 (38) |
| Center, n (%) | |||
| Forsyth, NC | 373 (26) | 333 (27) | 40 (19) |
| Jackson, MS | 273 (19) | 198 (16) | 75 (36) |
| Minneapolis, MN | 345 (24) | 311 (26) | 34 (17) |
| Washington, MD | 427 (30) | 370 (31) | 57 (28) |
| Smoking status, n (%) | |||
| Current | 63 (4) | 56 (5) | 7 (3) |
| Former | 736 (52) | 621 (51) | 115 (56) |
| Never | 619 (44) | 535 (44) | 84 (41) |
| Alcohol use, n (%) | |||
| Current | 692 (49) | 621 (51) | 71 (34) |
| Former | 394 (28) | 308 (25) | 86 (42) |
| Never | 332 (23) | 283 (23) | 49 (24) |
| Education, n (%) | |||
| <High school | 179 (13) | 130 (11) | 49 (24) |
| High school | 597 (42) | 518 (43) | 79 (38) |
| >High school | 642 (45) | 564 (47) | 78 (38) |
| BMI, kg/m2, mean (SD) | 28.25 (5.55) | 28.29 (5.44) | 28.01 (6.13) |
| Diabetes, n (%) | 461 (33) | 391 (32) | 70 (34) |
| Hypertension, n (%) | 1,057 (75) | 898 (74) | 159 (77) |
| Total cholesterol, mg/dL, mean (SD) | 183.24 (41.38) | 184.37 (41.18) | 176.59 (42.03) |
| Coronary heart disease, n (%) | 135 (10) | 110 (9) | 25 (12) |
| Stroke, n (%) | 49 (3) | 40 (3) | 9 (4) |
| Myocardial infarction, n (%) | 76 (5) | 62 (5) | 14 (7) |
| WMH volume, cm3, mean (SD) | 17.40 (16.84) | 16.76 (16.02) | 21.15 (20.66) |
| Estimated TIV, cm3, mean (SD) | 1,387.64 (157.99) | 1,385.92 (156.01) | 1,397.72 (169.22) |
| Loss of consciousness due to head injury, n (%) | 198 (14) | 177 (15) | 21 (10) |
| APOE ε4, n (%) | 401 (28) | 340 (28) | 61 (30) |
| Cognition status, n (%) | |||
| Normal | 830 (59) | 753 (62) | 77 (37) |
| Mild cognition impairment | 520 (37) | 426 (35) | 94 (46) |
| Dementia | 67 (5) | 32 (3) | 35 (17) |
Abbreviations: BMI = body mass index; TIV = total intracranial volume; WMH = white matter hyperintensity.
Cognitive status missing for n = 1.
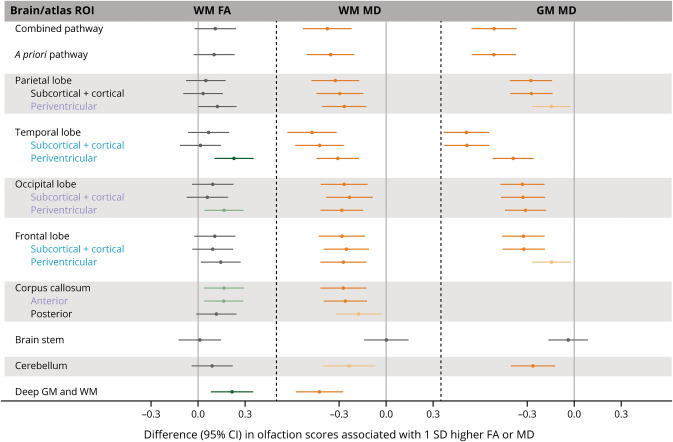
Figure 1 shows the results of the Lobar-22 atlas regions in the overall sample. In the FDR-corrected analysis (eTable 1A, links.lww.com/WNL/D26), higher WM FA (i.e., better WM microstructural integrity) in the temporal and occipital periventricular atlas regions, anterior corpus callosum, and deep brain regions were significantly associated with olfaction. The associations were stronger in the temporal periventricular ROI (β 0.23 [95% CI 0.10–0.36] per 1 SD higher FA) and deep brain regions (β 0.22 [95% CI 0.08–0.35]), which were similar to the difference in olfaction scores associated with approximately 2.8 years younger (lower) age; the β estimate for age (i.e., the difference in olfaction scores associated with 1 year older (higher) baseline age) was −0.08 (95% CI −0.10 to −0.06). Likewise, after FDR correction, higher WM MD and GM MD (i.e., lower microstructural integrity) in all atlas ROIs (except in the brain stem) and in the aggregated brain and olfaction pathway ROIs were significantly associated with lower olfaction score; the associations were strongest in the temporal lobe ROIs. After FWER correction, FA associations persisted only in the temporal periventricular and deep brain regions while MD associations were significant in nearly all atlas ROIs. We observed associations in additional regions in analysis that did not adjust for multiple comparisons.
Figure 1. Associations Between Microstructural Integrity of the Brain and Olfaction Score in the Lobar-22 Atlas Regions.
β Estimates were obtained from ROI-specific linear regression models with olfaction score as the outcome variable and standardized DTI measure as the independent variable, adjusting for age, sex, center race, education, smoking status, alcohol drinking status, prevalent heart disease, blood pressure status, diabetes, total cholesterol, body mass index, stroke, head injury, APOE ε4 carrier status, and estimated total intracranial volume. Positive β for FA indicates that better microstructural integrity (higher FA) is associated with better olfaction. Negative β for MD indicates that worse microstructural integrity (higher MD) is associated with worse olfaction. Blue text indicates that these regions formed the a priori olfaction pathway ROI. Blue and purple ROIs formed the combined olfaction pathway ROI. Light green and orange data points represent that the associations were significant after correcting for FDR (p ≤ 0.05) for FA and MD, respectively. Dark green and orange data points represent that the associations were significant after correcting for FWER (p ≤ 0.05) for FA and MD, respectively. Please see eTable 1A (links.lww.com/WNL/D26) for details. FA = fractional anisotropy; FDR = Benjamini-Hochberg false discovery rate; FWER = Bonferroni family-wise error rate; GM = gray matter; MD = mean diffusivity; ROI = region of interest; WM = white matter.
In the analysis that additionally adjusted for cognition (eTable 2A, links.lww.com/WNL/D26), associations with GM and WM MD, although generally attenuated (with β coefficients in the same direction as before), persisted in most regions. However, when adjusted for FDR, the associations with WM MD were supported only in the temporal and deep brain regions and the associations with GM MD were significant in all regions except for the parietal and frontal periventricular ROIs and brain stem. None of the WM FA associations were significant after FDR, but nominal associations (i.e., without multiple comparison adjustment) were seen in the temporal periventricular and deep brain ROIs.
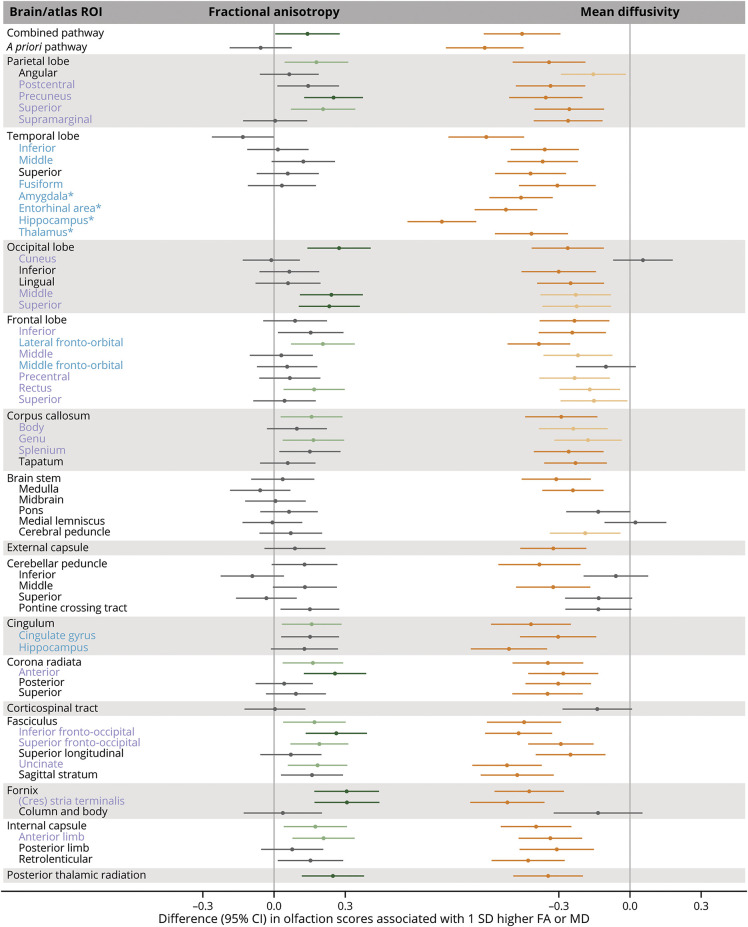
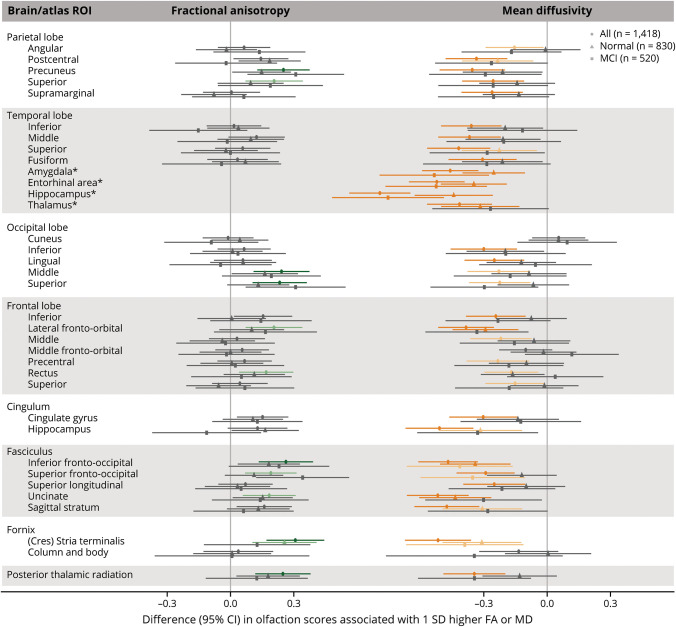
In the JHU atlas regions, higher WM FA in 21 atlas ROIs (of the 51 examined) was nominally associated with higher olfaction score (Figure 2, eTable 1B, links.lww.com/WNL/D26). However, after FDR correction, associations remained significant only in 15 regions, which included a few ROIs in the parietal, frontal, and occipital lobes; the corpus callosum; the association fibers including the cingulum, uncinate and fronto-occipital fasciculus; the stria terminalis of the fornix; and some projection fibers (e.g., the anterior corona radiata). Associations remained significant in 7 regions after FWER correction. Likewise, higher WM MD in 42 atlas ROIs (of the 50 examined) was associated with lower olfaction score after FDR correction, of which 32 remained significant after FWER correction. While the associations were generally comparable in magnitude across all atlas ROIs, we observed stronger associations with WM FA in the stria terminalis of the fornix and with WM MD in the stria terminalis, uncinate fasciculus, and hippocampus. Lower GM MD in the amygdala, entorhinal area, hippocampus, and thalamus was also associated with lower olfaction score (all corrected p values <0.05); β estimates ranged from −0.42 (95% CI −0.57 to −0.26) for the thalamus to −0.79 (95% CI −0.94 to −0.65) for the hippocampus, which correspond to the differences in olfaction scores associated with 5 and 9.5 years's higher baseline age, respectively.
Figure 2. Associations Between Microstructural Integrity of the Brain and Olfaction Score in the Johns Hopkins University Atlas Regions.
β Estimates were obtained from ROI-specific linear regression models with olfaction score as the outcome variable and standardized DTI measure as the independent variable, adjusting for age, sex, center race, education, smoking status, alcohol drinking status, prevalent heart disease, blood pressure status, diabetes, total cholesterol, body mass index, stroke, head injury, APOE ε4 carrier status, and estimated total intracranial volume. Positive β for FA indicates that better microstructural integrity (higher FA) is associated with better olfaction. Negative β for MD indicates that worse microstructural integrity (higher MD) is associated with worse olfaction. Blue text indicates that these regions formed the a priori olfaction pathway ROI. Blue and purple ROIs formed the combined olfaction pathway ROI. Light green and orange data points represent that the associations were significant after correcting for FDR (p ≤ 0.05) for FA and MD, respectively. Dark green and orange data points represent that the associations were significant after correcting for FWER (p ≤ 0.05) for FA and MD, respectively. Please see eTable 1B (links.lww.com/WNL/D26) for details. FA = fractional anisotropy; FDR = Benjamini-Hochberg false discovery rate; FWER = Bonferroni family-wise error rate; GM = gray matter; MD = mean diffusivity; ROI = region of interest; WM = white matter. Note: MD measures for all ROIs except for those with asterisk (*) are WM MD; the ROIs with * are GM MD.
The associations in the JHU atlas regions were also attenuated when adjusted for cognition (eTable 2B, links.lww.com/WNL/D26). Specifically, the associations with MD persisted in most regions (25 of the 50 after FDR correction), but those in some occipital and frontal regions and corpus callosum were no longer statistically significant. After FWER correction, the associations persisted in the MTL regions, lateral fronto-orbital, hippocampal cingulum, inferior fronto-occipital, uncinate, stria terminalis, and internal capsule regions. Of the 21 regions that were associated in the primary analysis of FA, none remained significant after FDR and FWER correction, but nominal associations were seen in the precuneus, superior occipital, anterior corona radiata and internal capsule, fronto-occipital and uncinate fasciculus, and stria terminalis.
The results were generally somewhat attenuated compared with those of our primary analyses when we excluded participants with dementia (eTables 3A and 3B, links.lww.com/WNL/D26) but inferences were similar (e.g., β −0.79 [95% CI −0.94 to −0.65] vs −0.66 [95% CI −0.81 to −0.51] for the hippocampus for with and without dementia, respectively). When additionally adjusted for WMH volume, infarcts, and microbleeds (eTables 4A and 4B), the results were similar to those of our primary analyses, suggesting these SVD markers do not explain the observed associations. Furthermore, we repeated the analysis adjusting a minimal set of covariates (eTables 5A and 5B), and the results were mostly similar. Likewise, the results of the analyses that accounted for sampling weights to select participants for MRI were generally comparable with those of the primary analyses (eTables 6A and 6B), although the associations in some olfactory regions were slightly attenuated.
Furthermore, the analyses that modeled the number of incorrectly identified odorants using negative binomial regressions yielded similar inferences (eTables 7A and 7B, links.lww.com/WNL/D26), supporting the robustness of our linear regression analyses. The analyses examining DTI markers and anosmia prevalence (defined using a clinical cut point) also yielded similar inferences as our primary analyses (e.g., the largest prevalence ratio 1.86 (95% CI 1.60–2.16) was for the hippocampus, eTables 8A and 8B).
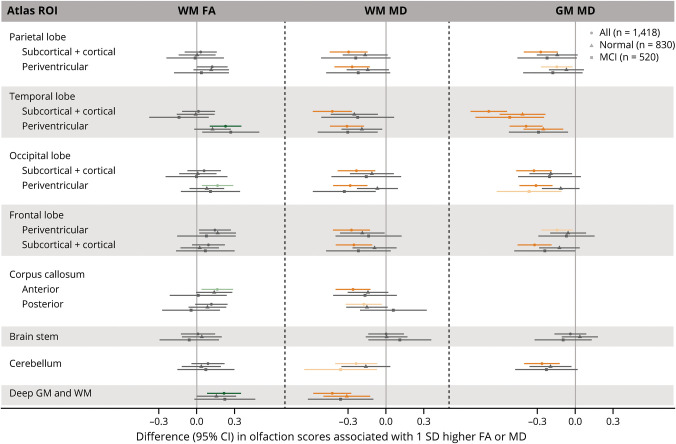
When stratified by cognitive status, the associations between microstructural integrity measures and olfaction were generally qualitatively similar between those with normal cognition and MCI (Figures 3 and 4, eTables 9A and 9B, links.lww.com/WNL/D26). Nonetheless, the associations with MD in the amygdala, entorhinal area, and hippocampus were stronger among those with MCI (all FDR/FWER corrected p values ≤0.05) (e.g., βs for the hippocampus −0.44 [95% CI −0.63 to −0.26] and −0.75 [95% CI −1.02 to −0.49] in individuals with normal cognition and MCI respectively, p interaction = 0.004). However, the statistical tests for interaction between MD and MCI status were not significant for the amygdala (β −0.25 [95% CI −0.40 to −0.105] vs −0.54 [95% CI −0.79 to −0.28], p interaction = 0.08) or the entorhinal area (β −0.35 [95% CI −0.50 to −0.19] vs −0.53 [95% CI −0.77 to −0.29], p = 0.13) for individuals with MCI and normal cognition, respectively.
Figure 3. Associations Between Microstructural Integrity of the Brain and Olfaction in the Lobar-22 Atlas Regions Stratified by Cognitive Status.
β Estimates were obtained from ROI-specific linear regression models with olfaction score as the outcome variable and standardized DTI measure as the independent variable, adjusting for age, sex, center race, education, smoking status, alcohol drinking status, prevalent heart disease, blood pressure status, diabetes, total cholesterol, body mass index, stroke, head injury, APOE ε4 carrier status, and estimated total intracranial volume. Positive β for FA indicates that better microstructural integrity (higher FA) is associated with better olfaction. Negative β for MD indicates that worse microstructural integrity (higher MD) is associated with worse olfaction. Solid circles represent β estimates for overall sample (n = 1,418), triangles for individuals with normal cognition (n = 830), and squares for individuals with MCI (n = 520). Light green and orange data points represent that the associations were significant after correcting for FDR (p ≤ 0.05) for FA and MD, respectively. Dark green and orange data points represent that the associations were significant after correcting for FWER (p ≤ 0.05) for FA and MD, respectively. Please see eTable 9B (links.lww.com/WNL/D26) for details. FA = fractional anisotropy; FDR = Benjamini-Hochberg false discovery rate; FWER = Bonferroni family wise-error rate; GM = gray matter; MCI = mild cognitive impairment; MD = mean diffusivity; ROI = region of interest; WM = white matter.
Figure 4. Associations Between Microstructural Integrity of the Brain and Olfaction in the Johns Hopkins University Atlas Regions Stratified by Cognitive Status.
β Estimates were obtained from ROI-specific linear regression models with olfaction score as the outcome variable and standardized DTI measure as the independent variable, adjusting for age, sex, center race, education, smoking status, alcohol drinking status, prevalent heart disease, blood pressure status, diabetes, total cholesterol, body mass index, stroke, head injury, APOE ε4 carrier status, and estimated total intracranial volume. Positive β for FA indicates that better microstructural integrity (higher FA) is associated with better olfaction. Negative β for MD indicates that worse microstructural integrity (higher MD) is associated with worse olfaction. Solid circles represent β estimates for overall sample (n = 1,418), triangles for individuals with normal cognition (n = 830), and squares for individuals with MCI (n = 520). Light green and orange data points represent that the associations were significant after correcting for FDR (p ≤ 0.05) for FA and MD, respectively. Dark green and orange data points represent that the associations were significant after correcting for FWER (p ≤ 0.05) for FA and MD, respectively. Please see eTable 9B (links.lww.com/WNL/D26) for details (only selected ROIs presented in Figure 4). FA = fractional anisotropy; FDR = Benjamini-Hochberg false discovery rate; FWER = Bonferroni family-wise error rate; GM = gray matter; MCI = mild cognitive impairment; MD = mean diffusivity; ROI = region of interest; WM = white matter. Note: MD measures for all ROIs except for those with asterisk (*) are WM MD; the ROIs with * are GM MD.
Discussion
In this community-based study of older adults, we found that microstructural integrity of multiple brain regions was associated with odor identification ability. The strongest associations with MD were observed in olfactory pathway regions (namely, the temporal lobe ROIs in the Lobar-22 atlas and the MTL ROIs in the JHU atlas) and some WM tracts connecting to these regions, although MD in most atlas regions was associated with olfaction. We also observed associations between higher WM FA and better olfaction in multiple regions, primarily in the periventricular regions in the Lobar-22 atlas and in selected JHU atlas regions in the parietal, frontal, and occipital lobes and WM tracts. Associations in many of these regions persisted even after adjusting for global cognition factor scores (although attenuated), suggesting these are not explained by cognition alone. Furthermore, we observed that the associations between GM MD and olfaction were particularly stronger in the MTL regions among individuals with MCI compared with those among individuals with normal cognition.
Microstructural integrity measures (particularly MD) in many brain regions, including those traditionally not associated with olfaction, were associated with odor identification ability. Nonetheless, stronger associations in the MTL regions (which include some central olfactory structures) underscore greater relevance of these regions for olfaction. Furthermore, our findings suggest that olfaction depends on microstructural integrity of the WM tracts connecting the central olfactory structures. Odor identification involves numerous aspects of cognitive processing including odor perception, memory, and semantic abilities.3,31 Further, older adults, compared with their younger counterparts, seem to demonstrate more widespread brain activation extending beyond primary olfactory networks during olfactory processing, possibly as a compensatory mechanism to age-related decline in olfactory and cognitive abilities.32 These findings could explain associations across diffuse brain regions in this older cohort (when compared with localized associations we expected). While these associations were attenuated after adjusting for cognition in some regions, the pattern persisted for MD. Furthermore, alterations in neurotransmitter systems have also been implicated in olfaction. It is possible that observed associations in some regions reflect neurodegenerative changes in neurotransmitter circuits.33 Nonetheless, we cannot rule out that associations of olfaction with anatomically broad-based regions simply reflect parallel co-occurring age-associated (or pathogenic) neurodegenerative processes and they do not have any functional relevance to olfaction. Furthermore, though we adjusted for a comprehensive set of covariates, some of these findings could be due to unexplained confounding. Moreover, we did not see associations in some regions that are likely relevant for olfaction—a potential explanation could be measurement error resulting from magnetic susceptibility artifacts because DTI uses echo planar imaging, which is prone to these kinds of artifacts near the olfactory tracts.
We observed that MD was more frequently associated with olfaction (and more often strongly) than FA. While the explanation for such differential associations is not clear, prior studies have also found MD to be more sensitive for detecting cognitive impairment and age-related reduction in microstructural integrity of the brain than FA.34,35 Global MD was in fact among the earliest MRI markers to change with increasing age in the Rotterdam study.35
Our findings are generally in line with those of prior studies on olfaction and brain neuropathology, although only a handful have examined associations with brain microstructure. For instance, in a subsample of the Health, Aging, and Body Composition study (n = 265), prior hyposmia was associated with higher GM MD in the right middle orbitofrontal gyrus and the amygdala 10 years later.21 In a study of 30 healthy adults aged 51–77 years, WM microstructural integrity in the splenium of the corpus callosum and the superior longitudinal fasciculi was correlated with better olfaction.20 In a study of 19 individuals with amnestic MCI (aMCI), lower mean diffusivity in multiple WM tract regions was associated with higher olfaction scores (somewhat diffused associations as observed in ours).36 Other studies were generally based on small clinical samples of individuals with PD or AD.19,37 Of relevance, several studies have linked odor identification ability with brain MRI markers, including volumes, hypometabolism, and tau pathology, specifically in the olfactory cortices and AD signature areas,7-10 although not consistently.11
Compared with individuals with normal cognition, we found stronger associations between cortical MD and olfaction in the MTL regions including the hippocampus, amygdala, and entorhinal areas among those with MCI, reflecting the later effects of dementia pathogenesis. This is in line with previous study findings (including the ARIC study) showing lower MTL volume activation in olfactory task–based MRI and greater MTL neurodegeneration (including diminished volumes and loss of functional connectivity and GM microstructural integrity) among individuals with MCI compared with cognitively normal individuals.18,38-42 MTL regions are involved in both memory and olfactory processing and are brain regions where AD-related tau and Lewy body disease–related α-synuclein pathologies appear early in the course of these diseases.2,43,44 In AD, particularly, tau pathology occurs initially in the MTL regions, which later progresses to other brain regions, and this temporal and spatial course of tauopathy progression has been suggested to coincide with cognitive deficit patterns.44,45 While it is possible that stronger associations in the MTL regions may be related to early tau-related neurodegeneration, we have not attempted to tease apart dementia etiology in these analyses. Relatively few studies have evaluated the association between brain microstructure and olfaction by cognitive status. For example, studies have reported correlations of WM integrity measures in the whole brain, in the orbitofrontal gyrus, and the inferior occipital gyrus with odor identification score in individuals with aMCI and/or AD, but not in cognitively normal individuals.19,46 In a study of the olfactory tract integrity and cortical glucose metabolism, those with MCI showed associations in the MTL and posterior cortical structures, while cognitively normal individuals showed associations in the anterior cerebral structures.47 We, however, were limited in that we were not able to explore patterns for prodromal PD.
Strengths of the study include large community-based sample of Black and White participants, availability of comprehensive information on potential confounders, and brain region DTI measures. Our study has also several limitations. First, its cross-sectional nature limits us from making inferences about temporality of the associations. We based our study on a premise that poor microstructural integrity of the brain would result in olfactory impairment; however, impaired olfaction can occur from damage to peripheral olfactory structures, and olfaction status has also been suggested to alter brain neuropathology, alluding to a possible bidirectional nature of the associations.48 Second, we used an odor identification test that is cognitively demanding. An odor sensitivity test, which gauges an individual's ability to detect odor concentration and is less cognitively demanding, would have shed additional light on the relationship between brain microstructures and olfaction independent of cognition. Third, the JHU atlas is based on a scan of a single 32-year-old person, and it thus has individual features (when compared with a population-averaged atlas). So this cohort being comparatively older, atlas ROIs may not have aligned well with anatomical features of some participants, potentially resulting in measurement error, especially for smaller atlas regions. Fourth, we excluded individuals with missing data on covariates for adjustment, which may have biased our findings. Finally, the inferior surface of the frontal lobe is an area of sharp changes in magnetic susceptibility and thus prone to signal attenuation and distortion in clinically compatible diffusion MRI sequences, unfortunately making the olfaction bulb and entorhinal cortex challenging regions for DTI.
In conclusion, our findings suggest that microstructural integrity of the brain is associated with olfaction, even after accounting for cognition. While we cannot generalize our findings to younger populations, our findings hint at the possibility of involvement of additional regions (outside of the currently recognized central olfactory structures) in olfaction processing in older populations, possibly to compensate for the age-associated loss in olfactory and cognitive abilities. Specifically, associations of olfaction with multiple brain regions, not detected in our prior volumetric analyses,18 likely reflect that DTI measures, MD and FA, are more sensitive than volumetric measures. Nonetheless, we cannot rule out that some of these regions do not have any functional relevance to olfaction and just reflect spatiotemporal correlation associated with co-occurring neurodegenerative processes. The differential associations in the MTL regions in individuals with normal cognition and MCI may reflect earlier and later effects of the dementia pathogenesis marked by olfactory loss. Future studies incorporating AD-related brain biomarkers may help shed more light on olfaction as an early manifestation of AD specifically and its utility in predicting individuals with high future AD risk. In addition, the current findings need further confirmation; specifically, longitudinal studies with repeated DTI and olfaction measures may help elucidate microstructural integrity changes and associated olfactory decline.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- AD
Alzheimer disease
- aMCI
amnestic MCI
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- BP
blood pressure
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FDR
Benjamini-Hochberg false discovery rate
- FWER
Bonferroni family-wise error rate
- GM
gray matter
- JHU
Johns Hopkins University
- MCI
mild cognitive impairment
- MD
mean diffusivity
- MTL
medial temporal lobe
- NCS
Neurocognitive Study
- PD
Parkinson disease
- ROI
region of interest
- SVD
small vessel disease
- WM
white matter
- WMH
white matter hyperintensity
Appendix. Authors

| Name | Location | Contribution |
| Srishti Shrestha, PhD | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Xiaoqian Zhu, PhD | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Kevin J. Sullivan, PhD | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Chad Blackshear, MS | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Jennifer A. Deal, PhD | Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| A. Richey Sharrett, MD, DrPH | Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Vidyulata Kamath, PhD | Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Andrea Lauren Christman Schneider, MD, PhD | Department of Neurology, and Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Clifford R. Jack, MD | Department of Radiology, Mayo Clinic, Rochester, MN | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Juebin Huang, MD | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, and Department of Neurology, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Priya Palta, PhD | Department of Neurology, University of North Carolina at Chapel Hill | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Robert I. Reid, PhD | Department of Radiology, Mayo Clinic, Rochester, MN | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| David S. Knopman, MD | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Rebecca F. Gottesman, MD, PhD | Stroke Branch, National Institute of Neurological Disorders and Stroke Intramural Research Program, Bethesda, MD | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Honglei Chen, MD, PhD | Department of Epidemiology and Biostatistics, Michigan State University, East Lansing | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| B. Gwen Windham, MD, MHS | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Michael E. Griswold, PhD | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Thomas H. Mosley Jr., PhD | The Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
The Atherosclerosis Risk in Communities Study is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I). Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke, NIA and NIDCD) and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI, and olfaction data collection was funded by the intramural research program of NIH, NIEHS (1ZIAES101986).
Disclosure
S. Shrestha, X. Zhu, K.J. Sullivan, C. Blackshear, A.R. Sharrett, J. Huang, R.I. Reid, D.S. Knopman, B.G. Windham, M.E. Griswold, and T.H. Mosley report no disclosures relevant to the manuscript. C.R. Jack serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from the NIH, the GHR Foundation, and the Alexander Family Alzheimer Disease Research Professorship of the Mayo Clinic. J.A. Deal is supported by NIH/NIA grant K01AG054693. A.L.C. Schneider is supported by NIH National Institute of Neurological Disorders and Stroke grant K23 NS123340. V. Kamath is supported by grants from the NIH (R01AG064093 and R01NS108452). R.F. Gottesman is supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. H. Chen is supported by the NIH grant R01AG071517. P. Palta is in part supported by NIH/NIA grant R00AG052830. Go to Neurology.org/N for full disclosures.
References
- 1.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307-2312. doi: 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 2.Fullard ME, Morley JF, Duda JE. Olfactory dysfunction as an early biomarker in Parkinson's disease. Neurosci Bull. 2017;33(5):515-525. doi: 10.1007/s12264-017-0170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy C Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. 2019;15(1):11-24. doi: 10.1038/s41582-018-0097-5 [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2016;88(5):456-462. doi: 10.1212/wnl.0000000000003558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RO, Christianson TJ, Kremers WK, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73(1):93-101. doi: 10.1001/jamaneurol.2015.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Shrestha S, Huang X, et al. Olfaction and incident Parkinson disease in US white and black older adults. Neurology. 2017;89(14):1441-1447. doi: 10.1212/wnl.0000000000004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassilaki M, Christianson TJ, Mielke MM, et al. Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol. 2017;81(6):871-882. doi: 10.1002/ana.24960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology. 2015;84(21):2153-2160. doi: 10.1212/wnl.0000000000001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dintica CS, Marseglia A, Rizzuto D, et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700-e709. doi: 10.1212/wnl.0000000000006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein J, Yan X, Johnson A, et al. Olfactory impairment is related to tau pathology and neuroinflammation in Alzheimer's disease. J Alzheimers Dis. 2021;80(3):1051-1065. doi: 10.3233/jad-201149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchanan SM, Parker TD, Lane CA, et al. Olfactory testing does not predict beta-amyloid, MRI measures of neurodegeneration or vascular pathology in the British 1946 birth cohort. J Neurol. 2020;267(11):3329-3336. doi: 10.1007/s00415-020-10004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316-329. doi: 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weston PS, Simpson IJ, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer's disease: a potential marker of early neurodegeneration. Alzheimers Res Ther. 2015;7(1):47. doi: 10.1186/s13195-015-0132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Groot M, Verhaaren BF, de Boer R, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44(4):1037-1042. doi: 10.1161/strokeaha.112.680223 [DOI] [PubMed] [Google Scholar]
- 15.Power MC, Su D, Wu A, et al. Association of white matter microstructural integrity with cognition and dementia. Neurobiol Aging. 2019;83:63-72. doi: 10.1016/j.neurobiolaging.2019.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabin JS, Perea RD, Buckley RF, et al. Global white matter diffusion characteristics predict longitudinal cognitive change independently of amyloid status in clinically normal older adults. Cereb Cortex. 2019;29(3):1251-1262. doi: 10.1093/cercor/bhy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Xu Y, Zhu B, Kantarci K. The role of diffusion tensor imaging in detecting microstructural changes in prodromal Alzheimer's disease. CNS Neurosci Ther. 2014;20(1):3-9. doi: 10.1111/cns.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath V, Senjem ML, Spychalla AJ, et al. The neuroanatomic correlates of olfactory identification impairment in healthy older adults and in persons with mild cognitive impairment. J Alzheimers Dis. 2022;89(1):233-245. doi: 10.3233/jad-220228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodward MR, Dwyer MG, Bergsland N, et al. Olfactory identification deficit predicts white matter tract impairment in Alzheimer's disease. Psychiatry Res Neuroimaging. 2017;266:90-95. doi: 10.1016/j.pscychresns.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segura B, Baggio HC, Solana E, et al. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013;246:148-153. doi: 10.1016/j.bbr.2013.02.025 [DOI] [PubMed] [Google Scholar]
- 21.Felix C, Chahine LM, Hengenius J, et al. Diffusion tensor imaging of the olfactory system in older adults with and without hyposmia. Front Aging Neurosci. 2021;13:648598. doi: 10.3389/fnagi.2021.648598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939-2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palta P, Chen H, Deal JA, et al. Olfactory function and neurocognitive outcomes in old age: the Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement. 2018;14(8):1015-1021. doi: 10.1016/j.jalz.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power MC, Tingle JV, Reid RI, et al. Midlife and late-life vascular risk factors and white matter microstructural integrity: the Atherosclerosis Risk in Communities Neurocognitive Study. J Am Heart Assoc. 2017;6(5):e005608. doi: 10.1161/jaha.117.005608, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oishi K, Faria A, Jiang H, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage. 2009;46(2):486-499. doi: 10.1016/j.neuroimage.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer's disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008;39(3):1186-1197. doi: 10.1016/j.neuroimage.2007.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross AL, Power MC, Albert MS, et al. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26(6):878-887. doi: 10.1097/ede.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement (Amst). 2016;2:1-11. doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han P, Zang Y, Akshita J, Hummel T. Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr. 2019;32(6):987-997. doi: 10.1007/s10548-019-00729-5 [DOI] [PubMed] [Google Scholar]
- 30.Kandel ER, Koester JD, Mack SH, Siegelbaum SA. Smell and Taste: The Chemical Senses. Principles of Neural Science, 6th ed. McGraw Hill; 2021. [Google Scholar]
- 31.Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32(10):1062-1067. doi: 10.1080/13803391003683070 [DOI] [PubMed] [Google Scholar]
- 32.Steffener J, Motter JN, Tabert MH, Devanand DP. Odorant-induced brain activation as a function of normal aging and Alzheimer's disease: a preliminary study. Behav Brain Res. 2021;402:113078. doi: 10.1016/j.bbr.2020.113078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker IM, Fullard ME, Morley JF, Duda JE. Olfaction as an early marker of Parkinson's disease and Alzheimer's disease. Handb Clin Neurol. 2021;182:317-329. doi: 10.1016/B978-0-12-819973-2.00030-7 [DOI] [PubMed] [Google Scholar]
- 34.Nir TM, Jahanshad N, Villalon-Reina JE, et al. Effectiveness of regional DTI measures in distinguishing Alzheimer's disease, MCI, and normal aging. Neuroimage Clin. 2013;3:180-195. doi: 10.1016/j.nicl.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinke EJ, de Groot M, Venkatraghavan V, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging. 2018;71:32-40. doi: 10.1016/j.neurobiolaging.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 36.Ryu SY, Lee DC, Lee SB, et al. Olfactory identification and white matter integrity in amnestic mild cognitive impairment: a preliminary study. Int J Imag Syst Tech. 2016;26(4):270-276. doi: 10.1002/ima.22198 [DOI] [Google Scholar]
- 37.Hummel T, Haehner A, Thaploo D, Georgiopoulos C, Falkenburger B, Whitcroft K. Advancement of PD is reflected by white matter changes in olfactory areas: a pilot study. Medicina (Kaunas). 2021;57(11):1183. doi: 10.3390/medicina57111183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jobin B, Boller B, Frasnelli J. Volumetry of olfactory structures in mild cognitive impairment and Alzheimer's disease: a systematic review and a meta-analysis. Brain Sci. 2021;11(8):1010. doi: 10.3390/brainsci11081010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasavada MM, Wang J, Eslinger PJ, et al. Olfactory cortex degeneration in Alzheimer's disease and mild cognitive impairment. J Alzheimers Dis. 2015;45(3):947-958. doi: 10.3233/jad-141947 [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Testa N, Jordan R, et al. Functional connectivity between the resting-state olfactory network and the hippocampus in Alzheimer's disease. Brain Sci. 2019;9(12):338. doi: 10.3390/brainsci9120338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77(1):26-34. doi: 10.1212/wnl.0b013e31822313dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oishi K, Mielke MM, Albert M, Lyketsos CG, Mori S. DTI analyses and clinical applications in Alzheimer's disease. J Alzheimers Dis. 2011;26(suppl 3):287-296. doi: 10.3233/jad-2011-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson's disease. Brain. 2002;125(11):2431-2445. doi: 10.1093/brain/awf251 [DOI] [PubMed] [Google Scholar]
- 44.Kantarci K, Murray ME, Schwarz CG, et al. White-matter integrity on DTI and the pathologic staging of Alzheimer's disease. Neurobiol Aging. 2017;56:172-179. doi: 10.1016/j.neurobiolaging.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shao Y, Wang Z, Ji B, et al. Diffusion tensor imaging study of olfactory identification deficit in patients with mild cognitive impairment. Front Aging Neurosci. 2021;13:765432. doi: 10.3389/fnagi.2021.765432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cross DJ, Anzai Y, Petrie EC, et al. Loss of olfactory tract integrity affects cortical metabolism in the brain and olfactory regions in aging and mild cognitive impairment. J Nucl Med. 2013;54(8):1278-1284. doi: 10.2967/jnumed.112.116558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Ain S, Poupon D, Hetu S, Mercier N, Steffener J, Frasnelli J. Smell training improves olfactory function and alters brain structure. Neuroimage. 2019;189:45-54. doi: 10.1016/j.neuroimage.2019.01.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ARIC study data used for this analysis are available to qualified investigators on request. Details on data availability and study protocols can be accessed at the ARIC website (sites.cscc.unc.edu/aric/).