Abstract
Objectives
To determine the timing and predictors of T2-lesion resolution in myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD).
Methods
This retrospective observational study using standard-of-care data had inclusion criteria of MOGAD diagnosis, ≥2 MRIs 12 months apart, and ≥1 brain/spinal cord T2-lesion. The median (interquartile range [IQR]) number of MRIs (82% at disease onset) per-patient were: brain, 5 (2–8); spine, 4 (2–8). Predictors of T2-lesion resolution were assessed with age- and sex-adjusted generalized estimating equations and stratified by T2-lesion size (small <1 cm; large ≥1 cm).
Results
We studied 583 T2-lesions (brain, 512 [88%]; spinal cord, 71 [12%]) from 55 patients. At last MRI (median follow-up 54 months [IQR 7–74]) 455 T2-lesions (78%) resolved. The median (IQR) time to resolution was 3 months (1.4–7.0). Small T2-lesions resolved more frequently and faster than large T2-lesions. Acute T1-hypointensity decreased the likelihood (odds ratio [95% CI]) of T2-lesion resolution independent of size (small: 0.23 [0.09–0.60], p = 0.002; large: 0.30 [0.16–0.55], p < 0.001), whereas acute steroids favored resolution of large T2-lesions (1.75 [1.01–3.03], p = 0.046). Notably, 32/55 (58%) T2-lesions resolved without treatment.
Discussion
The high frequency of spontaneous T2-lesion resolution suggests that this represents MOGAD's natural history. The speed of T2-lesion resolution and influence of size, corticosteroids, and T1-hypointensity on this phenomenon gives insight into MOGAD pathogenesis.
Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody–associated disease (MOGAD) is a CNS antibody–associated demyelinating disorder.1 Studies have demonstrated T2-hyperintense lesions in MOGAD more frequently resolve over time (brain, 67%–72%2,3; spinal cord, 79%3) than in multiple sclerosis (MS, 0%–16%) and aquaporin-4-immunoglobulin G (IgG) seropositive neuromyelitis optica spectrum disorder (0%–27%).2,3 Whether T2-lesion resolution in MOGAD reflects a treatment effect or the natural history of the disease is unclear.
In this study, we aimed to determine the timing and predictors of resolution of T2-hyperintense lesions (henceforth termed T2-lesion[s]) in patients with MOGAD as this could guide follow-up MRI timing and improve our understanding of its pathophysiology. We analyzed the association with patient and lesion factors through retrospective evaluation of brain and spinal cord T2-lesion dynamics in 55 patients with MOGAD.1
Methods
Inclusion Criteria and Study Design
This is a retrospective, observational study using standard-of-care data. Inclusion criteria were (1) MOGAD diagnosis,1 (2) ≥2 MRI scans available, and (3) ≥1 brain or spinal cord T2-lesion in ≥1 MRI scan. A final follow-up MRI ≥12 months without interval attacks in the same location was required to assess T2-lesion resolution and avoid underestimation due to short follow-up, but timing of T2-lesion resolution was also recorded for follow-up MRIs ≤12 months. We analyzed MRIs from May 2004 to June 2022.
We collected clinical and laboratory data (patient factors) from medical records including age, sex, disease duration, phenotype, disease course, expanded disability status scale, MOG-IgG titer, CSF oligoclonal bands, acute treatment within 1 month of the attack, and maintenance attack-prevention treatments. MOG-IgG1 detection was with live cell-based assays as previously described.4 We used the neuroradiology reports and a single rater with experience in neuroimaging blinded to the clinical history (L.C.), who evaluated the following MRI features (lesion factors): maximum transverse diameter (brain T2 fluid-attenuated inversion recovery [FLAIR]) or length (spine sagittal T2-weighted images), acute T1-hypointensity, enhancement on T1-weighted postgadolinium images, and diffusion restriction on diffusion-weighted images. Complete resolution of T2-lesions was evaluated on all available subsequent MRIs (brain, T2-FLAIR images; spine, T2-weighted images), and new T2-lesions that developed over time were analyzed for resolution when sufficient follow-up MRIs were available.
We used Pearson chi-squared and Kruskal-Wallis tests for between-group comparisons. Because patients could have multiple T2-lesions (median of 4/patient, interquartile range [IQR] 1–7) and outcomes potentially correlated, the association between patient and lesion factors and T2-lesion resolution was assessed with age- and sex-adjusted generalized estimating equations with an exchangeable matrix (IBM SPSS Statistics for Windows, version 25.0; IBM Corp., Armonk, NY) and stratified by large (≥1 cm, n = 362) and small (<1 cm, n = 221) diameter T2-lesions.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by Mayo Clinic's Institutional Review Board (IRB 08-006647). All participants gave written informed consent to the passive use of their medical records for research purposes.
Data Availability
Anonymized data from this study will be made available on request.
Results
Details of the study population, MRI scan number, and timing are presented in the Table. A total of 583 T2-lesions (brain, 512 [88%]; spinal cord, 71 [12%]) from 55 patients were analyzed. Five hundred thirty-four (92%) T2-lesions were present at baseline, and 49 (8%) developed during follow-up.
Table.
Demographic and Clinical Features of the Study Population, MRI Scan Number, and Timing

Timing and Frequency of T2-Lesion Resolution
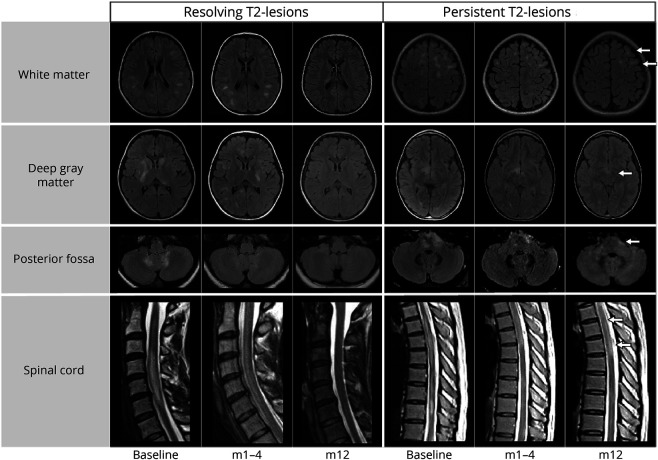
The median time to resolution per T2-lesion was 3 months (IQR 1.4–7.0). At the last available MRI scan, 455/583 T2-lesions (78%) resolved, with a frequency of 404/512 (79%) in the brain and 51/71 (72%) in the spinal cord (examples in Figure 1). In 24/55 (44%) patients all T2-lesions resolved. In the remaining 31/55 (56%), despite many of their T2-lesions resolving completely, at least one T2-lesion was still present at last follow up. T2-lesion resolution occurred within the first 12 months in 401/455 (89%) of the T2-lesions that had resolved. In 8/55 (15%) patients, new T2-lesions developed in addition to T2-lesion resolution at the first follow-up MRI. Small T2-lesions resolved more frequently (187/221 [85%] vs 268/362 [74%]; p = 0.003) and faster (2 [1–6] months vs 4 [1–9] months, p < 0.001) than large T2-lesions. Predictors of T2-lesion resolution varied by T2-lesion size (Figure 2). Data on timing of T2-lesion resolution stratified by phenotype, size, location, and treatment are shown as eAppendix 1 and eFigure 1 in the Supplement (links.lww.com/WNL/C912).
Figure 1. Examples of T2-Lesions Resolving or Persisting Over Time and in Different Locations in Patients With MOGAD.
T2-lesions are shown on T2 fluid-attenuated inversion recovery in the brain and T2-weighted images in the spinal cord. Those T2-lesions persisting at the last follow-up are indicated by a white arrow. An example of early resolution with concomitant development of new lesions is shown in the first follow-up of the resolving lesions in the deep gray matter. m1–4 = follow-up acquired between 1 and 4 months from the baseline imaging; m12 = follow-up acquired at 12 months from the baseline imaging; MOGAD = myelin oligodendrocyte glycoprotein antibody–associated disease.
Figure 2. Forest Plot of Patient and Lesion Factors Associated With T2-Lesion Resolution in MOGAD.
Bars correspond to odds ratio and 95% CIs derived from age- and sex-adjusted generalized estimating equations. The x-axis is in logarithmic scale to improve readability. For T2-lesion location, models were run separately for brain and spinal cord T2-lesions. Significant results are highlighted with * (red for negative associations and green for positive associations). The total number of T2-lesions presenting a certain feature and corresponding patients is also specified in the column on the right. #Odds ratio of T2-lesion resolution of T2-lesions <1 cm are missing in case of optic neuritis, brainstem syndrome, and diffusion restriction as none of the T2-lesions associated with optic neuritis resolved, and all T2-lesions observed during brainstem syndrome or with diffusion restriction resolved. ADEM = acute disseminated encephalomyelitis; EDSS = Expanded Disability Status Scale; IgG = immunoglobulin G; MOG = myelin oligodendrocyte glycoprotein; MOGAD = MOG antibody–associated disease.
Predictors of T2-Lesion Resolution Stratified by Size
Acute T1-hypointensity decreased the likelihood (odds ratio [95% CI]) of T2-lesion resolution independent of size (small: 0.23 [0.09–0.60], p = 0.002; large: 0.30 [0.16–0.55], p < 0.001) (Figure 2). For large T2-lesions acute steroid treatment (1.75 [1.01–3.03], p = 0.046) and deep gray matter location (2.94 [1.28–6.75], p = 0.011) increased the likelihood of resolution, but none of these factors influenced small T2-lesions (Figure 2). Concomitant optic neuritis (0.39 [0.17–0.9], p = 0.028) predicted persistence of large lesions and was associated with no resolution of small lesions. For small T2-lesions, they were more likely to resolve if occurring during acute disseminated encephalomyelitis (ADEM)/multifocal attacks (10.7 [1.8–62.2], p = 0.009). Many other factors analyzed did not influence T2-lesion resolution.
Spontaneous T2-Lesion Resolution
There were 32/55 (58%) T2-lesions that resolved spontaneously in the absence of any acute treatment.
Discussion
In MOGAD, over 75% of T2-lesions resolved often within 6 months. Small T2-lesions were more likely to resolve than large ones. Acute corticosteroids increased the likelihood of large T2-lesions disappearing, while T2-lesions with concomitant acute T1-hypointensity were more likely to persist independent of size. In subgroup analysis of a small minority of patients that did not receive acute treatment between MRIs, 58% of T2-lesions resolved spontaneously suggesting disappearance is part of MOGAD's natural history.3,5-7
Studies have also shown that MOGAD attacks often respond favorably to acute corticosteroid treatment,8 and thus, it is not surprising that it increased the likelihood of large T2-lesions resolving. The lack of impact on small T2-lesions may reflect the capacity of such lesions to spontaneously resolve. Maintenance attack prevention treatments trended toward predicting persistence potentially from a selection bias toward use in more severe cases.
Large deep gray matter T2-lesions were more likely to resolve, and frequent thalamic T2-lesion resolution was reported previously with ADEM in which 50% are typically MOG-IgG positive.9 This may also relate to greater gray matter remyelinating capacity as noted in MS10 or more difficulty in identifying T2-lesions in gray than white matter.
Acute T1-hypointensity coexisting with T2-lesions is a biomarker of tissue disruption11 and strongly predicted T2-lesion persistence independent of size. However, chronic T1-hypointensities remain very rare with MOGAD and are encountered much more often with multiple sclerosis. With concomitant optic neuritis, T2-lesions were less likely to resolve for unclear reasons and could reflect variability in small numbers. Small T2-lesions during cerebral attacks were more likely to resolve vs other phenotypes, which may indicate more active demyelinating T2-lesions occurring in that setting.12 Indeed, analysis of T2-lesion resolution was stratified by size given the overlap between small demyelinating and nondemyelinating T2-lesions, the latter occurring in 5% of all young adults (38% with migraine)13,14 and 47% 50 years and older.15 The explanation for T2-lesions on baseline MRI that did not resolve in follow-up include a few possibilities: (1) persistence of acute MOGAD demyelinating T2-lesions; (2) demyelinating T2-lesions of MOGAD already chronic at the time of baseline MRI; or (3) nondemyelinating T2-lesions from small vessel disease, migraine, or other reasons.
This study provides insight into potential mechanisms of T2-lesion resolution. First, steroids promoted T2-lesion resolution of large T2-lesions, suggesting a potential role of edema in this process. However, the speed of resolution may suggest remyelination as isolated edema would be expected to resolve faster than a median of 3 months. T1-hypointensity correlates with axonal damage on pathology,11 and the persistent T2-lesions could indicate a higher degree of axonal loss overwhelming the capacity for tissue recovery, remyelination, and healing.
For limitations, we acknowledge the retrospective design and the lack of a standardized MRI protocol at regular time intervals, although this reflects the heterogeneity of clinical practice in MRI surveillance in MOGAD disease. Future studies are needed to better characterize T2-lesion dynamics during attacks which was not the focus of this study.
To conclude, this study demonstrates that T2-lesions in MOGAD usually resolve within 1 year and follow-up MRI at 6–12 months showing resolution of all or most T2-lesions can help support a MOGAD diagnosis over competing etiologies.
Appendix. Authors

Study Funding
R01NS113828.
Disclosure
L. Cacciaguerra received speaker and consultant honoraria from ACCMED, Roche, BMS Celgene, and Sanofi. V. Redenbaugh reports no disclosures. J.J. Chen served as consultant for Roche, Horizon, and UCB. P. Morris, E. Sechi, and S.B. Syc-Mazurek report no disclosures. A.S. Lopez-Chiriboga has served on advisory boards for Genentech and Horizon Therapeutics. J.-M. Tillema is associate editor for Journal of Child Neurology. M.A. Rocca received speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla. M. Filippi is editor-in-chief of the Journal of Neurology, associate editor of Human Brain Mapping, Radiology, and Neurological Sciences; received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). S.J. Pittock reports grants, personal fees and nonfinancial support from Alexion Pharmaceuticals, Inc.; grants, personal fees, nonfinancial support and other support from MedImmune, Inc./Viela Bio, Inc.; personal fees for consulting from Genentech/Roche. He has a patent, Patent No. 8,889,102 (Application No. 12-678350, Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia)—issued; a patent, Patent No. 9,891,219B2 (Application No. 12-573942, Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an individual that is Aquaporin-4 [AQP4]-IgG Autoantibody positive)—issued. E.P. Flanagan has served on advisory boards for Alexion, Genentech and Horizon Therapeutics. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. E.P. Flanagan was a site primary investigator in a randomized clinical trial on Inebilizumab in neuromyelitis optica spectrum disorder run by Medimmune/Viela-Bio/Horizon Therapeutics. E.P. Flanagan has received funding from the NIH (R01NS113828). E.P. Flanagan is a member of the medical advisory board of the MOG project. E.P. Flanagan is an editorial board member of the Journal of the Neurological Sciences and Neuroimmunology Reports. A patent has been submitted on DACH1-IgG as a biomarker of paraneoplastic autoimmunity. Go to Neurology.org/N for full disclosures.
References
- 1.Lopez-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018;75(11):1355-1363. doi: 10.1001/jamaneurol.2018.1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks SA, Morris PP, Chen JJ, et al. Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J Neurol Neurosurg Psychiatry. 2020;92(4):384-390. doi: 10.1136/jnnp-2020-325121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sechi E, Krecke KN, Messina SA, et al. Comparison of MRI lesion evolution in different central nervous system demyelinating disorders. Neurology. 2021;97(11):e1097-e1109. doi: 10.1212/wnl.0000000000012467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sechi E, Buciuc M, Pittock SJ, et al. Positive predictive value of myelin oligodendrocyte glycoprotein Autoantibody testing. JAMA Neurol. 2021;78(6):741-746. doi: 10.1001/jamaneurol.2021.0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otani T, Irioka T, Igarashi S, Kaneko K, Takahashi T, Yokota T. Self-remitting cerebral cortical encephalitis associated with myelin oligodendrocyte glycoprotein antibody mimicking acute viral encephalitis: a case report. Mult Scler Relat Disord. 2020;41:102033. doi: 10.1016/j.msard.2020.102033 [DOI] [PubMed] [Google Scholar]
- 6.Takamatsu T, Yamanaka G, Uryu H, et al. Improvement in recurrent anti-myelin oligodendrocyte glycoprotein antibody-positive cerebral cortical encephalitis not requiring anti-inflammatory therapy following the decrease in cytokine/chemokine levels. Mult Scler Relat Disord. 2020;43:102168. doi: 10.1016/j.msard.2020.102168 [DOI] [PubMed] [Google Scholar]
- 7.Salama S, Khan M, Shanechi A, Levy M, Izbudak I. MRI differences between MOG antibody disease and AQP4 NMOSD. Mult Scler. 2020;26(14):1854-1865. doi: 10.1177/1352458519893093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi: 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224-1231. doi: 10.1212/wnl.59.8.1224 [DOI] [PubMed] [Google Scholar]
- 10.Chang A, Staugaitis SM, Dutta R, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72(6):918-926. doi: 10.1002/ana.23693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol. 1997;42(5):783-793. doi: 10.1002/ana.410420515 [DOI] [PubMed] [Google Scholar]
- 12.Sechi E, Buciuc M, Flanagan EP, et al. Variability of cerebrospinal fluid findings by attack phenotype in myelin oligodendrocyte glycoprotein-IgG-associated disorder. Mult Scler Relat Disord. 2021;47:102638. doi: 10.1016/j.msard.2020.102638 [DOI] [PubMed] [Google Scholar]
- 13.Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging. 2006;16(3):243-251. doi: 10.1111/j.1552-6569.2006.00047.x [DOI] [PubMed] [Google Scholar]
- 14.Messina R, Rocca MA, Colombo B, et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013;268(1):170-180. doi: 10.1148/radiol.13122004 [DOI] [PubMed] [Google Scholar]
- 15.Zhuang FJ, Chen Y, He WB, Cai ZY. Prevalence of white matter hyperintensities increases with age. Neural Regen Res. 2018;13(12):2141-2146. doi: 10.4103/1673-5374.241465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from this study will be made available on request.