Abstract
Background and Objectives
The APOE ε4 allele confers susceptibility to faster decline in odor identification and subsequently to Alzheimer disease (AD). Odor identification requires recognizing and naming odors and detecting them (odor sensitivity). Whether APOE ε4 is associated with decline of odor sensitivity and whether such decline serves as a harbinger of cognitive decline and AD remains unclear. We determined whether and when APOE ε4 affects decline in odor sensitivity, odor identification, and cognition in the National Social Life Health and Aging Project (NSHAP).
Methods
We used data from NSHAP, a nationally representative survey study of home-dwelling US older adults. Olfaction was measured over time (odor identification in 2005, 2010, and 2015; odor sensitivity in 2010 and 2015; both using validated tests). Cognition was measured with a modified version of the Montreal Cognitive Assessment in 2010 and 2015. Genotyping was performed using DNA samples collected in 2010. Odor sensitivity and identification were compared among APOE ε4 carriers and noncarriers stratified by age. Relationships between APOE ε4, odor sensitivity, odor identification, and cognition were analyzed in cross-section using ordinal logistic regression and longitudinally using mixed-effects models adjusted for confounders.
Results
Odor sensitivity was measured in 865 respondents, odor identification in 1,156 respondents, and cognition in 864 respondents; all these respondents had genetic data available. Odor sensitivity deficits in APOE ε4 carriers were apparent at ages 65–69 years, whereas odor identification deficits did not appear until ages 75–79 years. Subsequently, odor sensitivity did not decline more rapidly with aging in APOE ε4 carriers compared with that in noncarriers (carrier status and aging interaction: odds ratio [OR] 1.44, 95% CI 0.94–2.19, p = 0.092), whereas odor identification declined more rapidly in carriers (aging 10 years interaction: OR 0.26, 95% CI 0.13–0.52, p < 0.001). As expected, and in parallel to odor identification, cognition declined more rapidly in APOE ε4 carriers (interaction: OR 0.55, 95% CI 0.34–0.89, p = 0.015).
Discussion
APOE ε4 affects decline of odor sensitivity earlier than odor identification or cognition. Thus, testing odor sensitivity may be useful to predict future impaired cognitive function. Identifying the mechanism underlying these relationships will elucidate the key role of olfaction in neurodegeneration during aging.
Introduction
Alzheimer disease (AD) affects 6.2 million Americans aged 65 years or older, a number that is projected to increase to 13.8 million by 2060.1 Identifying early symptoms would enable intervention when expression of the disease is potentially modifiable and help elucidate pathologic mechanisms.2,3 Some of the earliest brain regions to be affected in AD (e.g., by β-amyloid plaques) are associated with olfactory information processing, including the olfactory bulb and the anterior olfactory nucleus.2 In particular, pathology in the entorhinal cortex interferes with the transmission of olfactory information to the hippocampus, a key vulnerable structure in AD.2
Whether the peripheral components (e.g., olfactory epithelium, olfactory bulb) and central components (e.g., entorhinal cortex, hippocampus) of olfaction are affected at the same time or at different points of the AD disease course is unknown. Odor sensitivity and odor identification rely on many overlapping sensory and neurologic processes. However, in a simplified framework, odor sensitivity relies more on intact peripheral sensory function, whereas odor identification requires additional central cognitive functioning (e.g., semantic ability and memory).4,5 In some cross-sectional studies, deficits in odor sensitivity (higher odor thresholds) and odor identification have been linked to AD.6-8 However, a recent meta-analysis asserted that odor identification is more strongly impaired than odor sensitivity in patients with AD.9 Data on odor sensitivity have been mixed. Early on, studies reported no deficits or a later onset of deficits than odor identification.10,11 More recently, AD studies report odor sensitivity deficits present at least as early as odor identification deficits.8,12 Moreover, although strong evidence demonstrates that poor odor identification precedes the development of dementia,13-15 the link between impaired odor sensitivity and dementia development has not been elucidated.
Measuring both aspects of olfaction in older adults at high risk of developing AD may help answer these questions. One important risk factor for developing AD is allelic variation in the APOE gene.16 Of the 3 common alleles (ε2, ε3, and ε4), APOE ε4 is associated with highest AD risk and earlier age at onset in a dose-dependent manner16,17 and with increased risk of cognitive impairment more broadly.18 Notably, ApoE protein is highly expressed in areas involved in olfaction, including the olfactory epithelium and olfactory bulb.19-21
Despite the predictive value of APOE genotype for developing AD, its effects on olfactory performance are still largely unknown because many small-scale studies examining only a single time point have provided inconsistent results. For example, impaired odor identification has been found in older adults with AD carrying 2 APOE ε4 alleles compared with those carrying 1 or no APOE ε4 alleles.22 Furthermore, older APOE ε4 carriers with normal cognition23,24 and mild cognitive impairment also have impaired odor identification.25 By contrast, a recent large (n = 757) cross-sectional multiethnic study in New York found no association between odor identification ability and the APOE ε4 allele.14
Few studies have assessed effects of APOE genotype on odor identification over time. One small study (n = 50) found a faster decline in odor identification across 4 years among older adults carrying 1 APOE ε4 allele relative to noncarriers.26 A study in Sweden reported that odor identification declined rapidly (measured as a simulated 10-year rate based on only 1 measurement) in APOE ε4 homozygotes across all age groups, but that the “rate of decline” was significantly higher in homozygotes relative to noncarriers only in middle age (45–60 years).27 To date, there have been no longitudinal studies using validated odor identification tests over multiple time points to describe the dynamics of odor identification decline in a large diverse sample of APOE ε4 carriers.
Of importance, odor identification deficits observed in APOE ε4 carriers could be explained, wholly or partly, by deficits in the central cognition required for identification. Comparing odor identification and odor sensitivity could help determine whether and when different components of olfactory physiology decline. This comparison may also elucidate whether and how these olfactory processes are related differently to the decline of central cognitive processes. So far, small studies (n = 27–114) exploring odor sensitivity in APOE ε4 carriers are contradictory. In adults without dementia, APOE ε4 did not affect odor sensitivity.23,28 By contrast, APOE ε4 carriers with mild cognitive impairment had worse odor sensitivity as did patients during the year before diagnosis of AD.29 Overall, the effect of APOE ε4 carrier status on odor sensitivity remains controversial.2
Large-scale longitudinal studies on odor sensitivity in APOE ε4 carriers using diverse well-characterized populations would be useful (1) to examine this component of olfactory function in patients at high genetic risk for AD and (2) to explore the mechanisms by which the olfactory system plays a role in the prodrome of AD. Therefore, to address these questions, we examined the relationships between APOE ε4 genotype and odor sensitivity, odor identification, and cognition in the National Social Life Health and Aging Project (NSHAP), an ethnically diverse, nationally representative longitudinal study of older US adults living at home.
Methods
Using data from NSHAP collected every 5 years between 2005 and 2015, we performed an epidemiologic study exploring odor sensitivity, odor identification, and cognition both cross-sectionally and over time in carriers and noncarriers of the APOE ε4 genotype.
NSHAP
NSHAP is a longitudinal study of health and social life in a nationally representative survey sample of home-dwelling US older adults. Field interviewers from the National Opinion Research Center (NORC) at the University of Chicago conducted in-home interviews and collected biomeasures (including olfaction and cognition) from 3,005 initial respondents born between 1920 and 1947 (respondents born in this time frame are considered “age eligible”) in 2005–2006.30 After eligible households from across the United States were identified using a classic multistage area probability sample, respondents were selected to produce a sample that was balanced across age and gender subgroups with an oversampling of African Americans and Latinos.30 Initial respondents were surveyed again 5 years later (2010–2011) when their partners were added to the survey (n = 3,377).31,32 In 2015–2016, the survey collected 10-year follow-up data from initial respondents and 5-year follow-up from their partners in addition to adding a new cohort born between 1948 and 1965 (n = 4,777; 2,409 returning respondents).33 Because NSHAP is a nationally representative sample, and survey data were weighted to correct for sampling strategy, the results reported reflect characteristics of the diverse US older adults living in their homes.30
Standard Protocol Approvals, Registrations, and Patient Consents
NSHAP was approved by the University of Chicago and NORC Institutional Review Boards, and all respondents provided written informed consent.
Genotyping
Oragene saliva samples were collected in 2010 (DNA Genotek Inc., Ontario, Canada). DNA was extracted by GenoFIND (DNA Genotek Inc.) and stored in aliquots at −80°C until genotyping was performed using Illumina's Infinium Global Screening Array v1.0 BeadChip at the Robert S. Boas Center for Genomics and Human Genetics at the Feinstein Institutes for Medical Research (Manhasset, NY). APOE genotypes were determined using imputed data for the rs429358 and rs7412 SNPs. Each APOE allele is defined by a haplotype of these 2 SNPs, as has been described elsewhere.34
Imputation was performed as part of a much larger set of samples from individuals with inflammatory bowel disease (IBD) and unrelated controls. Preimputation quality control involved removing the following: (1) A/T and C/G genotyped variants with minor allele frequency (MAF) ≥0.45 in 1000 Genomes Project (1000GP) EUR subset; (2) variants with a call rate <0.95 (or <0.98 call rate for variants with MAF <0.01); (3) variants with a significant difference in genotype call rate between cases with IBD and controls (p value <1 × 10−4); (4) variants with allele frequency differences vs those reported in gnomAD non-Finnish Europeans or TOPMed global MAF (using the criterion [(p1 − p0)^2]/[(p1 + p0) × (2 − p1 − p0)] >0.025 and >0.125, respectively, where p0 is the MAF in the reference panel, and p1 is the observed MAF in the study); (5) variants with a Hardy-Weinberg equilibrium p value <10−5 among controls (non-IBD patients) and 10–12 among cases; and (6) monomorphic variants. We also excluded entire samples (1) with a missing genotype rate >0.5 and/or (2) a heterozygosity estimate ±4 SDs from the mean (per 1000GP continental population). Nondirectly genotyped data were imputed within 1000GP PCA–derived continental ancestry groups using the TOPMed reference panel (r2@1.0.0) through the TOPMed imputation server (imputationserver@1.5.7). After the first round of imputation, genotyped variants with an empirical R2 <0.5 were excluded, and imputation was repeated after correcting strand issues at genotyped variants with an empirical R <−0.5. R2 was 0.99 for both rs429358 and rs7412.
PLINK version 1.0735 was used to convert genotype data for analysis in Stata 15.0 (StataCorp LLC, College Station, TX). 2,284 of the 3,377 total respondents in 2010 were randomly selected to provide Oragene saliva samples, 83% of whom (n = 1,876) successfully provided these saliva samples for genotyping.36 After genotyping and imputation, APOE genotypes were available for 98% (n = 1,830) of these respondents; 1,722 of these respondents were age eligible and included in analyses.
Olfaction
Odor Sensitivity
Odor sensitivity was quantified with the Olfactory Function Field Examination, which included a validated threshold test designed for in-home large-scale survey testing.37,38 Steadily increasing concentrations of n-butanol were presented in felt-tip pens in a triad with 2 blank pens (Sniffin-Sticks; Burghart Medical Technology, Wedel, Germany). The total number of correctly detected odors were scored (range 0–6), with higher scores indicating greater odor sensitivity.37,38 Odor sensitivity was first measured in 2010 (sensitivity baseline) and at a 5-year follow-up in 2015 (n = 865 initial respondents and their partners with APOE genotype data available).
Odor Identification
The ability to identify odors with words or pictures (odor identification) was also measured with the Olfactory Function Field Examination, which included a validated measure of a respondent's accuracy identifying common odorants presented in felt-tip pens (Sniffin' Sticks).37 The number of correctly identified odors was scored (range 0–5) and analyzed in cross-section from 1,156 respondents in 2010 (888 initial respondents and 268 partners with APOE genotype data available) and longitudinally for the 870 with a 5-year follow-up in 2015. A 10-year longitudinal analysis included data from 1,156 respondents with APOE genotype data available (602 initial respondents in 2005 (identification baseline), 2010, and 2015; 286 initial respondents in 2005 and 2010; 268 partners in 2010 and 2015). The results of this 10-year longitudinal analysis were confirmed by applying an identical model to only the 602 initial respondents with 3 data points. More detailed information regarding odor sensitivity and odor identification assessment in NSHAP is presented elsewhere.37,39
Cognition
Cognition was measured using an 18-item survey-adapted version of the Montreal Cognitive Assessment (MoCA-SA), a validated measure that can be administered easily by personnel without medical training32; scores closely correlate with those from the full MoCA.32,40,41 The MoCA-SA was added in 2010 (cognition baseline) and repeated in 2015 (5-year follow-up) for 864 initial respondents and partners with APOE genotype data available.
Statistical Analyses
Cross-sectional Analyses
Table 1 summarizes the population characteristics of age-eligible respondents providing genetic, olfactory, and cognitive data in 2010 (n = 1,275). In general, the reduction of sample sizes in our study relative to the total number of respondents in NSHAP is largely due to (1) random subsets of respondents being assigned to genetic testing and olfaction testing in Wave 2 (approximately 50% were assigned to both genetic and olfaction testing36) and (2) restricting analyses to respondents with cognition, olfaction, and genetic data available. While this strategy may have led to some missing data, the fact that this missing data were largely introduced through a randomized process makes it less likely to introduce bias.
Table 1.
Demographic Characteristics of Age-Eligible NSHAP Participants in 2010 With Genotype, Olfaction, and Cognition Data Available: The Estimated % of the US Population of Home-Dwelling Older Adults

Effect of APOE genotype on olfactory measures and cognition was tested using data from 2010, which is when odor sensitivity, odor identification, cognition, and genotype data were all collected. Ordinal logistic regression models were constructed to compare the associations of each APOE genotype with odor sensitivity, odor identification, and cognition. ε3/ε3, the most common genotype, was used as the reference genotype. All respondents with at least 1 APOE ε4 allele were included in the APOE ε4 carrier group. Of note, the carrier group included respondents with the ε2/ε4 genotype, who have both a protective allele and a pathogenic allele. Although the AD risk in this group is disputed, some evidence shows that this genotype is associated with increased AD risk.42
Unless otherwise stated, all analyses were adjusted for age, sex (self-identified), race/ethnicity (self-identified, standard NIH categories), and level of education (defined by highest degree or certification obtained). To help distinguish the effects of APOE genotype on the different components of the olfactory system, odor sensitivity and odor identification analyses were performed with and without adjustment for cognition (as a time-varying variable). A Spearman rank correlation coefficient was calculated between odor sensitivity and odor identification in 2010 based on the analytic sample (n = 1,275). To determine the ages when APOE ε4 carrier status affected odor sensitivity and odor identification change, we compared mean odor identification and odor sensitivity scores across 5-year age categories: 65–69, 70–74, 75–79, 80–84, and 85–89 years.
Longitudinal Analyses
Mixed-effects ordinal logistic regression models were used to assess the effects of APOE ε4 carrier status on odor sensitivity (aging over 5 years), odor identification (aging over 10 years), and cognition (aging over 5 years). The effect of APOE ε4 carrier status on the rate of olfactory decline was assessed with an interaction term between carrier status and years from baseline. Specifically, each model included terms for APOE ε4 carrier status (carrier vs. noncarrier), time (5-year follow-up vs. baseline and for the odor identification model, also 10-year follow-up vs. baseline), and the time by carrier status interaction plus covariates and a random subject effect. With this parameterization and using the odor sensitivity model as an example, the odds ratio (OR) for the APOE ε4 carrier status term can be interpreted as that for carriers (vs. noncarriers) at baseline, and the OR for the time term can be interpreted as that for aging 5 years among noncarriers. For ease of presentation in both tables and figures, the effect of aging 5 years among carriers, a function of the time and time by carrier status interaction terms, is also provided. Models for odor sensitivity and odor identification were each adjusted for cognition (as a time-varying variable) when data were available (2010 and 2015); the latter considered a sensitivity analysis of the model using all 10 years of data. All longitudinal analyses included the same covariates: age at baseline, sex, race/ethnicity, and education.
Data Availability
Nongenetic NSHAP data used in this study is currently available through the National Archive of Computerized Data on Aging (NACDA; icpsr.umich.edu/web/pages/NACDA/nshap.html). The APOE data are in the process of being submitted to the NACDA; in the meantime, requests can be directed to the corresponding author.
Results
Effects of APOE ε4 and Aging: Cross-sectional Analyses
Older US adults carrying an APOE ε4 allele had worse odor sensitivity compared with noncarriers (OR 0.63, 95% CI 0.49–0.81; Table 2). Specifically, heterozygotes had worse odor sensitivity relative to those with the ε3/ε3 reference genotype (ε3/ε4: OR 0.64, 95% CI 0.49–0.82; ε2/ε4: OR 0.31, 95% CI 0.15–0.64; Table 2). This association was already pronounced among APOE ε4 carriers aged 65–69 years (Figure 1A).
Table 2.
Effect of APOE ε4 on the Odds Ratios for Good Odor Sensitivity, Odor Identification, and Cognition Among Older US Adults in 2010
Figure 1. Age Differences Among US Older Adults (2010) in the Effect of APOE ε4 Status on (A) Odor Sensitivity (Mean # Correct ± SEM) and (B) Odor Identification (Mean # Correct ± SEM).

Unadjusted estimates are displayed. ε4+ = APOE ε4 carrier; ε4− = APOE ε4 noncarrier; SEM = standard error of the mean.
By contrast, there was no association between odor identification and APOE ε4 carrier status overall (Table 2). Rather, odor identification deficits in APOE ε4 carriers only became evident at 75–79 years of age, indicating that odor identification deficits in APOE ε4 carriers develop later in life than odor sensitivity deficits (Figure 1B). Of note, the calculated Spearman rank correlation coefficient (r = 0.27) indicated that odor sensitivity was weakly correlated with odor identification.
In cross-section, we did not detect worse cognitive function among APOE ε4 carriers living at home and capable of a 2-hour survey interview. We hypothesized that repeated measures of cognition within an individual would have more power to detect its decline and association with genotype and aging.
Effects of APOE ε4 Allele and Aging: Longitudinal Analyses
Longitudinal analyses confirmed worse odor sensitivity in APOE ε4 carriers at sensitivity baseline (OR 0.59, 95% CI 0.39–0.90; Figure 2A, eTable 1, links.lww.com/WNL/C985). On aging 5 years, carriers did not decline further, retaining their poor performance developed at younger ages (OR 0.90, 95% CI 0.58–1.40; eTable 1). By contrast, noncarriers did decline over 5 years (OR 0.63, 95% CI 0.47–0.85; eTable 1). The different aging trajectories for carriers and noncarriers persisted after adjusting for cognition (eTable 1), indicating a direct association between APOE ε4 and odor sensitivity beyond any effects on general cognitive function.
Figure 2. (A) Odor Sensitivity: Effect of APOE ε4 Carrier Status on Odds of Good Odor Sensitivity at Sensitivity Baseline and After Aging 5 Years; (B) Odor Identification: Effect of APOE ε4 Status on the Odds of Having Good Odor Identification at Identification Baseline and After Aging 5 or 10 Years.

Odor sensitivity: interaction of carrier status and aging 5 years: p = 0.092. Odor identification: interaction of carrier status and aging 5 years: p = 0.077; interaction of carrier status and aging 10 years: p < 0.001. Aging effects are independent of baseline differences. *p < 0.05; **p < 0.01; ***p < 0.001. ε4+ = APOE ε4 carrier; ε4− = APOE ε4 noncarrier.
By contrast, odor identification was similar in APOE ε4 carriers and noncarriers at identification baseline (OR 1.57, 95% CI 0.89–2.77; Figure 2B, eTable 2, links.lww.com/WNL/C985) but declined more rapidly in APOE ε4 carriers over 10 years (interaction of carrier status and aging 10 years: OR 0.26, 95% CI 0.13–0.52, p < 0.001; Figure 2B, eTable 2). Although APOE ε4 carriers tended toward losing odor identification more rapidly during the first 5 years (interaction of carrier status and aging 5 years: OR 0.57, 95% CI 0.31–1.06, p = 0.077), their decline accelerated in the next 5 years (interaction of carrier status and aging 10 years: OR 0.26, 95% CI 0.13–0.52, p < 0.001) (Figure 2B, eTable 2). That is, the effect of genotype became larger with aging. As with odor sensitivity, increased rate of decline in odor identification during the final 5 years of follow-up persisted after adjusting for cognition (eTable 3).
As with odor identification, cognition was not worse in APOE ε4 carriers at cognition baseline (OR 1.08, 95% CI 0.62–1.91; Figure 3, eTable 4, links.lww.com/WNL/C985) but declined more rapidly over the next 5 years in carriers (interaction of APOE ε4 carrier status and aging 5 years: OR 0.55, 95% CI 0.34–0.89, p = 0.015; Figure 3, eTable 4). As expected, better cognition was associated with better odor identification (OR 1.17, 95% CI 1.11–1.23; eTable 3, links.lww.com/WNL/C985), but there was no association between cognition and odor sensitivity (OR 1.01, 95% CI 0.98–1.06; eTable 1).
Figure 3. Cognition: The Effect of APOE ε4 Carrier Status on the Odds of Having Good Cognition (Higher MoCA-SA Score) at Cognition Baseline and on Aging 5 Years.
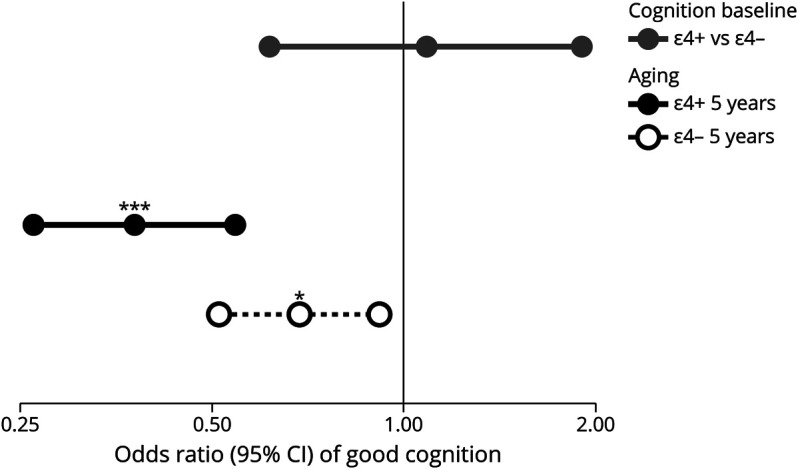
Interaction of carrier status and aging 5 years: p = 0.015. Aging effects are independent of baseline differences. *p < 0.05; **p < 0.01; ***p < 0.001. ε4+ = APOE ε4 carrier; ε4− = APOE ε4 noncarrier; MoCA-SA = survey-adapted Montreal Cognitive Assessment.
Discussion
This nationally representative study describes temporal relationships between APOE ε4 status and declines in odor sensitivity, odor identification, and cognition among diverse older adults living at home in the United States. Odor sensitivity deficits manifested 10 years earlier than odor identification deficits in APOE ε4 carriers in cross-sectional analysis. Specifically, odor sensitivity was already worse in APOE ε4 carriers at 65–69 years of age. Over time, carriers did not experience additional odor sensitivity loss, whereas odor sensitivity of noncarriers declined significantly. Thus, previous studies focused on older ages23,26,28,29 would have been unlikely to detect the accelerated decline of odor sensitivity in APOE ε4 carriers, which happens before the age of 65 years, a difference that is subsequently obscured by age-related decline in sensitivity among noncarriers. These results suggest that earlier odor sensitivity testing may identify at-risk patients, which could inform studies of the early stages of AD and identify patients for early intervention therapies.
By contrast, APOE ε4 had no detectable effect on odor identification until 75–79 years of age in cross-sectional analyses. Longitudinally, the odor identification of APOE ε4 carriers declined more rapidly over 5 and 10 years. These findings, based on repeated olfaction measurements from the large, diverse population of US adults, help clarify prior discrepancies in the literature surrounding odor identification deficits in APOE ε4 carriers because many prior studies have been conducted in small,23,25,26 ethnically homogenous,24,25,27 or geographically isolated14,24-27 populations. As expected,3 a more rapid decline in cognition, rather than baseline differences, was most strongly associated with APOE ε4 in this diverse population.
These results indicate that odor sensitivity was impaired before a rapid decline in odor identification in APOE ε4 carriers. Furthermore, this sequence persisted in APOE ε4 carriers even after adjusting for cognition, indicating that the olfactory deficits observed in APOE ε4 carriers are initially independent of a decline in general cognitive function. It should be noted that the classification of odor sensitivity as a “peripheral” process and odor identification as a “central” process is likely an oversimplification that ignores significant overlap in the sensory and neurologic processes underlying these olfactory processes.43,44 However, dichotomizing the 2 processes in this way can help illuminate the differences we demonstrated between these 2 processes in APOE ε4 carriers.
The peripheral mechanisms underlying the initial decline in olfactory sensitivity likely involve the presence of ApoE in the nerve fascicles, Schwann cells, supporting cells and vasculature of the olfactory epithelium, and the olfactory nerve and glomeruli of the olfactory bulb.19-21 In transgenic mouse models, carriers of the human APOE ε4 allele had impaired nerve regeneration and olfactory processing. Odor-evoked responses in their olfactory bulbs were markedly higher at young ages, indicating attenuated inhibition in peripheral olfactory pathways. By late middle age, APOE ε4–carrying mice had also developed attenuated inhibition in the piriform cortex, the next node communicating olfactory information bidirectionally with the olfactory bulb through entrained oscillations.45,46
In humans, young and middle aged APOE ε4 carriers also had altered olfactory event–related potentials (OERPs) during an odor detection task,47 consistent with the odor sensitivity changes observed in the carriers of our study. By contrast, another study of OERP using an odor identification task as the olfactory stimulus across 3 different age groups revealed increased OERP changes in APOE ε4 carriers in the oldest age group, with these changes increasing more quickly in APOE ε4 carriers over time.48 Together, these findings support the concept that peripheral olfactory structures (e.g., the olfactory epithelium and bulb) are affected first in APOE ε4 carriers and that more advanced cortical pathology is superimposed on top of the already damaged peripheral olfactory systems. This concept requires further testing in the future.
One of the major strengths of our study is the multiethnic makeup of the NSHAP survey population, especially because different demographic groups are known to exhibit different patterns of olfactory and cognitive decline. For example, deficits in odor identification have been noted to be more prevalent and to emerge earlier in African Americans even when adjusting for sex, education, memory, and various aspects of physical and mental health.49 There is a critical need to identify social and environmental insults that accelerate aging of olfactory function. Indeed, social and environmental risk factors obscure the contribution of APOE ε4 to dementia risk in underserved populations.50 Unfortunately, our sample sizes were too small to contribute to the needed evaluation of genetic, social, and environmental factors driving these health disparities. Our next round of NSHAP data collection (2021–2023) will provide a 10-year follow-up for odor sensitivity and cognition and a 15-year follow-up for odor identification. While our survey represents US older adults living at home, we could not survey those with the frank dementia found in clinical studies, which obviates participation in a challenging 2-hour interview. The statistical models reported in this study did not correct for the dropout of participants who developed dementia severe enough to prevent continuing in the study. Correcting for this dropout would likely further increase the effect size of APOE ε4 on odor sensitivity, odor identification, and cognition. Last, we acknowledge that, although our models were adjusted for many confounders, there are likely relevant confounders that we were unable to include.
In summary, we present evidence that APOE ε4 carriers among diverse US older adults living at home lose their odor sensitivity well before rapid declines in odor identification and cognition. Future work focused on the neural mechanisms underlying these early manifestations are needed to further understand the effect of APOE allelic variation on both components of olfaction and neurodegeneration. Although rates of decline in odor sensitivity and identification need to be quantified as predictors of AD, these longitudinal data nonetheless provide compelling evidence for the clinical utility of repeatedly testing olfactory and cognitive function in at-risk populations. In APOE ε4 carriers, odor sensitivity particularly may be a useful early indicator of further olfactory dysfunction, cognitive decline, and ultimately neurodegenerative disease, especially in studies aiming to identify at-risk patients early in the disease course.
Acknowledgment
The authors thank the operations team of NORC at the University of Chicago for their vital role in conducting NSHAP field surveys. The authors also gratefully acknowledge the contributions of NSHAP respondents. Members of the Olfactory Research Group provided useful feedback and encouragement. The authors gratefully acknowledge the NIDDK-funded Inflammatory Bowel Disease Genetics Consortium Data Coordinating Center (Judy Cho, PI; U24DK062429) for genotyping the samples analyzed here, and Laura Fachal of the Wellcome Sanger Institute for performing the QC and imputation.
Glossary
- 1000GP
1000 Genomes Project
- AD
Alzheimer disease
- IBD
inflammatory bowel disease
- MAF
minor allele frequency
- MoCA
Montreal Cognitive Assessment
- MoCA-SA
survey-adapted Montreal Cognitive Assessment
- NACDA
National Archive of Computerized Data on Aging
- NORC
National Opinion Research Center
- NSHAP
National Social Life Health and Aging Project
- OERP
olfactory event–related potential
- OR
odds ratio
- SNP
single-nucleotide polymorphism
Appendix. Authors

Study Funding
National Institute on Aging and the NIH (AG021487; AG030481; AG033903; AG043538); and National Institutes of Diabetes, Digestive and Kidney Diseases (U24DK062429).
Disclosure
M.S. GoodSmith discloses research funding from the University of Chicago Pritzker School of Medicine's Scholarship and Discovery program. K.E. Wroblewski discloses research funding from the following: NIA R01 AG048511; NIA R01 AG060756; NIA R01 AG043538; NIA R01 AG033903. L.P. Schumm discloses research funding from the following: NIA R01 AG048511, NIDDK U24 DK062429, NIA 2R01AG043538-11. M.K. McClintock discloses research funding from the following: NIA R37 AG030481; NIA R01 AG043538; NIA R01 AG048511, NIA 2R01AG043538-11. J.M. Pinto discloses research funding from the following: NIA T35 AG029795; NIA R01 AG043538; AHRQ T32 HS000084; NIA R01 AG048511; NIA R01 AG067497; and NIA P30AG066619-01. Go to Neurology.org/N for full disclosures.
References
- 1.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327-406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 2.Murphy C. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. 2019;15(1):11-24. doi: 10.1038/s41582-018-0097-5 [DOI] [PubMed] [Google Scholar]
- 3.Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol. 2013;9(2):106-118. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32(10):1062-1067. doi: 10.1080/13803391003683070 [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Liu J, Wroblewski KE, McClintock MK, Pinto JM. Odor sensitivity versus odor identification in older US adults: associations with cognition, age, gender, and race. Chem Senses. 2020;45(4):321-330. doi: 10.1093/chemse/bjaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol Aging. 1990;11(4):465-469. doi: 10.1016/0197-4580(90)90014-q [DOI] [PubMed] [Google Scholar]
- 7.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55(1):84-90. doi: 10.1001/archneur.55.1.84 [DOI] [PubMed] [Google Scholar]
- 8.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2008;29(5):693-706. doi: 10.1016/j.neurobiolaging.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 9.Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer's disease and Parkinson's disease on olfaction: a meta-analysis. Behav Brain Res. 2012;231(1):60-74. doi: 10.1016/j.bbr.2012.02.047 [DOI] [PubMed] [Google Scholar]
- 10.Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry. 1991;148(3):357-360. doi: 10.1176/ajp.148.3.357 [DOI] [PubMed] [Google Scholar]
- 11.Larsson M, Semb H, Winblad B, Amberla K, Wahlund L-O, Bäckman L. Odor identification in normal aging and early Alzheimer's disease: effects of retrieval support. Neuropsychology. 1999;13(1):47-53. doi: 10.1037/0894-4105.13.1.47 [DOI] [PubMed] [Google Scholar]
- 12.Förster S, Vaitl A, Teipel SJ, et al. Functional representation of olfactory impairment in early Alzheimer's disease. J Alzheimers Dis. 2010;22(2):581-591. doi: 10.3233/JAD-2010-091549 [DOI] [PubMed] [Google Scholar]
- 13.Schubert CR, Carmichael LL, Murphy C, Klein BEK, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56(8):1517-1521. doi: 10.1111/j.1532-5415.2008.01826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015;84(2):182-189. doi: 10.1212/WNL.0000000000001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams DR, Kern DW, Wroblewski KE, McClintock MK, Dale W, Pinto JM. Olfactory dysfunction predicts subsequent dementia in older U.S. adults. J Am Geriatr Soc. 2018;66(1):140-144. doi: 10.1111/jgs.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med. 1996;47(1):387-400. doi: 10.1146/annurev.med.47.1.387 [DOI] [PubMed] [Google Scholar]
- 17.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921-923. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 18.Henderson AS, Jorm AF, Korten AE, et al. Apolipoprotein E allele ɛ4, dementia, and cognitive decline in a population sample. Lancet. 1995;346(8987):1387-1390. doi: 10.1016/S0140-6736(95)92405-1 [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi M, Getchell ML, Takami S, Getchell TV. Increased density of olfactory receptor neurons immunoreactive for apolipoprotein E in patients with Alzheimer's disease. Ann Otol Rhinol Laryngol. 1998;107(5):421-426. doi: 10.1177/000348949810700511 [DOI] [PubMed] [Google Scholar]
- 20.Nathan BP, Nannapaneni S, Gairhe S, Nwosu I, Struble RG. The distribution of apolipoprotein E in mouse olfactory epithelium. Brain Res. 2007;1137:78-83. doi: 10.1016/j.brainres.2006.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Struble RG, Short J, Ghobrial M, Nathan BP. Apolipoprotein E immunoreactivity in human and mouse olfactory bulb. Neurosci Lett. 1999;267(2):137-140. doi: 10.1016/S0304-3940(99)00352-3 [DOI] [PubMed] [Google Scholar]
- 22.Oleson S, Murphy C. Olfactory dysfunction in ApoE ɛ4/4 homozygotes with Alzheimer's disease. J Alzheimers Dis. 2015;46(3):791-803. doi: 10.3233/JAD-150089 [DOI] [PubMed] [Google Scholar]
- 23.Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann NY Acad Sci. 1998;855:744-750. doi: 10.1111/j.1749-6632.1998.tb10654.x [DOI] [PubMed] [Google Scholar]
- 24.Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-ɛ4 is independent of clinical dementia. Neurobiol Aging. 2010;31(4):567-577. doi: 10.1016/j.neurobiolaging.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 25.Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN. Olfactory identification and apolipoprotein E ε4 allele in mild cognitive impairment. Brain Res. 2002;951(1):77-81. doi: 10.1016/S0006-8993(02)03137-2 [DOI] [PubMed] [Google Scholar]
- 26.Calhoun-Haney R, Murphy C. Apolipoprotein ε4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cogn. 2005;58(2):178-182. doi: 10.1016/j.bandc.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 27.Josefsson M, Larsson M, Nordin S, Adolfsson R, Olofsson J. APOE-ɛ4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Sci Rep. 2017;7(1):1286. doi: 10.1038/s41598-017-01508-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert PE, Murphy C. The effect of the ApoE ε4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer's disease, probable Alzheimer's disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26(6):779-794. doi: 10.1080/13803390490509439 [DOI] [PubMed] [Google Scholar]
- 29.Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfactiona. Ann NY Acad Sci. 1998;855:723-731. doi: 10.1111/j.1749-6632.1998.tb10651.x [DOI] [PubMed] [Google Scholar]
- 30.O'Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2009;64B(suppl 1):i12-i19. doi: 10.1093/geronb/gbp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Muircheartaigh C, English N, Pedlow S, Kwok PK. Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S15-S26. doi: 10.1093/geronb/gbu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotwal AA, Schumm P, Kern DW, et al. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 2015;29(4):317-324. doi: 10.1097/WAD.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Muircheartaigh C, English N, Pedlow S, Schumm LP. Sample design and estimation in the National Social Life, Health, and Aging Project: round 3 (2015-2016). J Gerontol B Psychol Sci Soc Sci. 2021;76(suppl 3):S207-S214. doi: 10.1093/geronb/gbab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seripa D, Matera MG, Daniele A, et al. The missing ApoE allele. Ann Hum Genet. 2007;71(4):496-500. doi: 10.1111/j.1469-1809.2006.00344.x [DOI] [PubMed] [Google Scholar]
- 35.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaszczak A, O'Doherty K, Colicchia M, et al. Continuity and innovation in the data collection protocols of the second Wave of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S4-S14. doi: 10.1093/geronb/gbu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern DW, Wroblewski KE, Schumm LP, Pinto JM, McClintock MK. Field survey measures of olfaction: the Olfactory Function Field Exam (OFFE). Field Methods. 2014;26(4):421-434. doi: 10.1177/1525822X14547499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern DW, Schumm LP, Wroblewski KE, Pinto JM, Hummel T, McClintock MK. Olfactory thresholds of the U.S. population of home-dwelling older adults: development and validation of a short, reliable measure. PLoS One. 2015;10(3):e0118589. doi: 10.1371/journal.pone.0118589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Chen RC, McClintock MK. Olfactory function in Wave 2 of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S134-S143. doi: 10.1093/geronb/gbu093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 41.Dale W, Kotwal AA, Shega JW, et al. Cognitive function and its risk factors among older US adults living at home. Alzheimer Dis Assoc Disord. 2018;32(3):207-213. doi: 10.1097/WAD.0000000000000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- 43.Doty RL, Smith R, McKeown DA, Raj J. Tests of human olfactory function: principal components analysis suggests that most measure a common source of variance. Percept Psychophys. 1994;56(6):701-707. doi: 10.3758/bf03208363 [DOI] [PubMed] [Google Scholar]
- 44.Martinez BA, Cain WS, de Wijk RA, Spencer DD, Novelly RA, Sass KJ. Olfactory functioning before and after temporal lobe resection for intractable seizures. Neuropsychology. 1993;7(3):351-363. doi: 10.1037/0894-4105.7.3.351 [DOI] [Google Scholar]
- 45.Peng KY, Mathews PM, Levy E, Wilson DA. Apolipoprotein E4 causes early olfactory network abnormalities and short-term olfactory memory impairments. Neuroscience. 2017;343:364-371. doi: 10.1016/j.neuroscience.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iravani B, Arshamian A, Lundqvist M, Kay LM, Wilson DA, Lundström JN. Odor identity can be extracted from the reciprocal connectivity between olfactory bulb and piriform cortex in humans. Neuroimage. 2021;237:118130. doi: 10.1016/j.neuroimage.2021.118130 [DOI] [PubMed] [Google Scholar]
- 47.Corby K, Morgan CD, Murphy C. Abnormal event-related potentials in young and middle-aged adults with the ApoE ε4 allele. Int J Psychophysiol. 2012;83(3):276-281. doi: 10.1016/j.ijpsycho.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan CD, Murphy C. Individuals at risk for Alzheimer's disease show differential patterns of ERP brain activation during odor identification. Behav Brain Funct. 2012;8(1):37. doi: 10.1186/1744-9081-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014;69A(3):323-329. doi: 10.1093/gerona/glt063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang MX, Stern Y, Marder K, et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751-755. doi: 10.1001/jama.279.10.751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nongenetic NSHAP data used in this study is currently available through the National Archive of Computerized Data on Aging (NACDA; icpsr.umich.edu/web/pages/NACDA/nshap.html). The APOE data are in the process of being submitted to the NACDA; in the meantime, requests can be directed to the corresponding author.



