Abstract
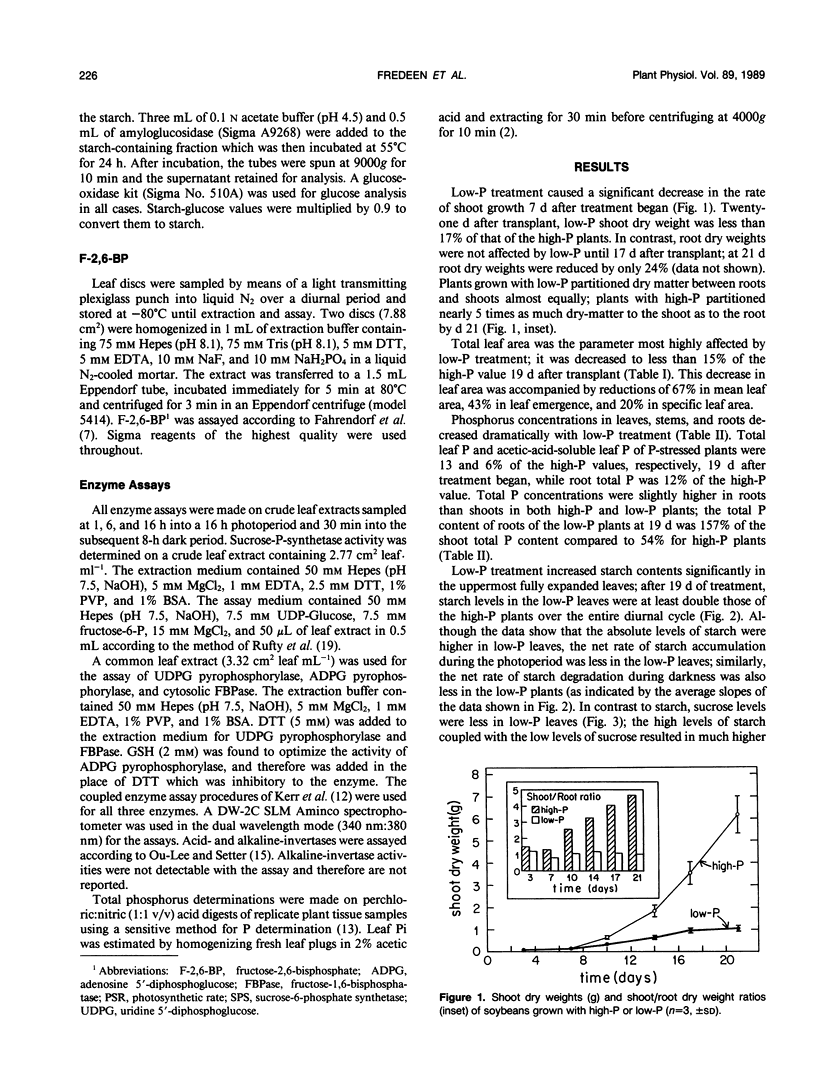
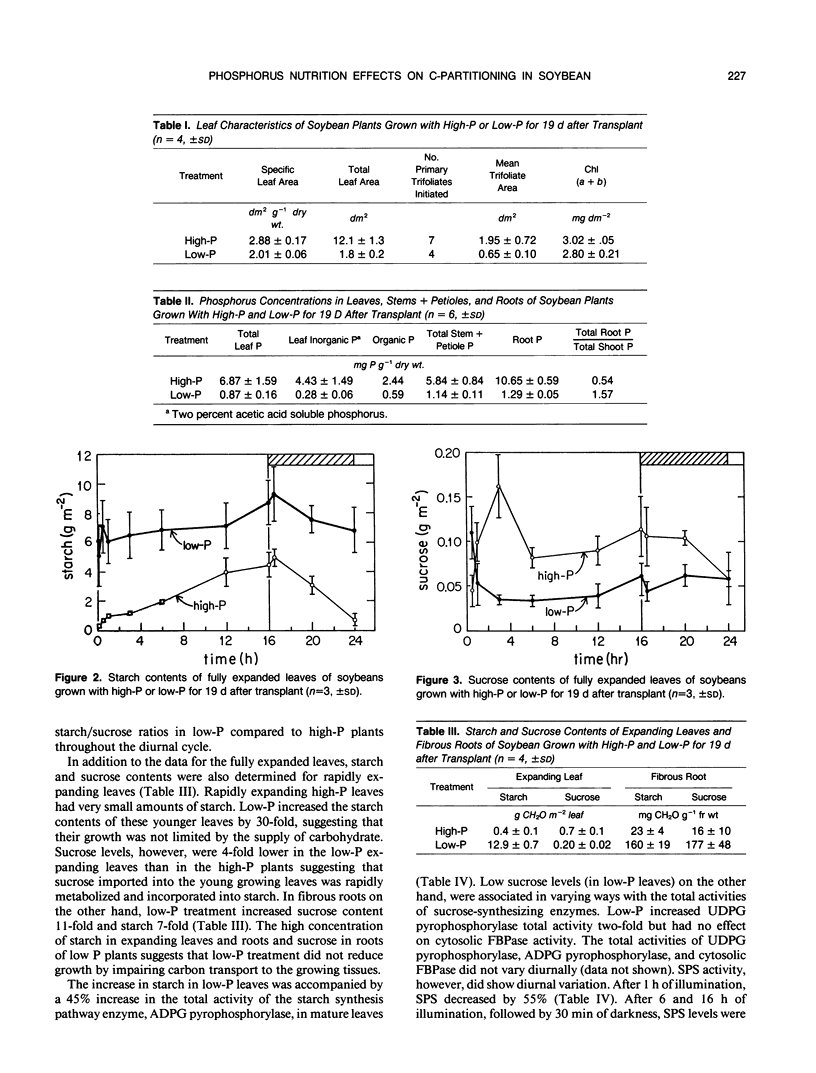
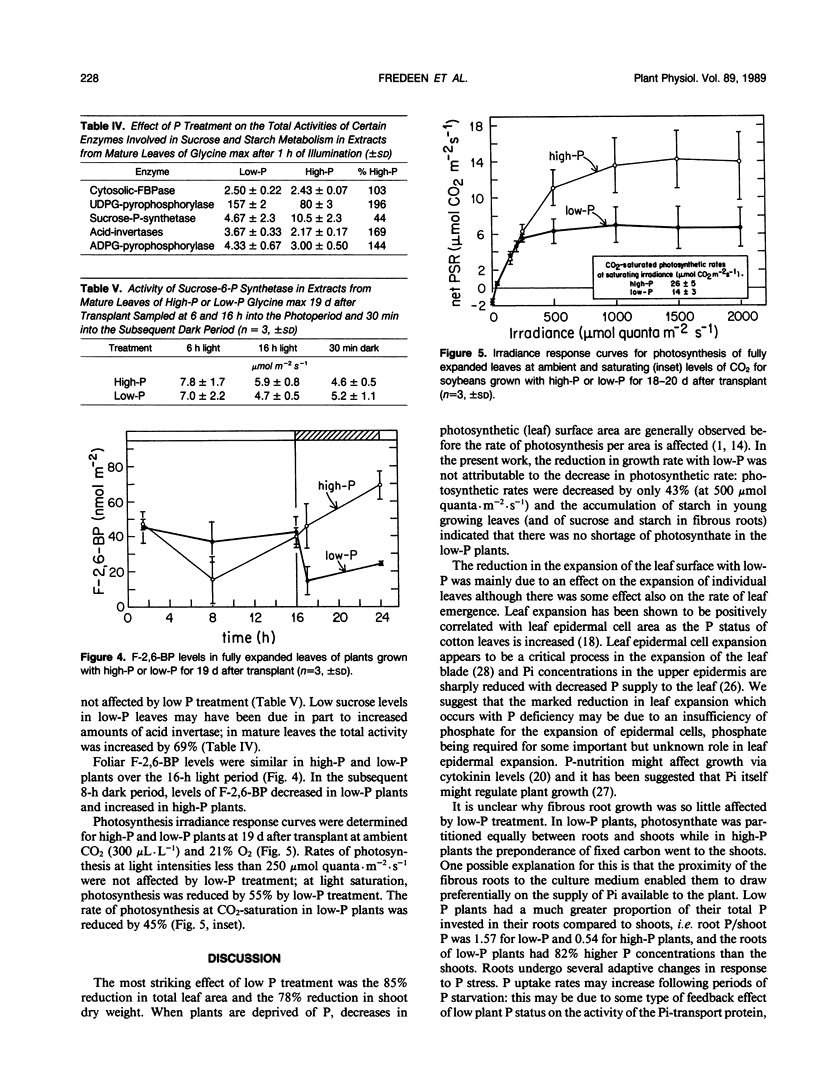
Soybean plants (Glycine max [L.] Merr var Amsoy 71) were grown in growth chambers with high-phosphorus (high-P) and low-phosphorus (low-P) culture solutions. Low-P treatment reduced shoot growth significantly 7 days after treatment began. Root growth was much less affected by low-P, there being no significant reduction in root growth rate until 17 days had elapsed. The results suggest that low-P treatment decreased soybean growth primarily through an effect on the expansion of the leaf surface which was diminished by 85%, the main effect of low-P being on the rate of expansion of individual leaves. Low-P had a lesser effect on photosynthesis; light saturated photosynthetic rates at ambient and saturating CO2 levels were lowered by 55 and 45%, respectively, after 19 days of low-P treatment. Low-P treatment increased starch concentrations in mature leaves, expanding leaves and fibrous roots; sucrose concentrations, however, were reduced by low-P in leaves and increased in roots. Foliar F-2,6-BP levels were not affected by P treatment in the light but in darkness they increased with high-P and decreased with low-P. The increase in the starch/sucrose ratio in low-P leaves was correlated primarily with changes in the total activities of enzymes of starch and sucrose metabolism.
Full text
PDF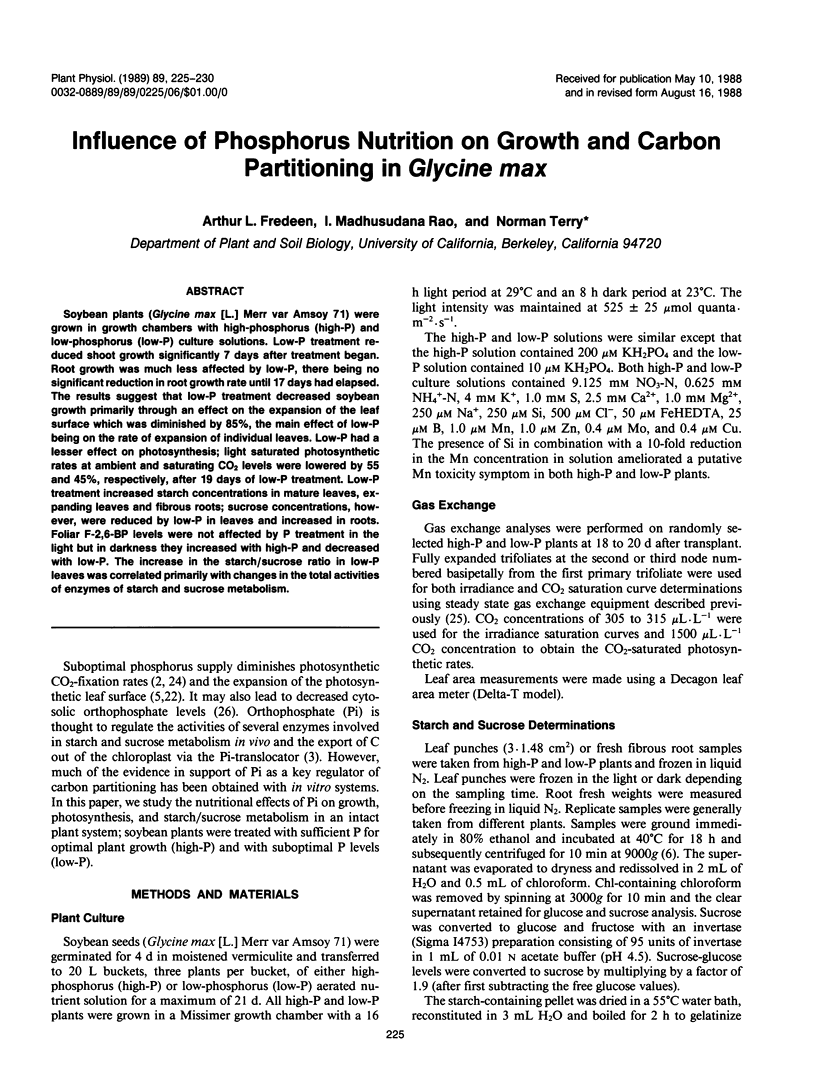


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fader G. M., Koller H. R. Relationships between Respiration Rate and Adenylate and Carbohydrate Pools of the Soybean Fruit. Plant Physiol. 1984 Jul;75(3):694–699. doi: 10.1104/pp.75.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrendorf T., Holtum J. A., Mukherjee U., Latzko E. Fructose 2,6-bisphosphate, carbohydrate partitioning, and crassulacean Acid metabolism. Plant Physiol. 1987 May;84(1):182–187. doi: 10.1104/pp.84.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Huber S. C., Israel D. W. Effect of N-source on soybean leaf sucrose phosphate synthase, starch formation, and whole plant growth. Plant Physiol. 1984 Jun;75(2):483–488. doi: 10.1104/pp.75.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Ou-Lee T. M., Setter T. L. Enzyme activities of starch and sucrose pathways and growth of apical and Basal maize kernels. Plant Physiol. 1985 Nov;79(3):848–851. doi: 10.1104/pp.79.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Eidenbock M. P. Carbon Accumulation during Photosynthesis in Leaves of Nitrogen- and Phosphorus-Stressed Cotton. Plant Physiol. 1986 Nov;82(3):869–871. doi: 10.1104/pp.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Kerr P. S., Huber S. C. Characterization of diurnal changes in activities of enzymes involved in sucrose biosynthesis. Plant Physiol. 1983 Oct;73(2):428–433. doi: 10.1104/pp.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Baysdorfer C., Kremer D. F. A comparative analysis of fructose 2,6-bisphosphate levels and photosynthate partitioning in the leaves of some agronomically important crop species. Plant Physiol. 1987 Apr;83(4):768–771. doi: 10.1104/pp.83.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M., Peavey D. G., Gibbs M. The regulation of starch metabolism by inorganic phosphate. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1554–1561. doi: 10.1016/s0006-291x(76)80191-x. [DOI] [PubMed] [Google Scholar]
- Terry N., Ulrich A. Effects of phosphorus deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1973 Jan;51(1):43–47. doi: 10.1104/pp.51.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeby M. T., van Steveninck R. F., de Vries H. M. Quantitative Estimates of Phosphorus Concentrations within Lupinus luteus Leaflets by Means of Electron Probe X-ray Microanalysis. Plant Physiol. 1987 Oct;85(2):331–334. doi: 10.1104/pp.85.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]


