Abstract
Like other phthalates, diethyl phthalate (DEP) is considered as a contaminant of emerging concern (CEC) due to its ease in migrating from a package to water and food, and hence contaminate consumers, being metabolized and excreted in the urine. Its presence has a negative impact on aquatic ecosystems, especially with respect to disruption of the endocrine system and to reproductive disorders in humans. It mainly enters water bodies via sewage effluents from effluent treatment plants, due to its incomplete or inefficient removal. The objective of this work was to evaluate the toxicity of DEP at different trophic levels and to analyze data on the incidence and concentration of DEP according to its solubility. The concentrations ranged from 12.5 mg L−1 to 500 mg L−1 considering the response for toxicity at each trophic level and to determine the lethal concentration in 50% of the following organisms (LC50) (in mg L−1): Lactuca sativa seeds, Artemia salina Leach nauplii and Zebrafish embryo larval stage (Danio rerio), being 41,057.58 after 120 h; 401.77 after 48 h; and 470 after 96 h of exposure, respectively. As expected, higher organisms were more affected even at low concentrations, which shows the anthropological contribution of CECs to water bodies.
Keywords: CECs, Plasticizers, Flexible food packaging, DEP, Toxicity, Water environment
Graphical abstract
Highlights
-
•
Lactuca sativa showed resistance to DEP toxicity
-
•
Seeds germinated and the roots grew at the concentrations studied.
-
•
Significant inhibition of the growth of zebrafish at the concentration of 50 mg L-1 of DEP
-
•
All over the world, plastic requires strict control of its environmental impact.
1. Introduction
Since the nineteen nineties, environmental pollution by contaminants of emerging concern (CECs) has been gaining importance in the scientific literature. However, many countries, especially Brazil, are a long way from establishing sanitary control limits for treated effluents with regular monitoring. CECs are detected and quantified in all environmental matrices, especially in surface and subterranean drinking water, but there is a lack of control requisites due to the high analytical costs [[1], [2], [3]].
Pesticides, personal hygiene pharmaceutical products, hormones, endocrine deregulators, sun shields/ultraviolet filters, illegal drugs, perfluoride compounds, disinfection subproducts, nanomaterials, microplastics, antibiotic-resistant genes and industrial compounds, amongst others [[4], [5], [6], [7], [8], [9]] are amongst the most investigated CECs. They are identified by the USA Environmental Protection Agency (US-EPA) as having no regulatory status and of having a negative impact on the environment, human health and the health of all other live organisms.
Phthalates are introduced into the food chain by way of plants that absorb them from the soil and have stimulated interest since they have been shown to present hepatotoxic, mutagenic and carcinogenic effects ′ [[10], [11], [12], [13]]. For human beings, phthalates come from foods, beverages [[14], [15], [16], [17], [18]] and water [19], which represent more than 67% of the contribution for human exposure [20]. Phthalates and their metabolites have already been detected in the human body (breast milk, blood, urine, semen) [[21], [22], [23]] and their ingestion can interrupt the normal functioning of the endocrine system [24,25] amongst other adverse effects for human health [[26], [27], [28], [29], [30], [31]].
A very limited number of studies with organisms as the target, dealing with acute phthalate toxicity, can be found in the scientific literature, using Vibrio fischeri [32,33], Danio rerio [34], and Daphnia magna [35,36], all little focused on long-term exposures and the observation of abnormalities in the behavior of the target organism. Studies on the toxic effects of phthalates in humans carried out in vitro as also the results obtained with animals, show that the adverse effects observed are related to the reproductive system, compromised spermatic functions [37,38], embryonic toxicity [39]; ocular irritation [40], and changes in the blood cell constituents [[41], [42], [43]]. The toxicity can act in different ways according to the target organism under study, and the conclusions made based on one target organism may not be applicable to others, requiring that specific tests be carried out for different species for a final evaluation of the risks [44].
Thus, the objective of the present work was to analyze the acute toxic effects of diethyl phthalate at three trophic levels Lactuca sativa seeds, Artemia salina Leach nauplii and the embryo-larval stage of Danio rerio (Zebrafish) and the lethal concentration in 50% (LC50) of the individuals exposed to the CEC using DEP as the model.
2. Material and methods
2.1. Reagents
The following reagents were acquired: diethyl phthalate (DEP) and 3,4-dichloroaniline from Sigma-Aldrich®, zinc sulfate (ZnSO4) from Isofar®, sodium chloride (marine NaCl) from Maxxi Reef Plus® and sodium dodecyl sulfate (SDS) from Neon®.
2.2. Toxicology of DEP using a bioassay with Lactuca sativa
2.2.1. Preparation of solutions and obtaining of Lactuca sativa seeds
Five concentrations of DEP (330; 260; 200; 132 and 66 mg L−1) were prepared in deionized water for the bioassay with Lactuca sativa seeds, according to the methodology proposed by IRAM (2003) [45] and Young et al. (2012) [46]. A non-chemically treated seed batch of the variety L. sativa was acquired from the local market and used for the test. The species Lactuca sativa (lettuce) is one of the vegetable species recommended by USEPA (1996) [47] to determine the ecological effects of pesticides and toxic substances and is commonly used in phytotoxicity studies [[48], [49], [50], [51], [52]]. The mean germination rate of all plant seeds should be above 90% [48]. The seeds were homogenized before carrying out the toxicity test. The parameters of pH and conductivity were evaluated for each solution using an AKSO Water Quality pH meter.
2.2.2. Toxicity bioassay methodology
The bioassay with Lactuca sativa seeds was carried out according to the methodology proposed by USEPA (1996) [47] adapted by Utzig et al. (2019) [53]. The American lettuce (Lactuca sativa L.) was selected as a common lettuce variety for salads and the study provided important information to evaluate the potential danger of plasticizers, specifically from DEP, on agricultural food safety.
Seeds: 15 seeds were selected and placed on top of filter paper in Petri dishes with at least 1 cm distance between each seed [54]. The tests were carried out using filter paper lining the base of 90 mm diameter Petri dishes containing 4 mL of each sample of the positive (400 mg L−1 zinc sulfate) and negative (deionized water) controls. The Petri dishes were covered, sealed with adhesive tape and placed in an incubator. Germination was interrupted after 120 h in the dark at room temperature, and the seed germination rate calculated by measuring the length of the rootlet (using a pachymeter).
The final points of toxicity evaluated were the relative growth index = RGI, germination index = GI, and the lethal concentration in 50% individuals = LC50. The seed was considered germinated when the appearance of the rootlet could be visibly detected [46]. The quality controls were germination above 90% and a variation coefficient for root lengthening below 30% in the control treatments [55]. The toxicity tests were carried out in triplicate at 22 ± 2 °C in the dark, taking the reading after 120 h of exposure.
Calculations of the phytotoxicity indexes: The number of germinated seeds was used to calculate the LC50 of the effluent [56]. The root lengthening data were used to calculate the germination index (GI) according to Zucconi et al. (1981, 1985) [57,58] and the relative growth index (RGI) according to Alvarenga et al. (2007) [59] and Varnero et al. (2007) [60]. The following equations show these phytotoxicity indexes:
| (1) |
| (2) |
Where SRL is the sample rootlet length, CRL the control rootlet length, SGS the number of seeds germinated in the sample and SGC the number of seeds germinated in the control. The RGI values were differentiated into three categories according to the effects of toxicity (Table 1) (see Table 2).
Table 1.
Differentiation of the RGI values into three categories.
| Inhibition of root lengthening (IRL) | 0 < x > 0.8 |
|---|---|
| No significant effects (NSE) | 0.8 ≤ x ≥ 1.2 |
| Stimulation of root lengthening (SRL) | x > 1.2, where x is the value obtained for the RGI (Eq. (1)) |
Table 2.
Toxicity classification.
| TU | Toxicity |
|---|---|
| <0.4 | No acute toxicity |
| 0.4 < TU < 1 | Mild acute toxicity |
| 1 < TU < 10 | Acute toxicity |
| 10 < TU < 100 | High acute toxicity |
| TU > 100 | Very high acute toxicity |
The effective concentration which reduced root growth by 50% (LC50) was estimated by linear regression (y = ax + b), and for comparative purposes, the linear regression data were adjusted by logarithmic application. The model proposed by Persoone et al. (2003) [61] was used to classify the toxicity, and all the toxicity values transformed into toxicity units (TU) according to the RGI (Eq. (1)).
| (3) |
2.3. Toxicology of DEP using a bioassay with Artemia salina
2.3.1. Preparation of solutions
Eight different DEP concentrations were used for the bioassay with Artemia salina as follows: 500; 357; 255; 182; 130; 93; 66 and 47 mg L−1, prepared in a 3.4% saline solution using Milliq water, shaking for 2 h. The parameters of pH and conductivity were evaluated for each solution using an AKSO Water Quality pH meter.
2.3.2. Experimental procedure
The toxicity of DEP was evaluated using the bioassay with Artemia salina Leach of Meyer et al. (1982) [62], ABNT 16530 (2021) [63]. The assays evaluated the immobilization of A. Salina when faced with the acute toxicity [64]. All experiments were carried out in quintuplicate.
Eggs: an egg batch estimated to contain billions of eggs (in 5 g) of the variety Artemia salina Leach was acquired from the local market and used for the test.
Incubation: 0.15 g of A. salina eggs were weighed on an analytical balance and mixed with 100 mL of a 3.4% NaCl (marine) solution in a recipient with no photoperiod and no aeration for 48 h at a temperature of 27 ± 2 °C up to egg hatched.
Immobility evaluation: After egg cracking, 15 test tubes were taken to evaluate the different plasticizer concentrations (500; 357; 255; 182; 130; 93; 66; 47 mg L−1). Aliquots of 10 mL of each test solution previously prepared in 3.4% marine solution (sodium chloride) were placed in the test tubes and 10 Artemia salina nauplii added with the aid of Pasteur pipettes. After 24 h of exposure of the nauplii to the different concentrations, the number of live and dead nauplii were counted, the percent survival calculated, and the dose-response curves constructed. Acute toxicity was evaluated by observing the effects of the compounds in the A. salina mobility test. The microcrustaceans were considered immobile when remaining at the bottom of the test recipient after 48 h of incubation and not starting to swim during 15 s of observation. The negative toxicity control was a 3.4% marine solution and the positive control different concentrations of sodium dodecyl sulfate (4.38; 3.13; 2.23; 1.59; 1.14; and 0.81 mg L−1).
Toxicity index calculations: the number of live nauplii in relation to the increase in concentration of the micropollutants was used to calculate the LC50 values. The data obtained were formed by the Probits method [65] and expressed as the LC50 (mean lethal concentration) and percent mortality. The concentration causing lethality in 50% of the nauplii (LC50) was calculated using the Probit method by way of statistical software with 95% of confidence.
2.4. Toxicology of DEP using a bioassay with Zebrafish
2.4.1. Preparation of solutions
A stock diethyl phthalate solution was prepared and diluted to the chosen concentrations (12.5; 25; 50; 100 and 200 mg L−1) based on the solubility limit of the compound, using water from the fish maintenance system. The positive control solution was prepared with 3,4-dichloroaniline (4 mg L−1) and the negative control was just water from the fish maintenance system. The parameters of pH and conductivity were evaluated for each solution using an AKSO Water Quality pH meter.
2.4.2. Maintenance of the adult Zebrafish (Danio rerio) and obtaining of the eggs
The adult male and female Zebrafish were maintained in a Hydrus rack (Alesco) in the Environmental Toxicology Research Laboratory (EnvTox) of the Pharmacy Faculty of the Federal University of Goiás (Brazil). The rack had a water recirculation system with automatic regulation of the water quality parameters, such as a temperature of 26 °C, pH value of 7.5 ± 0.5 and electric conductivity of 0.7 ± 0.1 mS. The fish were fed three times a day with commercial fish food (Tetra Color Flakes) and live Artemia salina organisms, and maintained in a 12:12 h light:dark cycle using a temporizer. To obtain eggs, the day before spawning, females with bulging bellies and slender males were selected and placed in reproduction aquariums in a proportion of 1 female to 2 males. This pre-contact of adult fish is fundamental, since it stimulates an increase in the reproduction rate. In addition, for reproduction to occur, the water quality and feeding parameters must be rigidly followed [66,67]. Mating started soon after illumination of the reproduction aquariums the next morning, and spawning occurred about 30 min later. The eggs were collected and transferred to Petri dishes in system water to be analyzed using a Leica S9i stereomicroscope (Wetzlar, Germany). Viable fertilized eggs were selected for the tests and non-viable (non-fertilized) eggs discarded.
2.4.3. Acute toxicity test using the test compounds with Zebrafish embryos and larvae
The acute toxicity test with Zebrafish embryos and larvae was carried out according to the guidelines nº 236 of the Organization for Economic Cooperation and Development (OECD) (OECD, 2013) [68]. Twenty-four well plates were used for the tests, and for each plate 4 wells were used for the internal control, adding 2 mL of system water to each, while the other 20 wells were used for exposure to diethyl phthalate, adding 2 mL of each solution per well for each of the concentrations (12.5; 25; 50; 100; and 200 mgL−1). The embryos were added to all the plates, one embryo per well.
The experiments were carried out in triplicate, each replicate being one plate with 20 embryos: negative control (maintenance system water), each diethyl phthalate concentration tested and a positive control (4 mg L−1 3,4-dichloroaniline). The plates were incubated at 26 °C ± 1 with a 12:12 h light:dark photoperiod and the development of the Zebrafish embryos and larvae evaluated daily at 24 h intervals up to 96 h post-fertilization (hpf). The following lethal parameters were evaluated every 24 h: coagulation of the fertilized egg, non-somite formation, loss of heartbeat and the non-detachment of the tail from the yolk sack. Other sublethal parameters were evaluated according to the development such as: non-eye formation, absence of body pigmentation, problems with the yolk sack absorption, hatching failures, backbone deformities and alteration of larval size [69,70], amongst other development abnormalities.
The embryo (n = 20) heart rates were also evaluated after 48 hpf, the heartbeats being counted for 15 s and multiplied by 4 to analyze a 1 min period [69,71]. The larval lengths were measured using an adaptation of Chen et al. (2017) [72], whereby the larvae (n = 20) were photographed using a Leica S9i stereomicroscope (Wetzlar, Germany), the length being measured using the internal scale of the microscope.
2.4.4. Ethics Commission
The project was first submitted to the Ethics Commission for the Use of Animals of UFG (CEUA/UFG) where it was approved under the protocol number of 072/2017.
2.5. Statistical analysis
All statistical procedures were carried out using the GraphPad Prism software version for Windows (version 5.0, GraphPad Software, San Diego, CA, USA). The Student t-test with 5% significance was used for the bioassay with Lactuca sativa seeds; the linear model for the analysis of variance (ANOVA) followed by Tukey's test for the bioassay with Artemia salina; and for the Zebrafish bioassay, comparisons between the different experimental groups were obtained by ANOVA followed by the Dunnett multiple comparison tests (α = 0.05). Each experiment was compared with its respective negative control. Toxicity was expressed as the mean lethal concentration (LC50) values determined on the GraphPad Prism.
3. Results and discussion
3.1. DEP toxicology in the bioassay with Lactuca sativa
Fig. 1 shows the germination index (GI) of the Lactuca sativa seeds exposed to different concentrations of diethyl phthalate (DEP) for 120 h, and Table 3 shows the results obtained for relative germination (RG), relative growth index (RGI) and germination index (GI).
Fig. 1.
Growth index induced by Diethyl phthalate in Lactuca sativa seeds after 120 h of exposure at 22 ± 2 °C with no photoperiod.
Table 3.
Variation in the diethyl phthalate (DEP) concentration and its impact on the germination and development of Lactuca sativa roots, expressed as relative germination (RG), relative growth index (RGI) and germination index (GI).
| DEP Concentrations (mg L−1) | RG | RGI | GI |
|---|---|---|---|
| 330 | 0.93 | 0.33 | 30.66 |
| 260 | 1.00 | 0.41 | 41.40 |
| 200 | 1.00 | 0.49 | 51.14 |
| 132 | 1.00 | 0.57 | 59.23 |
| 66 | 1.00 | 0.65 | 64.76 |
| NC | 0.93 | 0.91 | 85.65 |
| PC | 0.90 | 1.0 | 90 |
*NC = negative control (ZnSO4 - 40 mg L−1); PC = positive control (water).
The results obtained experimentally for the impact of the exposure of Lactuca sativa seeds to the plasticizer diethyl phthalate (DEP) at concentrations of 66 to 330 mg L−1 showed no significant negative impacts. There were no significant effects for the inhibition of root lengthening of the species Lactuca sativa, with the RGI values varying from 0.33 to 0.65 and more than 93% of the seeds germinating. According to Ref. [73], the phytotoxicity of crude and treated beer effluents when applied to L. sativa showed a coefficient of variation (CV) of 5.35% for the root length of the control sample with 97% seed germination, so the criteria established for the validation of the bioassay were accepted, and the GI for the crude effluent was 74 ± 5.9%. Also found higher GI values in the more diluted samples of industrial landfill leachate (treated, non-treated) and glycerin (mixtures) in the proportion of 95:5 (v:v), and dilutions (1; 1/2; 1/5) with values above 60%, and the lowest indexes, close to zero, were found in the crude samples [74].
For DEP, the lethal concentration that reduced root growth by 50% (LC50) can be estimated by linear regression (y = ax + b), obtaining a value for R2 = 0.9996 and for LC50 after 120 h of 41,057.58 mg L−1. The model proposed by Persoone et al. (2003) [61] was used to classify the acute toxicity of DEP for Lactuca sativa, and the toxicity unit (TU) was below 0.4, showing there was no acute toxicity at DEP concentrations varying from 66 to 330 mg L−1. In this study, exposure to DEP promoted a decrease in total root length, in root surface area, when compared with positive and negative controls, which from 200 mg L−1 DEP, there was no significant difference, because it did not increase phytotoxicity in lettuce roots.
Also observed that microplastics inhibited root growth of broad beans (Vicia faba), interfering with the absorption of water and nutrients by the roots and, therefore, making root growth and development difficult [75]. Root activity generally refers to absorption, synthesis and the oxidation and reduction capacities of the roots, representing a physiological index.
Zhang et al. (2021) [76] stated that the maximum dose of dibutyl phthalate (DBP) for ingestion is 0.01 mg kg−1. day−1 [[77], [78], [79]], assuming a man of medium weight (≈60 kg) with a daily ingestion of fruits and vegetables of 0.345 kg, considering a fraction of the mean weight of the foods of 4.23 mg kg−1 to be phthalates. The highest DBP residue found in lettuce leaves was 0.055 mg kg−1, indicating that the DBP content in lettuce leaves did not exceed the standard. According to the carcinogenicity of phthalates, the non-carcinogenic risk of DBP, reference dose of DBP (0.1 mg kg−1 dia−1 [80] and mean daily dose of DBP (mg kg−1 dia−1) were evaluated. The non-carcinogenic risk of DBP was 0.0013 and a non-carcinogenic risk index >1 is considered harmful to human health [81], suggesting that the DBP in lettuce leaves is not harmful to human health. Nevertheless, the potential risk to human health caused by the bioaccumulation of phthalates cannot be ignored.
3.2. DEP toxicology in the bioassay with Artemia salina
The results obtained in the mortality assay for Artemia salina nauplii in the II-III instar stage were considered valid since the percent mortality of the control sample was 0%. It was noted that in the negative control (3400 mg L−1 of marine salt), carried out in quintuplicate, the movement of the nauplii was not affected by the exposure time. This experiment was carried out in quintuplicate for each contaminant concentration and the results showed that nauplii mobility remained constant after 24 h exposure at concentrations of 47 to 255 mg L−1. Nauplii mobility was only affected by the existence of toxic compounds from 24 h of exposure at concentrations above 357 mg L−1 of DEP.
Fig. 2 shows the mortality rate for Artemia salina nauplii exposed to DEP for 48 h. The mortality rate was concentration-time-dependent, with significant lethality as from a concentration of 357 mg L−1.
Fig. 2.
Mortality induced by toxicity of Diethyl phthalate for Artemia salina nauplii after 48 h of exposure.
The results obtained for the mean lethal concentration showed a LC50–48h of 401.77 mg L−1 greater than that found by Call et al. (2001) [82] for aquatic organisms (LC50 4.21–102 mg L−1), which classifies it as non-toxic. Artemia acute toxicity tests have low sensitivity when compared to other aquatic organisms. However, the present study wanted to obtain the LC50 due to the importance of studying the consequences of biocides on Artemia and their aquatic environment that could endanger the survival of other organisms [83]. The LC50–24h for exposure to the positive control (SDS) was estimated as 5195.78 mg L−1 by the model with the best fit, which is within the limits proposed by Svensson et al. (2005) [84]. The toxic effect of DSS was as from 3130 mg L−1, where the nauplii showed 66% lethality after 48 h. The LC50 after 48h was estimated as a LC50–48h of 2952.74 mg L−1, and the LC50 after 48 h for DEP was 7.35 times smaller than the value of LC50 for SDS. In addition, the values obtained in the experiment were significantly different from the hypothetical values according to the test carried out (p < 0.05). The nauplii were not affected at concentrations of 182; 130; 93; 66 and 47 mg L−1 and maintained 100% of their mobility according to the hypothetical curve when exposed to DEP above 500 mg L−1.
In study the toxic effect of organophosphate pesticides on the lethality for Artemia salina, also used a limited range of from 0.2 mg L−1 to 3000 mg L−1 depending on the toxic compound tested. In this study the values for LC50 were calculated with high sensitivity using more extended scales [85].
Also, Artemia salina species have been reported as being organisms that accumulate toxic compounds with no effect on their life cycle [86]. reported that the Artemia species are tolerant to exposure to cadmium with LC50 values varying from 93.3 to 280 mg L−1 and showed differences in acute cadmium toxicity between nauplii belonging to different species of Artemia and among populations of the same species.
Artemia salina species have been used in many scientific experiments in toxicity tests for toxic materials including pesticides [87], leached materials [84], dental materials [88], fungal toxins [89] and anti-fouling biocides [90].
3.3. DEP toxicology in the bioassay with Zebrafish
The lethality rate of Zebrafish embryos and larvae exposed to diethyl phthalate in the period of 96 h is shown in Fig. 3. It was observed that the mortality rate is concentration-time-dependent, where there is a significant lethality from the concentration of 50 mg L−1.
Fig. 3.
Diethyl phthalate-induced mortality rate on Zebrafish embryos and larvae during 96h of exposure.
For the analysis of the average lethal concentration, it is possible to observe Table 4, in which Diethyl phthalate has an LC50–96h equal to 470 mg L−1.
Table 4.
Mean lethal concentration values (LC50) and confidence interval after 96 h of exposure to Diethyl phthalate (LC50–96h).
| Composto | CL50–96h (mg L−1) | Intervalo de confiança |
|---|---|---|
| Diethyl phthalate | 470 | 0.041–0.053 |
Fig. 4 shows the hatching rate of Zebrafish embryos in the periods of 72 and 96h. It is observed that there was an increase in the hatching rate and normal development of the fish.
Fig. 4.
Hatching rate of Zebrafish embryos exposed to Diethyl phthalate in the period of 72 and 96 h of exposure. At concentrations of 100 and 200 mg L−1, there was lethality (#) of the embryos before the period of 72 and 96 h.
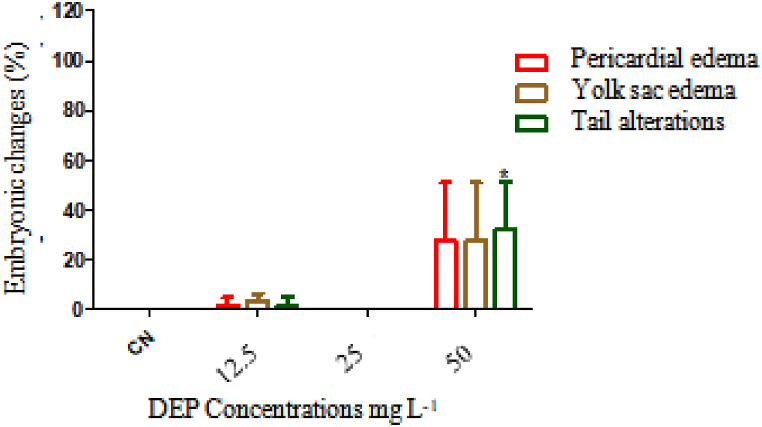
For the sublethal effects, Fig. 5 shows the embryonic alterations in the period of 96 h of exposure to DEP, being that pericardial edema, yolk sac edema, and tail alterations were found. The tail change is significant at the concentration of 50 mg L−1 DEP when compared to the negative control.
Fig. 5.
Embryonic changes caused by Diethyl phthalate during 96 h of exposure.
The length of Zebrafish larvae exposed to DEP is shown in Fig. 6, it was observed that there is significant inhibition of the growth of Zebrafish larvae at the concentration of 50 mg L−1 of DEP negative control.
Fig. 6.
Mean length of Zebrafish larvae exposed to Diethyl phthalate after 96 h of exposure.
The heartbeat of Zebrafish embryos and larvae exposed to DEP at different concentrations, analyzed over 48 h, can be seen in Fig. 7. It is possible to identify that there was no significant difference in the heart rate of embryos and larvae exposed to DEP when compared to negative control.
Fig. 7.
Mean heart rate of Zebrafish embryos exposed to Diethyl phthalate in 48 h.
In the research by [100] DEP was applied to Zebrafish embryos through the microinjection technique, using concentrations of 5 and 10 μM, which presented mortality of 55 and 48%, respectively, in the period of 48 h. They also observed skeletal changes in embryos and larvae exposed to DEP. Still in this research by [91], another form of exposure was evaluated, in water, in which embryos were exposed to DEP at concentrations of 11 and 22 ppm, which showed a mortality of 64 and 94%, respectively, within 72 h. Skeletal changes were also found in this form of exposure in embryos and larvae exposed to DEP, as in the present work, indicating that DEP causes teratogenicity during embryonic development. In the study by [92] mortality was evaluated up to the period of 168 hpf, in which a lethality of 25% was found at a concentration of 100 mg L−1, unlike the present study, which presents an LC50–96h of 47 mg L−1. Another evaluation that obtained different results was heart rate, where at concentrations of 10 and 100 mg L−1, [92] found a significant difference when compared to the negative control, but in the present study, there was no difference between the concentrations (12.5; 25; 50 mg L−1 respectively) tested. Regarding body length, [92] found a significant difference in the concentrations of 10 and 100 mg L−1 when compared to the control, with the larvae showing an increase in growth. In the present study, there was also a significant difference in body length, at a concentration of 50 mg L−1, but there was an inhibition of larval growth. [93] observed in their study that DEP has a CL50 in 7 days close to 10 mg L−1. This also observed inhibition of heart rate at the highest concentrations (8 and 10 mg L−1) tested, as in the present study (50 mg L−1). Furthermore, [102] also found tail changes, pericardial, and yolk sac edema as teratogenic changes, these changes being significant at the concentration of 8 mg L−1. While the present study shows significant results for the tail change at the concentration of 50 mg L−1.
4. Conclusion
The analysis can provide information about the plasticizer contaminant Diethyl phthalate (DEP) which varied according to the bioassay. For Lactuca sativa seeds to the plasticizer showed no significant negative impacts and for Artemia salina and Zebrafish the LC50 obtained were close values of 401.77 mg L−1 and 470 mg L−1, respectively. Although these values were higher than those reported in the literature, the bioassays are important to study the consequences of plasticizers in aquatic environment, moreover the possibility of a cumulative effect of the toxic compounds cannot be ignored. Therefore, there is a need for research and partnership investments, and search for new ways to deal with residues, which will surely have more value and preponderance in the productive processes of the future.
Author contribution statement
Maria Carolina de Almeida, M.D.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Michele Resende Machado, M.D.; Taís Aragão Ishizawa, M. D: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gessyca Gonçalves Costa, M.D.: Performed the experiments; Analyzed and interpreted the data.
Gisele Augusto Rodrigues de Oliveira, Dr.: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Hugo Freire Nunes, Dr.; Danillo Fabrini Maciel Costa Veloso, Dr; Julião Pereira, Dr: Contributed reagents, materials, analysis tools or data.
Tatianne Ferreira de Oliveira, Dr.: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the CNPQ for supporting the research (Universal Project No. 4054962018-4), the Federal University of Goiás for infrastructure, for financial support, highlighting the support of Environmental Toxicology Research Laboratory-ENVTOX, Pharmacy College, from the Laboratory of Extraction and Separation Methods-LAMES, Faculty of Chemistry, from the Center for Research, Technological Development and Innovation in Pharmaceuticals, Medicines and Cosmetics - FARMATEC, Faculty of Pharmacy and the Federal Institute of Education, Science and Technology of Goiás (IFG), Inhumas Campus for their relevant contributions to this work.
References
- 1.Cabeza Y., Candela L., Ronen D., Teijon G. Monitoring the occurrence of emerging contaminants in treated wastewater and groundwater between 2008 and 2010. The BaixLlobregat (Barcelona, Spain) J. Hazard Mater. 2012;239–240:32–39. doi: 10.1016/j.jhazmat.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Montes-Grajales D., Fennix-Agudelo M., Miranda-Castro W. Occurrence of personal care products as emerging chemicals of concern in water resources: a review. Sci. Total Environ. 2017;595:601–614. doi: 10.1016/j.scitotenv.2017.03.286. [DOI] [PubMed] [Google Scholar]
- 3.Montagner C.C., Sodré F.F., Acayaba R.D., Vidal C., Campestrini I., Locatelli M.A., Pescara I.C., Albuquerque A.F., Umbuzeiro G.A., Jardim W.F. Ten years-snapshot of the occurrence of emerging contaminants in drinking, surface and ground waters and wastewaters from são paulo state, Brazil. J. Braz. Chem. Soc. 2019;30(3):614–632. doi: 10.201577/0103-5053.20180232. [DOI] [Google Scholar]
- 4.Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B., et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 5.Comerton A.M., Andrews R.C., Bagley D.M. Practical overview of analytical methods for endocrine-disrupting compounds, pharmaceuticals and personal care products in water and wastewater. Philos. Trans. R. Soc. London, Ser. A Math. Phys. Eng. Sci. 2009;367:3923–3939. doi: 10.1098/rsta.2009.0111. [DOI] [PubMed] [Google Scholar]
- 6.Brausch J.M., Rand G.M. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere. 2011;82:1518–1532. doi: 10.1016/j.chemosphere.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Bo L., Shengen Z., Chang C.-C. Emerging pollutants-part II: treatment. Water Environ. Res. 2016;88:1876–1904. doi: 10.2175/106143016X14696400495857. [DOI] [PubMed] [Google Scholar]
- 8.Magi E., Di Carro M. Marine environment pollution: the contribution of mass spectrometry to the study of seawater. Mass Spectrom. Rev. 2016;37(4):492–512. doi: 10.1002/mas.21521. [DOI] [PubMed] [Google Scholar]
- 9.Fadare O.O., Wan B., Guo L.-H., Zhao L. Microplastics from consumer plastic food containers: are we consuming it? Chemosphere. 2020;253 doi: 10.1016/j.chemosphere.2020.126787. [DOI] [PubMed] [Google Scholar]
- 10.Zurmühl T. Development of a method for the determination of phthalate esters in sewage sludge including chromatographic separation from polychlorinated biphenyls, pesticides and polyaromatic hydrocarbons. Analyst. 1990;115:1171–1175. doi: 10.1039/AN9901501171. [DOI] [Google Scholar]
- 11.Ma T., Christie P., Teng Y., Luo Y. Rape (Brassica chinensis L.) seed germination, seedling growth, and physiology in soil polluted with di-n-butyl phthalate and bis (2-ethylhexyl) phthalate. Environ. Sci. Pollut. Res. 2013;20(8):5289–5298. doi: 10.1007/s11356-013-1520-5. [DOI] [PubMed] [Google Scholar]
- 12.Yue Q., Huang Y.-Y., Shen X.-F., Yang C., Pang Y.-H. In situ growth of covalet organic framework on titanium fiber for headspace solid-phase microextraction of 11 phthalete esters in vegetables. Food Chem. 2020;318 doi: 10.1016/j.foodchem.2020.126507. [DOI] [PubMed] [Google Scholar]
- 13.Tian M., Wu S., Wang Y.-X., Liu L., Zhang J., Shen H., Lu Yanyang, Bao H., Qingyu Huang Q. Associations of environmental phthalate exposure with male steroid hormone synthesis and metabolism: an integrated epidemiology and toxicology study. J. Hazard Mater. 2022;436 doi: 10.1016/j.jhazmat.2022.129213. [DOI] [PubMed] [Google Scholar]
- 14.Yang T.C., Peterson K.E., Meeker J.D., Sánchez B.N., Zhang Z., Cantoral A., Solano M., Tellez-Rojo M.M. Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environ. Res. 2017;156:326–333. doi: 10.1016/j.envres.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim S. The associations between personal care products use and urinary concentrations of phthalates, parabens, and triclosan in various age groups: the Korean National Environmental Health Survey Cycle 3 2015-2017. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140640. [DOI] [PubMed] [Google Scholar]
- 16.Adenuga A.A., Ayinuola O., Adejuyigbe E.A., Ogunfowokan A.O. Biomonitoring of phthalate esters in breast milk and urine samples as biomarkers for neonates' exposure, using modified quechers method with agricultural biochar as dispersive solid-phase extraction absorbent. Microchem. J. 2020;152 doi: 10.1016/j.microc.2019.104277. [DOI] [Google Scholar]
- 17.Anda-Flores Y.B., Cordon-Cardona B.A., Gonzalez-León A., Valenzuela-Quintanar A.I., Peralta E., Soto-Valdez H. Effect of essay conditions on the migration of phthalates from polyvinyl chloride cling films used for food packaging in Mexico. Food Packag. Shelf Life. 2021;29 doi: 10.1016/j.fpsl.2021.100684. [DOI] [Google Scholar]
- 18.Tian L., Zheng J., Pineda M., Yargeau V., Furlong D., Chevrier J., Bornman R., Obida M., Goodyer C.G., Bayen S. Targeted screening of 11 bisphenols and 7 plasticizers in food composites from Canada and South Africa. Food Chem. 2022;385 doi: 10.1016/j.foodchem.2022.132675. [DOI] [PubMed] [Google Scholar]
- 19.Khaustov A., Redina M., Goryainov S. Migration of PAHs and phthalates from package materials during water storage: glass or plastic? Polycycl. Aromat. Comp. 2022;42(2):358–370. doi: 10.1080/10406638.2020.1734033. [DOI] [Google Scholar]
- 20.Das M.T., Ghosh P., Thakur I.S. Intake estimates of phthalate esters for South Delhi population based on exposure media assessment. Environ. Pollut. 2014;189:118–125. doi: 10.1016/j.envpol.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Chen J.A., Liu H., Qiu Z., Shu W. Analysis of di-n-butyl phthalate and other organic pollutants in Chongqing women undergoing parturition. Environ. Pollut. 2008;156(3):849–853. doi: 10.1016/j.envpol.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Liou S.-H., Yang G.C.C., Wang C.-L., Chiu Y.-H. Monitoring of PAEMs and beta agonists in urine for a small group of experimental subjects and PAEs and beta-agonists in drinking water consumed by the same subjects. J. Hazard Mater. 2014;277:169–179. doi: 10.1016/j.jhazmat.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Rocha B.A., Asimakopoulos A.G., Barbosa J.R.F. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ. 2017;586:152–162. doi: 10.1016/j.scitotenv.2017.01.193. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin S., Masai E., Kamimura N., Takahashi K., Anderson R.C.A., Faisal P.A. Phthalates impact human health: epidemiological evidence and plausible mechanism of action. J. Hazard Mater. 2017;340:360–383. doi: 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Duan C., Fang Y., Sun J., Li Z., Wang Q., Bai J., Peng H., Liang J., Gao Z. Effects of fastfood packaging plasticizers and their metabolites on steroid hormone synthesis in H295R cells. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138500. [DOI] [PubMed] [Google Scholar]
- 26.Hauser R., Calafat A. Phthalates and human health. Occup. Environ. Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamai Y.A., Ikeda-Araki A., Kawai T., Tsuboi T., Yoshioka E., Kanazawa A., Cong S., Kishi R. Comparisons of urinary phthalate metabolites and daily phthalate intakes among Japanese families. Int. J. Hyg Environ. Health. 2015;218:461–470. doi: 10.1016/j.ijheh.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Ejaredar M., Nyanzaa E.C., Eycke K.T., Dewey D. Phthalate exposure and children's neurodevelopment: a systematic review. Environ. Res. 2015;142:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Johns L.E., Cooper G.S., Galizia A., Meeker J.D. Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 2015;85:27–39. doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariana M., Feiteiro J., Verde I., Cairrao E. The effects of phthalates in the cardiovascular and reproductive systems: a review. Environ. Int. 2016;94:758–776. doi: 10.1016/j.envint.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Mariana M., Cairrao E. Phthalates implications in the cardiovascular system. J. Cardiovas. Develop. Dis. 2020;7:26. doi: 10.3390/jcdd7030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang G.Z., Cao T., Sun S.H., Bi Q., Li P. Study of the adverse effects of phthalates found in children's toys on Vibrio fischeri lethality. Toxicol. Environ. Chem. 2017;99:848. doi: 10.1080/02772248.2017.132005. [DOI] [Google Scholar]
- 33.Wei S., Wang F.H., Chen Y.J., Lan T., Zhang S.T. The joint toxicity effect of five antibiotics and dibutyl phthalate to luminescent bacteria (Vibrio fischeri) Environ. Sci. Pollut. Res. 2018;25 doi: 10.1007/s11356-018-2720-9. [DOI] [PubMed] [Google Scholar]
- 34.Hamid N., Junaid M., Manzoor R., Jia P.P., Pei D.S. Prioritizing phthalate esters (PAEs) using experimental in vitro/vivo toxicity assays and computational in silico approaches. J. Hazard Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122851. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Wang T.Y., Ban Y.L., Shen C.C., Shen Q., Chai X.J., Zhao W., Wei J. Di-(2-ethylhexyl) phthalate exposure modulates antioxidant enzyme activity and gene expression in juvenile and adult Daphnia magna. Arch. Environ. Contam. Toxicol. 2018;75:145. doi: 10.1007/s00244-018-0535-9. [DOI] [PubMed] [Google Scholar]
- 36.Shen C.C., Wei J., Wang T.Y., Wang Y. Acute toxicity and responses of antioxidant systems to dibutyl phthalate in neonate and adult Daphnia magna. PeerJ. 2019;7 doi: 10.7717/peerj.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pant N., Pant A.B., Shukla M., Mathur N., Gupta Y.K., Saxena D.K. Environmental and experimental exposure of phthalate esters: the toxicological consequence on human sperm. Hum. Exp. Toxicol. 2011;30(6):507. doi: 10.1177/0960327110374205. [DOI] [PubMed] [Google Scholar]
- 38.Sun X., Chen W., Weng S., Pan T., Hu X., Wang F., Xia T., Chen H., Luo T. Effects of the environmental endocrine disruptors di-2-ethylhexyl phthalate and mono-2-ethylhexyl phthalate on human sperm function in vitro. Reprod. Fertil. Dev. 2020;32(6):629. doi: 10.1071/RD19164. [DOI] [PubMed] [Google Scholar]
- 39.Fang H., Fang W., Cao H., Luo S., Cong J., Liu S., Pan F., Jia X. Di-(2-ethylhexyl)-phthalate induces apoptosis via the PPARγ/PTEN/AKT pathway in differentiated human embryonic stem cells. Food Chem. Toxicol. 2019;131 doi: 10.1016/j.fct.2019.05.06. [DOI] [PubMed] [Google Scholar]
- 40.Kruger T., Yi Cao Y., Kjærgaard S.K., Knudsen L.E., Bonefeld-Jørgensen E.C. Effects of phthalates on the human corneal endothelial cell line B4G12. Int. J. Toxicol. 2012;31(4):364–371. doi: 10.1177/1091581812449660. [DOI] [PubMed] [Google Scholar]
- 41.Sicińska P. Di-n-butyl phthalate, butylbenzyl phthalate and their metabolites induce haemolysis and eryptosis in human erythrocytes. Chemosphere. 2018;203:44. doi: 10.1016/j.chemosphere.2018.03.161. [DOI] [PubMed] [Google Scholar]
- 42.Sicińska P. Di-n-butyl phthalate, butylbenzyl phthalate, and their metabolites exhibit different apoptotic potential in human peripheral blood mononuclear cells. Food Chem. Toxicol. 2019;133 doi: 10.1016/j.fct.2019.110750. [DOI] [PubMed] [Google Scholar]
- 43.Sicińska P., Kik K., Bukowska B. Human erythrocytes exposed to phthalates and their metabolites alter antioxidant enzyme activity and hemoglobin oxidation. Int. J. Mol. Sci. 2020;21(12):4480. doi: 10.3390/ijms21124480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habert R., Livera G., Rouiller-Fabre V. Man is not a big rat: concerns with traditional human risk assessment of phthalates based on their anti-androgenic effects observed in the rat foetus. Basic Clin. Androl. 2014;24:14. doi: 10.1186/2051-4190-24-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.IRAM . IRAM 29012-16, firsted; Argentina: 2003. Environmental Quality. Water Quality Sampling Guidance on Biotesting of Samples. [Google Scholar]
- 46.Young B.J., Riera N.I., Beily M.E., Bres P.A., Crespo D.C., Ronco A.E. Toxicity of the effluent from ananaerobic bioreactor treating cereal residues on Lactuca sativa. Ecotoxicol. Environ. Saf. 2012;76:182–186. doi: 10.1016/j.ecoenv.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Environmental Protection Agency - USEPA . 1996. Ecological Effects Test Guidelines (OPPTS 850.4200): Seed Germination/Root Elongation Toxicity Test. [Google Scholar]
- 48.Lin D., Xing B.A. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243. doi: 10.1016/j.envpol.2007.01.016. 25. [DOI] [PubMed] [Google Scholar]
- 49.Migliore L., Cozzolino S., Fiori M. Phytotoxicity to and uptake of enrofloxacin in crop plants. Chemosphere. 2003;52(7):1233–1244. doi: 10.1016/S0045-6535(03)00272-8. [DOI] [PubMed] [Google Scholar]
- 50.Sverdrup L.E., Krogh p.H., Nielsen T., Kjær C., Stenersen J. Toxicity of eight polycyclic aromatic compounds to red clover (Trifolium pratense), ryegrass (Lolium perenne), and mustard (Sinapsis alba) Chemosphere. 2003;53(8):993–1003. doi: 10.1016/S0045-6535(03)00584-8. [DOI] [PubMed] [Google Scholar]
- 51.Rivetta A., Negrini N., Cocucci M. Involvement of Ca2+-calmodulin in Cd2+ toxicity during the early phase of radish (Raphanus sativus L.) seed germination. Plant Cell Environ. 1997;20:600–608. doi: 10.1111/j.1365-3040.1997.00072.x. [DOI] [Google Scholar]
- 52.Wierzbicka M., Obidzinska J. The effect of lead on seed imbibition and germination in different plant species. Plant Sci. 1998;137:155–171. doi: 10.1016/S0168-9452(98)00138-1. [DOI] [Google Scholar]
- 53.Utzig L.M., Lima R.M., Gomes M.F., Ramsdorf W.A., Martins L.R., Liz M.V., Freitas A.M. Ecotoxicity response of chlorpyrifos in Aedes aegypti larvae and Lactuca sativa seeds after UV/H2O2 and UVC oxidation. Ecotoxicol. Environ. Saf. 2019;169:449–456. doi: 10.1016/j.ecoenv.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Yang L., Watts D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Sobrero C., Ronco A. In: Ensayos Toxicológicos y Métodos de Evaluación de Calidad de Aguas: Estandarización, Intercalibración, Resultados y Aplicaciones. Castillo G., editor. México; 2004. Ensayo de toxicidad aguda consemillas de L. sativa; p. 71. –79pp. [Google Scholar]
- 56.Finney D.J. third ed. Cambridge University Press; Cambridge, UK: 1971. Probit Analysis. [Google Scholar]
- 57.Zucconi F., Pera A., Forte M., De Bertoldi M. Evaluating toxicity of immature compost. Biocycle. 1981;22:54–57. ISSN: 0276–5055. [Google Scholar]
- 58.Zucconi F., Monaco A., Forte M., De Bertoldi M. In: Composting of Agricultural and Other Wastes. Gasser J.K.R., editor. Elsevier; London: 1985. Phytotoxins during the stabilization of organic matter; pp. 73–85. [Google Scholar]
- 59.Alvarenga P., Palma P., Gonçalves A., Fernandes R., Cunha-Queda A., Duarte E., Vallini G. Evaluation of chemical and ecotoxicological characteristics of biodegradable organic residues for application to agricultural l and. Environ. Int. 2007;33:505–513. doi: 10.1016/j.envint.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Varnero M., Rojas A.C., Orellana R.R. Índices de fitotoxicidad en residuos organicos durante el compostaje. Rev. Ciencia del Suelo y Nutrición Veg. 2007;7(1):28–37. doi: 10.4067/S0718-27912007000100003. [DOI] [Google Scholar]
- 61.Persoone E., Perrier A., Tuzet A. Simulating water uptake in the root zone with a microscopic-scale model of root extraction. Agronomie. 2003;23:153–168. [Google Scholar]
- 62.Meyer B.N., Ferrigni N.R., Putnam L.B., Jacobsen L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45(5):31–34. doi: 10.1055/s-2007-971236. 1982. [DOI] [PubMed] [Google Scholar]
- 63.ABNT NBR 16530 Aquatic ecotoxicology – acute toxicity – test method with Artemia sp (Crustacea, Brachiopoda) Norma Brasileira. 2021;2 23 de setembro de 2021. 24pp. 978-85-07-08700-7. [Google Scholar]
- 64.Trovó A.G., Silva T.F.S., Gomes J.R.O., Machado A.E.H., Borges Neto W., Muller Junior P.S., Daniel D. Degradation of caffeine by photo-fenton process: optimization of treatment conditions using experimental design. Chemosphere. 2013;90:170–175. doi: 10.1016/j.chemosphere.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 65.Finney D.J. Cambridge University Press; Cambridge: 1962. Probit Analysis; p. 318p. [Google Scholar]
- 66.Schneider A.C.R., Maurer R.L., Matte U., Porawski M., Schaefer P.G., Dos Santos J.L., Da Silveira T.R. Implementation of a new experimental animal model - zebrafish. Revista HCPA. 2009;29(2):2357–9730. 100–103. [Google Scholar]
- 67.Zorzetto R., Guimarães M. Um peixe modelo. Pesquisa FAPESP. São Paulo. 2013;209:16. 19pp. [Google Scholar]
- 68.Organization for Economic Co-operation and Development – OECD . vol. 236. OECD Guideline; France: 2013. (Fish Embryo Toxicity (FET) Test). [Google Scholar]
- 69.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann L.B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dynam. 1995;203:255–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 70.Nagel R.DarT. The embryo test with the Zebrafish Danio rerio--a general model in ecotoxicology and toxicology. ALTEX. 2002;19(Suppl 1):38–48. PMID: 12096329. [PubMed] [Google Scholar]
- 71.Fraysse B., Mons R., Garric J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006;63(2):253–267. doi: 10.1016/j.ecoenv.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Chen Q., Gundlach M., Yang S., Jiang J., Velki M., Yin D., Hollert H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017;584:1022–1031. doi: 10.1016/j.scitotenv.2017.01.156. [DOI] [PubMed] [Google Scholar]
- 73.Vianna L.O., Martins K.G., Souza K.V., Stroparo E.C. Fitotoxicidade de efluente da indústria cervejeira em sementes de Lactuca sativa L. Revista Internacional de Ciências. 2017;7(2):265–275. doi: 10.12957/ric.2017.30072. [DOI] [Google Scholar]
- 74.Recio L.V., Pereira N.R., Arantes E.J., Gomes S.D., Castro T.M. Phyto-toxicity in lettuce seeds in anaerobic coding of leachate landfill industry and glycerin. Braz. J. Ani. Environ. Res. 2019;2(3):982–989. ISSN 2595-573X. [Google Scholar]
- 75.Jiang X., Chen H., Liao Y., Ye Z., Li M., Klobučar G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019;250:831–838. doi: 10.1016/j.envpol.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y.-J., Guo J.-L., Xue J.-C., Bai C.-L., Guo Y. Phtalate metabolites: characterization, toxicities, global distribuition, and exposure assessment. Environ. Pollut. 2021;291 doi: 10.1016/j.envpol.2021.118106. [DOI] [PubMed] [Google Scholar]
- 77.Balafas D., Shaw K.J., Whitfield F.B. Phthalate and adipate estres in Australian packaging materias. Food Chem. 1999;65:279–287. doi: 10.1016/S0308-8146(98)00240-4. [DOI] [Google Scholar]
- 78.EFSA Opinion of the scientific painel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to bis-(2-ethylhexil) phtalate (DEHP) for use in food contact materials. EFSA J. 2005;243:1–20. doi: 10.2903/j.efsa.2005.243. [DOI] [Google Scholar]
- 79.EFSA Opinion of the scientific painel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to di butyl phtalate (DBP) for use in food contact materials. EFSA J. 2005;242:1–17. doi: 10.2903/j.efsa.2005.242. [DOI] [Google Scholar]
- 80.Risk Assessment Informantion System - RAIS . Departamente of Energy; Washington DC: 2013. Risk Exposure Models for Chemicals User's Guide (EB/OL) [Google Scholar]
- 81.Wang J., Chen G., Christie P., Zhang M., Luo Y., Ying Teng Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Sci. Total Environ. 2015;523:129–137. doi: 10.1016/j.scitotenv.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 82.Call D.J., Markee T.P., Geiger D.L., Brooke L.T., Vandeventer F.A., Cox D.A., Genisot K.I., Robillard K.A., Gorsuch J.W., Parkerton T.F., Reiley M.C., Ankley G.T., Mount D.R., et al. An assessment of the toxicity of phthalate esters to freshwater benthos. 1. Aqueous exposures. Environ. Toxicol. Chem. 2001;20:1798–1804. PMID: 11491565. [PubMed] [Google Scholar]
- 83.Epole N., Domínguez-Martín E.M., Roberto A., Tavares J., Rijo P., Isca V., Pereira P., Cebola M.J. Artemia species: an important tool to screen general toxicity samples. Curr. Pharmaceut. Des. 2020;26(24):2892–2908. doi: 10.2174/1381612826666200406083035. [DOI] [PubMed] [Google Scholar]
- 84.Svensson B., Mathiasson L., Mårtensson L., Bergström S. Artemia salinaas test organism for assessment of acute toxicity of leachate water from landfills. Environ. Monit. Assess. 2005;102:309–321. doi: 10.1007/s10661-005-6029-z. [DOI] [PubMed] [Google Scholar]
- 85.Venkateswara Rao J., Kavitha P., Jakka N.M., Sridhar V., Usman P.K., P. K. Toxicity of organophosphates on morphology and locomotor behavior in brine shrimp, Artemia salina. Arch. Environ. Contam. Toxicol. 2007;53:227–232. doi: 10.1007/s00244-006-0226-9. [DOI] [PubMed] [Google Scholar]
- 86.Sarabia R., Del Ramo J.J., Varo I., Dias-Mayans J. Comparing the acute response to cadmium toxicity of nauplii from different populations of Artemia. Environ. Toxicol. Chem. 2002;21(2):437–444. doi: 10.1002/etc.5620210229. [DOI] [PubMed] [Google Scholar]
- 87.Barahona M.V., Sánchez-Fortún S. Comparative sensitivity of three age classes of Artemia salina larvae to several phenolic compounds. Bull. Environ. Contam. Toxicol. 1996;56:271–278. doi: 10.1007/s001289900041. [DOI] [PubMed] [Google Scholar]
- 88.Pelka M., Danzl C., Distler W., Petschelt A. A new screening test for toxicity testing of dental materials. J. Dent. 2000;28:341–345. doi: 10.1016/S0300-5712(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 89.Harwing J., Scott P.M. Brine Shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl. Environ. Microbiol. 1971;21:1011–1016. doi: 10.1128/am.21.6.1011-1016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koutsaftis A., Aoyama I. Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina. Sci. Total Environ. 2007;387:166–174. doi: 10.1016/j.scitotenv.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 91.Kim S.M., Yoo J.A., Baek J.M., Cho K.H. Diethyl phthalate exposure is associated with embryonic toxicity, fatty liver changes, and hypolipidemia via impairment of lipoprotein functions. Toxicol. Vitro. 2015;30(1):383–393. doi: 10.1016/j.tiv.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 92.Lee H., Lee J., Choi K., Kim K.T. Comparative analysis of endocrine disrupting effects of major phthalates in employed two cell lines (MVLN and H295R) and embryonic zebrafish assay. Environ. Res. 2019;172:319–325. doi: 10.1016/j.envres.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 93.Pu S.Y., Hamid N., Ren Y.W., Pei D.S. Effects of phthalate acid esters on zebrafish larvae: development and skeletal morphogenesis. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.