Abstract
In vitro and in vivo antidermatophyte activities of NND-502, a new imidazole antimycotic agent, were compared with those of two existing antifungal agents, lanoconazole and terbinafine. NND-502 exhibited strong in vitro antifungal activity against Trichophyton spp.; its MIC was 1 to 4 times lower than that of lanoconazole or terbinafine. In an in vivo study with a guinea pig model of tinea pedis, 7-day topical treatment with a 0.5% solution of NND-502 (dissolved in polyethylene glycol 400) was more effective than that with a 0.5% solution of either lanoconazole or terbinafine for eradicating fungi from the infected feet. When the duration of treatment was shortened to 3 days, a topical 1% solution of NND-502 achieved a complete mycological cure, while topical 1% solutions of lanoconazole and terbinafine did not.
A large number of antifungal imidazoles, among them, lanoconazole, an imidazole compound with a ketene dithioacetal structure, have been clinically introduced for the topical treatment of dermatomycoses (6, 9, 11–13, 20, 22, 24). Clinical trials of lanoconazole completed in Japan with preparations of 1% cream and 1% solution demonstrated that they are highly effective in the treatment of various dermatomycoses, including tinea pedis, tinea corporis, and cutaneous candidiasis (22). Topical lanoconazole preparations were successfully launched in 1994 and are commercially available in Japan.
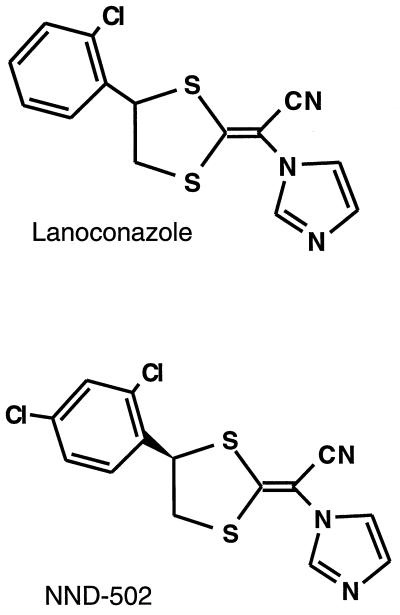
NND-502, (−)-(E)-[4-(2,4-dichlorophenyl)-1,3-dithiolan-2- ylidene]-1-imidazolylacetonitrile, whose structure is shown in Fig. 1, is a novel analog of lanoconazole that is being developed for the therapy of dermatomycoses. Since one of the major differences between lanoconazole and NND-502 is that the former is a racemic compound and the latter is an R-enantiomer (the S-enantiomers of both compounds are inactive as antimycotic agents), NND-502 is expected to be the more potent antifungal agent. In this study, we compare the in vitro and in vivo antifungal activities of NND-502 against dermatophytes with those of lanoconazole and terbinafine, which are members of an allylamine class of antifungal agents (17).
FIG. 1.
Chemical structures of lanoconazole and NND-502.
NND-502, lanoconazole, and terbinafine were synthesized according to methods described previously (10, 18, 21). Their chemical structures were identified by their melting points and nuclear magnetic resonance spectra, and the purity of all test materials was more than 99.0% as measured by high-pressure liquid chromatography. Twenty stock cultures of Trichophyton mentagrophytes and Trichophyton rubrum were used for in vitro study, and T. mentagrophytes TIMM 2789 was used for in vivo study. Male Hartley guinea pigs (Japan SLC Inc., Shizuoka, Japan) weighing 350 to 500 g were used to study infection. The animals were maintained in an air-conditioned room at 23 ± 2°C (set temperature ± standard deviation) and were allowed access to feed and water ad libitum throughout the experiment.
The MICs for Trichophyton spp. were determined by a macrobroth dilution technique with Sabouraud dextrose broth. The conidial suspension of each strain was prepared in sterile physiological saline containing 0.1% (wt/vol) Tween 80 from cultures grown on Sabouraud dextrose agar slants at 27°C for 1 to 4 weeks. Following filtration through sterilized gauze to remove hyphal fragments and agar blocks, the final suspension was adjusted to 106 conidia/ml. To each tube containing 9.8 ml of the broth, 0.1 ml of each drug being tested dissolved in dimethyl sulfoxide and 0.1 ml of a conidial suspension were added. All of the tubes were incubated with shaking (120 strokes/min) at 27°C for 7 days. The MIC was defined as the lowest drug concentration which prevented visual fungal growth.
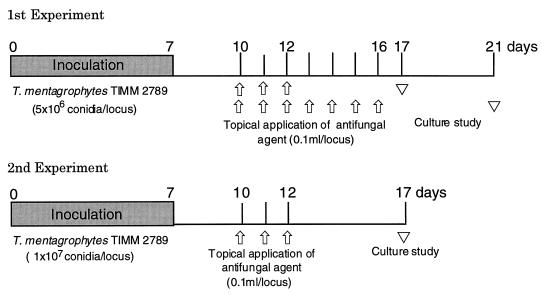
Two experiments with a guinea pig model of tinea pedis were conducted to evaluate the in vivo antifungal activity of NND-502. The procedure that was used for infection in the first experiment is essentially identical to the method of Fujita and Matsuyama (7) and Fujita, Matsuyama, and Sato (8). The conidial suspension of T. mentagrophytes TIMM 2789 was prepared in the same way as in the in vitro study to obtain a final suspension of 108 conidia/ml. One side of a paper disc (1.5 mm thick and 8 mm in diameter) was covered with a piece of aluminum foil, while the other side held the inoculum suspension. The disc was wetted with 50 μl of the inoculum suspension and then fixed onto the sole of the guinea pig foot with elastic adhesive tape. The disc was removed on the 7th day postinfection. Each agent, dissolved in polyethylene glycol 400 (0.1 ml/locus), was topically applied to the whole soles of guinea pigs once a day for 3 or 7 consecutive days starting on the 10th day postinfection. Five days after the last treatment, the skin from the soles of the guinea pigs’ feet was divided into two parts, and the tissue from the half closer to the toe (toe portion) and that from the corresponding heel portion for each animal were cut into small blocks (about 2 by 2 mm). Ten tissue blocks obtained from each portion of the foot were implanted on a Sabouraud dextrose agar plate containing 500 μg of cycloheximide per ml, 50 μg of chloramphenicol per ml, and 50 μg of sisomicin per ml and cultured at 27°C for 14 days. The tissue blocks yielding fungal growth were considered to be culture positive, and feet (toe and heel portions combined) with more than one culture-positive tissue block were considered to be fungus positive. Intensity of infection was assessed with scores ranging from 0 to 10, based on the number of culture-positive tissue blocks of the 10 tissue blocks studied (14). The mean of the data for the toe portion and the corresponding heel portion was considered to be a representative value for each foot.
The procedure for infecting and treating the animals in the second experiment was essentially identical to the method of Uchida et al. (23). A sheer plaster (Band-Aid; Johnson & Johnson Co., Ltd., Tokyo, Japan) was wetted with 100 μl of the inoculum suspension and then fixed onto the sole of an animal’s foot with elastic adhesive tape. The plaster was removed on the 7th day postinfection. Each agent, dissolved in polyethylene glycol 400 (0.1 ml/locus), was topically applied to the whole soles of the guinea pigs once a day for 3 consecutive days. The latter part of the procedure was the same as that for the first experiment. Experimental schedules are shown in Fig. 2.
FIG. 2.
Experimental schedule for study of the therapeutic efficacy of topical treatment with polyethylene glycol 400 solution, NND-502, lanoconazole, and terbinafine in a guinea pig model of tinea pedis.
Statistical analyses for the rate of fungus-positive feet and the average intensity of infection were assessed by Fisher’s exact probability test and nonparametric Tukey’s multiple comparison test in combination with Kruskal-Wallis’s rank test, respectively. P values of <0.05 were considered to be significant.
In vitro susceptibilities of 20 strains of Trichophyton spp. to NND-502, lanoconazole, and terbinafine are summarized in Table 1. The MIC of NND-502 for each strain was 2 to 4 times lower than that of lanoconazole and almost equal to or slightly lower than that of terbinafine. The MICs of NND-502, lanoconazole, and terbinafine for T. mentagrophytes TIMM 2789, the strain used for in vivo study, were 0.0025, 0.01, and 0.0025 μg/ml, respectively.
TABLE 1.
In vitro antifungal activities of NND-502, lanoconazole, and terbinafine against stock cultures of Trichophyton spp.
| Organism (no. of strains) | Compound | MIC (μg/ml)
|
|
|---|---|---|---|
| Range | Geometric mean | ||
| T. mentagrophytes (10) | NND-502 | 0.0025–0.02 | 0.0093 |
| Lanoconazole | 0.01–0.04 | 0.025 | |
| Terbinafine | 0.0025–0.02 | 0.0093 | |
| T. rubrum (10) | NND-502 | 0.00063–0.0025 | 0.0015 |
| Lanoconazole | 0.0013–0.01 | 0.0054 | |
| Terbinafine | 0.00063–0.005 | 0.0027 | |
In the first experiment with the tinea pedis guinea pig model, the efficacy of 3- or 7-day therapy with a 0.5% solution of NND-502 was compared with those of 0.5% solutions of lanoconazole and terbinafine (Table 2). In the 3-day treatment regimen, T. mentagrophytes was recovered from the feet of all the untreated control and solvent (polyethylene glycol 400)-treated control animals. All these animals’ feet were culture positive, with average intensity of infection scores higher than 5 (scores higher than 6 and 4 in the toe and heel portions, respectively). These results indicate that the infection spread over almost the whole soles of the infected animals. In the animals treated once daily for 3 consecutive days with a 0.5% solution of NND-502, 7 of 10 infected feet became culture negative and the rate of fungus-positive feet and the average infection intensity scores were significantly lower than those in the untreated and solvent-treated control animals. When treatment with the 0.5% solution of NND-502 was prolonged to 7 consecutive days, all 10 infected feet became culture negative. Although the 0.5% solutions of lanoconazole and of terbinafine were both highly effective in this animal model of tinea pedis, complete mycological cure of all the infected feet was not achieved with either, even after 7 days of treatment.
TABLE 2.
Therapeutic efficacy of topically applied NND-502, lanoconazole, and terbinafine in the guinea pig model of tinea pedis (first experiment)d
| Treatment | No. of culture-positive feet/total no. of feet (%) | Average intensity of infection score |
|---|---|---|
| 3-Day | ||
| Untreated control | 10/10 (100) | 5.8 |
| PEG400a | 10/10 (100) | 5.6 |
| 0.5% NND-502 | 3/10 (30)b,c | 0.25b,c |
| 0.5% Lanoconazole | 7/10 (70) | 0.8b,c |
| 0.5% Terbinafine | 5/10 (50)b,c | 0.7b,c |
| 7-Day | ||
| Untreated control | 10/10 (100) | 6.1 |
| PEG400 | 10/10 (100) | 5.7 |
| 0.5% NND-502 | 0/10 (0)b,c | 0b,c |
| 0.5% Lanoconazole | 2/10 (20)b,c | 0.1b,c |
| 0.5% Terbinafine | 1/10 (10)b,c | 0.1b,c |
Polyethylene glycol 400.
Significantly different (P < 0.05) from values obtained for the comparable untreated control group.
Significantly different from values obtained for the comparable PEG400 group.
No significant differences were found in any of the parameters among the comparable 0.5% NND-502, 0.5% lanoconazole, and 0.5% terbinafine groups.
In the second experiment, the therapeutic efficacies of three concentrations of NND-502 solution topically applied for 3 consecutive days were compared with each other and with those of reference agents (Table 3). For animals treated once daily with 0.25 and 0.5% solutions of NND-502, 3 of 10 and 8 of 10 infected feet became culture negative, respectively, and the rate of fungus-positive feet and the average intensity of infection scores were significantly lower than those for untreated and solvent-treated control animals. When the animals were treated with a 1% solution of NND-502, all infected feet became culture negative. Under the same experimental conditions, lanoconazole and terbinafine solutions also showed concentration-dependent therapeutic effects but did not achieve a mycological cure of all infected feet.
TABLE 3.
Therapeutic efficacy of topically applied NND-502, lanoconazole, and terbinafine in the guinea pig model of tinea pedis (second experiment)d
| Treatment | No. of culture-positive feet/total no. of feet (%) | Average intensity of infection score |
|---|---|---|
| Control | ||
| Untreated | 10/10 (100) | 8.2 |
| PEG400a | 10/10 (100) | 8.15 |
| NND-502 (%) | ||
| 0.25 | 7/10 (70) | 0.7b,c |
| 0.5 | 2/10 (20)b,c | 0.1b,c |
| 1 | 0/10 (0)b,c | 0b,c |
| Lanoconazole (%) | ||
| 0.25 | 9/10 (100) | 1.65 |
| 0.5 | 6/10 (60)b,c | 0.95b,c |
| 1 | 3/10 (30)b,c | 0.25b,c |
| Terbinafine (%) | ||
| 0.25 | 9/10 (90) | 1.4 |
| 0.5 | 5/10 (50)b,c | 0.45b,c |
| 1 | 3/10 (30)b,c | 0.25b,c |
Polyethylene glycol 400.
Significantly different (P < 0.05) from values obtained for the untreated control group.
Significantly different from values obtained for the PEG400 group.
No significant differences were found in any of the parameters among the comparable NND-502, lanoconazole, and terbinafine groups.
Recently, a major advance in antifungal chemotherapy, the successful short-term therapy for dermatophytoses, was achieved by the use of topical terbinafine (3–5). The 1% cream of terbinafine, applied twice daily for 1 week, was reported to be significantly superior to a 4-week course of 1% cream of clotrimazole for treating tinea pedis in humans (4). Treatment of this fungal infection with the 1% cream of terbinafine once daily for 1, 3, 5, or 7 days was also effective (5). Preclinical studies with the imidazole antifungal agent lanoconazole demonstrated that short-term treatment with this drug resulted in excellent therapeutic efficacy in a guinea pig model of tinea pedis, suggesting that the antifungal potential of lanoconazole is almost equal to that of terbinafine (14). NND-502 is a newly synthesized antifungal imidazole compound whose structure is closely related to that of lanoconazole; thus, we decided to compare its in vitro and in vivo antidermatophyte activities with those of lanoconazole and terbinafine. The in vitro activity of NND-502 against T. rubrum and T. mentagrophytes was shown to be almost equal to or higher than those of lanoconazole and terbinafine. To evaluate in vivo activity, experimental infection with T. mentagrophytes in guinea pigs’ feet was adopted because this model mimics naturally occurring tinea pedis in humans in terms of histopathology, symptomatology, and chronicity and has been successfully used for evaluation of the activities of several antifungal agents (1, 2, 14–16, 23). In this tinea pedis model, topical NND-502 solutions exhibited activity which seemed to be more potent than those of lanoconazole and terbinafine, and a 3-day treatment with a 1% solution of the agent achieved complete mycological cure. Although both reference agents were also highly effective in the model, neither their efficacies nor their in vitro anti-Trichophyton activities exceeded those of NND-502.
These results suggest that NND-502 is a promising candidate for clinical development as a topical antifungal agent that may be useful for the short-term treatment of dermatophytoses.
REFERENCES
- 1.Arika T, Yokoo M, Yamaguchi H. Butenafine, a new benzylamine derivative: in vitro effect on arthrospores of T. mentagrophytes and therapeutic efficacy on experimental tinea pedis in guinea pigs. Jpn J Med Mycol. 1992;33:541–547. [Google Scholar]
- 2.Arika T, Yokoo M, Maeda T, Amemiya K, Yamaguchi H. Effects of butenafine hydrochloride, a new benzylamine derivative, on experimental tinea pedis in guinea pigs. Antimicrob Agents Chemother. 1990;34:2254–2255. doi: 10.1128/aac.34.11.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordero, C., I. de la Rosa, Z. Espinosa, R. F. Rojas, and N. Zaias. 1992. Short-term therapy of tinea cruris/corporis with topical terbinafine. J. Dermotol. Treatment 3(Suppl. 1):23–24.
- 4.Evans E G V, Dodman B, Williamson D M, Brown G J, Bowen R G. Comparison of terbinafine and clotrimazole in treating tinea pedis. Br Med J. 1993;307:645–647. doi: 10.1136/bmj.307.6905.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans E G V, Seaman R A, James I G. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83–87. doi: 10.1111/j.1365-2133.1994.tb06888.x. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson, T. 1983. Treatment of dermatomycoses with topical tioconazole and miconazole. Dermatologica 66(Suppl. 1):20–33. [DOI] [PubMed]
- 7.Fujita S, Matsuyama T. Experimental tinea pedis by non-abrasive inoculation of Trichophyton mentagrophytes arthrospores on the plantar part of guinea pig foot. J Med Vet Mycol. 1987;25:203–213. doi: 10.1080/02681218780000541. [DOI] [PubMed] [Google Scholar]
- 8.Fujita S, Matsuyama T, Sato Y. Experimental tinea pedis in guinea pig feet-scanning electron microscopic and histological study of the infection. Jpn J Med Mycol. 1988;29:163–168. [Google Scholar]
- 9.Hirsch H A. Clinical evaluation of terconazole. European experience. J Reprod Med. 1989;34:593–596. [PubMed] [Google Scholar]
- 10.Kodama, H., Y. Niwano, K. Kanai, and M. Yoshida. April 1997. Japanese patent (Jpn Koukai Tokkyo Kouhou) H9-100279.
- 11.Kokoschka E M, Niebauer G, Mounari M, Monici Preti P. Treatment of dermatomycoses with topical fenticonazole and econazole. Mykosen. 1986;29:45–50. [PubMed] [Google Scholar]
- 12.Kuokkanen K. Topical tioconazole in dermatomycosis. Mykosen. 1982;25:274–280. [PubMed] [Google Scholar]
- 13.McVie D H, Littlewood S, Allen B R, Pollock A C, Wood P, Milne L J. Sulconazole versus clotrimazole in the treatment of dermatophytosis. Clin Exp Dermatol. 1986;11:613–618. doi: 10.1111/j.1365-2230.1986.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 14.Niwano Y, Tabuchi T, Kanai K, Hamaguchi H, Uchida K, Yamaguchi H. Short-term topical therapy of experimental tinea pedis in guinea pigs with lanoconazole, a new imidazole antimycotic agent. Antimicrob Agents Chemother. 1995;39:2353–2355. doi: 10.1128/aac.39.10.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohmi T, Konaka S, Uchida M, Yamaguchi H. Antifungal activity of a new agent latoconazole in two tinea models. Arzneim-Forsch/Drug Res. 1991;41:847–851. [PubMed] [Google Scholar]
- 16.Oka H, Niwano Y, Ohmi T, Tanaka T, Uchida M, Yamaguchi H. Therapeutic efficacy of latoconazole in formulations of clinical use on experimental dermatophytosis in guinea pigs. Arzneim-Forsch/Drug Res. 1992;42:345–349. [PubMed] [Google Scholar]
- 17.Petranyi G, Ryder N S, Stütz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984;224:1239–1241. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- 18.Seo, A., H. Kanno, N. Hasegawa, Y. Miyagi, and K. Ikeda. November 1985. Japanese patent (Jpn Koukai Tokkyo Kouhou) S60-218387.
- 19.Smith E B, Graham J L, Ulrich J A. Topical clotrimazole in tinea pedis. South Med J. 1977;70:47–48. doi: 10.1097/00007611-197701000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Stettendorf S. Tolerability and efficacy of bifonazole in dermatomycoses. Arzneim-Forsch/Drug Res. 1983;33:750–754. [PubMed] [Google Scholar]
- 21.Stütz A, Petranyi G. Synthesis and antifungal activity of (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethanamine (SF 86-327) and related allylamine derivatives with enhanced oral activity. J Med Chem. 1984;27:1539–1543. doi: 10.1021/jm00378a003. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H. New topical imidazoles under development in Japan. TJN-318 (NND-318): evaluation of antifungal activities and the results of clinical open study on dermatomycoses. In: Yamaguchi H, et al., editors. Recent progress in antifungal chemotherapy. New York, N.Y: Mercel Dekker Inc.; 1991. pp. 103–112. [Google Scholar]
- 23.Uchida K, Kudoh M, Yamaguchi H. A study on effectiveness of treatment and prevention of relapse using topical administration of terbinafine in a guinea pig model for tinea pedis. Jpn J Antibiot. 1994;47:1407–1412. [PubMed] [Google Scholar]
- 24.Wagner W, Reckers-Czaschka R. Oxiconazole in dermatomycosis—a double-blind, randomized therapy compared with bifonazole. Mykosen. 1987;30:484–492. [PubMed] [Google Scholar]