Abstract
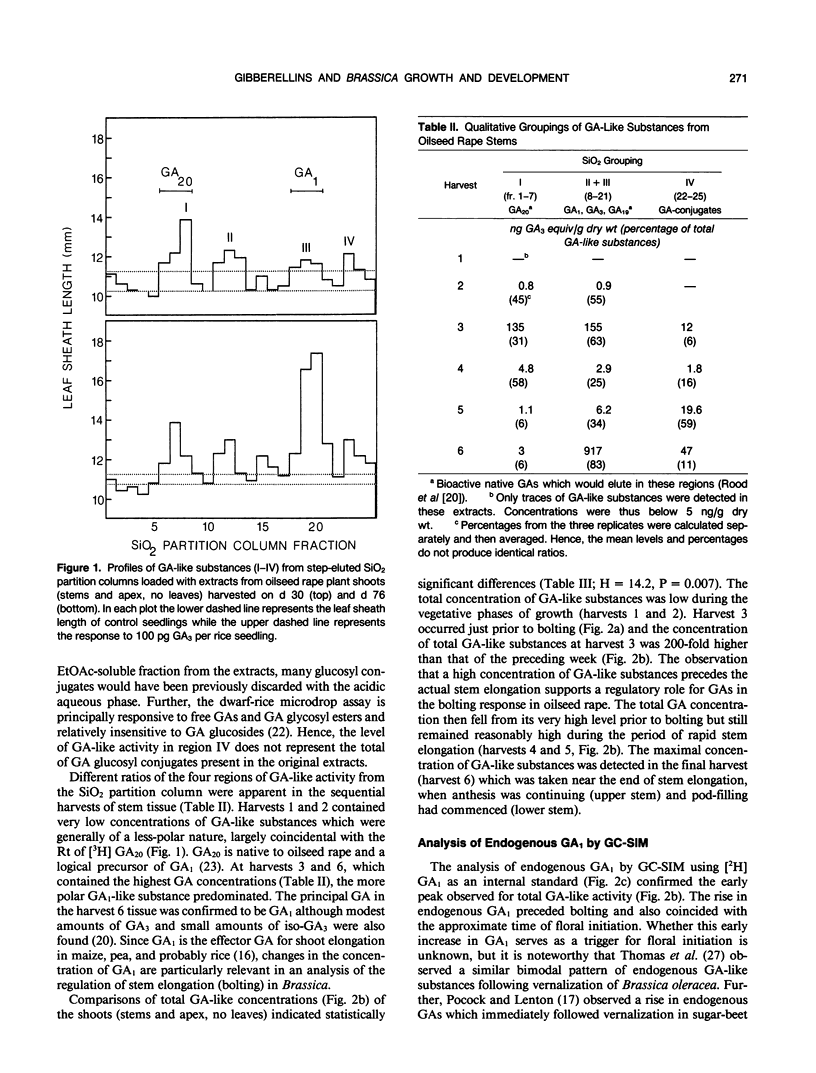
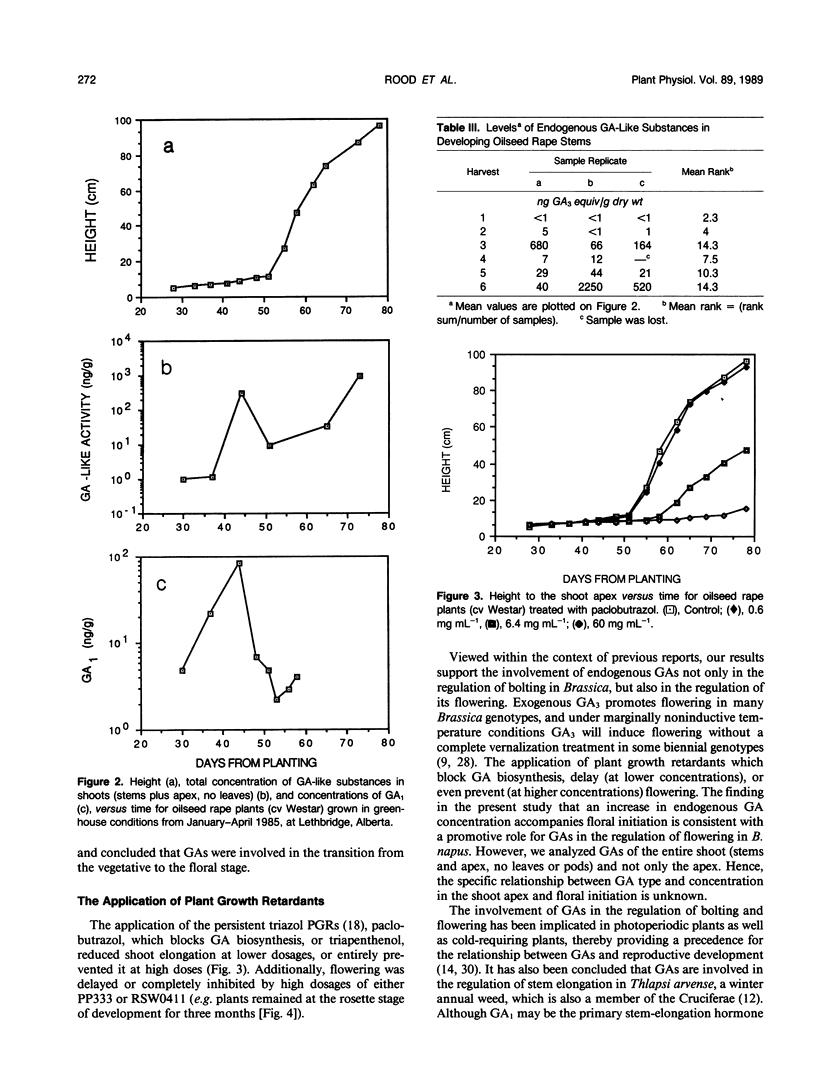
Greenhouse-grown oilseed rape (Brassica napus, annual Canola variety `Westar') plants were harvested at six dates from the vegetative phase until the early pod (silique)-fill/late flowering stage. Endogenous gibberellin (GA)-like substances were extracted from stems, purified, and chromatographed on silica gel partition columns prior to bioassay in serial dilution using the `Tan-ginbozu' dwarf rice microdrop assay. The concentrations of total endogenous GA-like substances were low during vegetative stages (1 nanogram GA3 equivalents/gram dry weight), and rose 300-fold by the time of floral initiation. After floral initiation the concentration of GA-like substances fell, then rose again during bolting to maximal levels during the early pod-fill stage (940 nanograms per gram dry weight). The qualitative profiles of GA-like substances varied across harvests, with higher proportions of a GA1-like substance at the early pod-fill stage. In a second study stems were similarly harvested at eight dates and the concentrations of endogenous GA1, the principal bioactive native GA of oilseed rape, were determined by gas chromatography-selected ion monitoring using [17,17-2H]GA1 as a quantitative internal standard. The concentration of GA1 increased at about the time of floral initiation and then subsequently fell, thus confirming the pattern noted above for total GA-like substances. The exogenous application of paclobutrazol (PP333), a persistent triazole plant growth regulator (PGR) which blocks GA biosynthesis, or another triazole, triapenthenol (RSW0411), prevented flowering as well as bolting; plants remained at the vegetative rosette stage. These results imply a causal role for endogenous GA, in the control of bolting, which normally precedes anthesis. Further, the rise in the concentration of total endogenous GA-like substances, including GA1, which was associated with floral initiation, and the prevention of visable floral development by the triazole PGRs, also indicates a role for endogenous GAs in the regulation of flowering in B. napus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- King R. W., Pharis R. P., Mander L. N. Gibberellins in Relation to Growth and Flowering in Pharbitis nil Chois. Plant Physiol. 1987 Aug;84(4):1126–1131. doi: 10.1104/pp.84.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A. THE EFFECT OF GIBBERELLIN UPON FLOWER FORMATION. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):709–717. doi: 10.1073/pnas.43.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D. Role of Gibberellins in the Environmental Control of Stem Growth in Thlaspi arvense L. Plant Physiol. 1985 May;78(1):8–13. doi: 10.1104/pp.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis R. P., Evans L. T., King R. W., Mander L. N. Gibberellins, Endogenous and Applied, in Relation to Flower Induction in the Long-Day Plant Lolium temulentum. Plant Physiol. 1987 Aug;84(4):1132–1138. doi: 10.1104/pp.84.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Larsen K. M., Mander L. N., Abe H., Pharis R. P. Identification of endogenous gibberellins from sorghum. Plant Physiol. 1986 Sep;82(1):330–332. doi: 10.1104/pp.82.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pearce D., Pharis R. P. Identification of endogenous gibberellins from oilseed rape. Plant Physiol. 1987 Nov;85(3):605–607. doi: 10.1104/pp.85.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pharis R. P., Koshioka M. Reversible conjugation of gibberellins in situ in maize. Plant Physiol. 1983 Oct;73(2):340–346. doi: 10.1104/pp.73.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suge H., Rappaport L. Role of gibberellins in stem elongation and flowering in radish. Plant Physiol. 1968 Aug;43(8):1208–1214. doi: 10.1104/pp.43.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer S. H., Bukovac M. J. Gibberellin Effects on Temperature and Photoperiodic Requirements for Flowering of Some Plants. Science. 1957 Jul 5;126(3262):30–31. doi: 10.1126/science.126.3262.30. [DOI] [PubMed] [Google Scholar]



