Abstract
Purpose
Variations of cytokines and gut microbiota diversity with improved cognitive function in patients with obesity following bariatric surgery were poorly understood. The aim of this study was to testify the relationship among gut microbiota, cytokines and cognitive function in patients with obesity before and after laparoscopic sleeve gastrectomy (LSG).
Methods
Forty patients were enrolled in this study. Demographics, and serum and stool specimens were collected from all patients before and 3 months after LSG. The Montreal Cognitive Assessment (MoCA) scale, as well as assessment of immediate and delayed memory were used to evaluate self-perceived cognitive improvement after LSG.
Results
LSG resulted in significant weight loss and improvement in cognitive functions, as measured by questionnaires. Bariatric surgery tended to increase gut microbiota relative abundance and diversity. The intestinal flora increased in the proportion of Bacteroidetes and Fusobacteria phyla, and decreased in the proportion of Firmicutes, Proteobacteria, and Actinobacteria phyla after LSG. Plasma IL-1β and TNF-α levels were significantly decreased following LSG, while IL-4 was significantly increased. MoCA test scores were significant correlated with IL-4, TNF-α and IL-1β. In addition, Firmicutes had a positive correlation with TNF-α, while Fuscobacteria had a negative correlation with IL-1β. Bacteroidetes was negatively correlated with IL-4.
Conclusion
Changes in gut microbiota were positive relationship with cognitive function improvement following LSG. Inflammation cytokines maybe played as a mediator between gut microbiota and cognitive function through gut-microbiota-brain axis.
Keywords: Laparoscopic sleeve gastrectomy, Cognitive function, Gut microbiota, Cytokine
Highlights
-
•
Bariatric surgery increased the gut bacterial diversity, as well as improved the cognitive function in patients with obesity.
-
•
Modifications in gut microbiota were positive relationship with cognitive function improvement following LSG.
-
•
Cytokines, such as IL-1β, IL-4 and TNF-α, played an important role in the positive relationship between gut microbiota and cognitive function.
1. Introduction
The global rise in obesity and overweight individuals has become a major health issue, causing a range of adverse health effects [1]. Obesity is closely linked to various diseases such as hypertension, coronary heart disease, type 2 diabetes, and cancer [2]. In addition to these health problems, obesity has also been identified as a potential cause of cognitive impairment that can significantly impair the quality of life [3]. While calorie restriction and exercise are the primary treatment modes to combat obesity, maintaining significant and long-term weight loss can be challenging and often ineffective for most individuals. Bariatric surgery is currently proved to be one of the most successful and reliable techniques capable of inducing significant and sustained weight reduction over time [4]. Laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB) are the two main bariatric surgery procedures used to treat obesity. Research has shown that bariatric surgery not only promotes weight loss, but it can also improve neurocognitive function in obese individuals. Nonetheless, the precise mechanism whereby bariatric surgery improves neurocognitive function has yet to be fully explored.
One possible explanation for the observed improvement in cognitive function following bariatric surgery is related to the gut microbiota. Recent research has suggested that the bacteria in the gastrointestinal tract can interact with the brain through the gut-brain axis, specifically affecting cognitive function [5]. The human gut contains a significant amount of bacteria, which, interestingly, is almost equal in weight to the human brain [6]. Studies in animals have shown that the gut microbiome is involved in brain development, behavior, and neurotransmitter signaling [7]. Conversely, the brain can also alter the gut microbiome through changes in mucus production, motility, and immune signaling [8]. In the context of obesity, variations in the gut microbiome have been linked to modifications in eating behavior, recovery of metabolic abnormalities, and alterations in endogenous hormone secretion [9,10]. Bariatric surgery has been shown to alter eating behavior and endogenous hormone levels and previous studies have demonstrated significant and sustained changes in the gut microbiome following LSG [11,12]. However, it remains unclear how changes in the gut microbiome may influence the observed improvement in cognitive function following bariatric surgery. In our study, we compared pre- and post-surgery data, while also investigating the role of bariatric surgery in improving cognitive function through microbial analysis and serum metabolomics.
2. Materials and methods
2.1. Patient selection and study design
This was a prospective observational study which included forty patients with obesity who treated with LSG from July 1st to December 31st of 2020 in our department. The inclusion criteria included: 1) patients between the age of 18 and 65 who considering bariatric surgery; 2) eligibility criteria of the Chinese Surgical Guidelines for Obesity and Diabetes are met; and 3) educational status was primary school or higher. The exclusion criteria included the following: 1) a history of gastrointestinal surgery; 2) inability to complete neuropsychiatric tests for any reason before or after surgery; 3) use of drugs affecting satiety, hunger or intestinal motility; 4) current or past alcohol or drug abuse, pregnancy, probiotic within 1 month before enrollment; 5) a history of mental illness, cognitive dysfunction, or malignant tumors; 6) genetical disorders. We compare the outcomes of patients with obesity before and after surgery. Demographic information, weight, the Montreal Cognitive Assessment (MoCA) scale, delayed memory evaluation, serum for metabolomics and inflammation cytokine, and stool samples for 16S rRNA were collected from patients before and after surgery. Before the surgery and 3 months after the surgery, fasting blood samples and stool samples were collected. All patients written the informed consent. Ethical Committee of our hospital approved this study.
2.2. Anthropometrics and body composition
A certified case manager measured patients’ height, weight, and body mass index (BMI) at baseline and 3 months after surgery in duplicate.
2.3. Patient questionnaire
General information was collected from the patients before and 3 months after surgery. The Montreal Cognitive Assessment (MoCA) test, as well as functional testing, parallel instant memory, and delayed memory evaluation were applied by trained cognitive testers.
The MoCA test-Beijing version was utilized in strict accordance with its guiding methods and carried out in an environment without external interference. The following eight cognitive domains were evaluated: attention and concentration; executive function; memory; language; structural skills; abstract thinking; computational skills; and orientation. The highest possible score on the MoCA test-Beijing version is 30 points; scores <27 points correspond to cognitive dysfunction. If the degree is less than 12 years, one point will be added to the recorded score [13].
The World Health Organization-University of California-Los Angeles Auditory Verbal Learning Test (WHO-UCLA AVLT) was used to evaluate memory. This scale consists of two lists (A and B), each containing 15 words from 5 categories (body parts, animals, tools, furniture, and vehicles) and 3 random words from each. List A was presented five times, subsequently, the list B was presented once. The immediate memory score was the average accurate recall of list A. After 30 min, the subject was asked to recall list A, with the accurate recall for delayed memory score.
2.4. 16S ribosomal RNA gene sequencing and analysis
16S rRNA amplicon sequencing was performed by Genesky Biotechnologies Inc. Total DNA was extracted by using FastDNA® SPIN Kit. The integrity of DNA was detected by Agarose gel electrophoresis. The concentration and purity of DNA were tested by Nanodrop 2000 and Qubit3.0 Spectrophotometer. The V3–V4 hypervariable regions of the 16S rRNA gene were amplified with the following primers: 341F (5′-CCTACGGGNGGCWGCAG-3′); and 805R (5′-GACTACHVGGGTATCTAATCC-3′).
The raw read sequences were processed in QIIME2. The adaptor and primer sequences were trimmed using the cutadapt plugin. The quality control was confirmed with DADA2 plugin which identify amplicon sequence variants (ASVs). Taxonomic assignments of ASV representative sequences were performed with a confidence threshold of 0.8 by a pre-trained Naive Bayes classifier, which was trained on RDP. 16S ribosomal RNA sequencing were tested in triplicate.
2.5. Inflammation cytokine measurements
Each enrolled patient was drawn 2 ml blood sample in an ethylene diaminotetraacetic tactic acid (EDTA) tube. Blood samples were centrifuged with 2500 RPM and 4000 RPM for 10 min. Consequently, the plasma was separated from the blood. The plasma sample was stored aseptically at −80 °C until analysis. Three inflammation cytokines (TNF-α, IL-1β and IL-4) were detected by ELISA in triplicate.
2.6. Statistical analysis
Values are expressed as the mean ± standard deviation, median with interquartile ranges or numbers (percentages). Datas of cytokine, MoCA scale score, immediate memory score and delayed memory score were analyzed with one-sample t-test. The differences of these parameters were compared using paired-sample t-test. Difference in diversity were tested using the Wilcoxon rank-sum test, and the Kruskal-Wallis rank-sum test for alpha diversity and analysis of similarities for beta diversity. Differences in KEGG and MetaCyc were tested using Welch's t-test. The correlation between cytokine and microbiota were evaluated by Spearman rank correlation tests. The p value < 0.05 was regard as statistically significant. IBM SPSS Statistics 22 were utilized for statistical analyses.
3. Results
3.1. Changes in physical parameters following LSG
Forty subjects who underwent LSG were enrolled in this study. The patients included 25 males and 15 females, with an average age of 30.3 ± 6.7 years (range, 21–49 years; Table 1). Four patients completed primary school, 20 completed middle school, and 16 completed university studies. Before surgery, the average weight was 126.8 ± 10.2 kg and BMI was 42.7 ± 4.2 kg/m2, respectively. LSG led to significant reductions in weight, BMI, and other biochemical measures at 3 months (Table 1). After surgery, the average weight was 98.8 ± 12.4 kg and BMI was 31.3 ± 5.6 kg/m2 (P < 0.05), respectively.
Table 1.
General characteristics of patients between before and three months after surgery.
| LSG |
||
|---|---|---|
| Pre-Surgery | Post-Surgery | |
| Age (years) | 30.3 ± 6.7 | |
| Gender (M: F) | 5:3 | |
| Weight (kg) | 126.8 ± 10.2 | 98.8 ± 12.4 * |
| BMI (kg/m2) | 42.7 ± 4.2 | 31.3 ± 5.6 * |
| Systolic blood pressure (mmHg) | 125.8 ± 12.1 | 122.5 ± 11.8 |
| Diastolic blood pressure (mmHg) | 72.7 ± 15.3 | 71.9 ± 12.2 |
| Fasting glucose (mmol/L) | 6.6 ± 2.3 | 6.2 ± 3.5 |
| HbA1C (%) | 6.3 ± 1.2 | 5.8 ± 1.3 |
| T-CHOL (mmol/L) | 5.5 ± 1.1 | 4.9 ± 4.2 |
| HDL-CHOL (mmol/L) | 1.2 ± 0.3 | 1.3 ± 0.3 |
| LDL-CHOL (mmol/L) | 2.9 ± 0.9 | 2.8 ± 0.8 |
BMI: Body Mass Index; M: male; F: female.
3.2. Changes in cognitive function following LSG
Cognitive function improved postoperatively. Analysis of variance showed the differences in cognitive function pre- and post-operatively were statistically significant (P < 0.05; Table 2).
Table 2.
Montreal Cognitive Assessment scale score, Immediate memory score and Delayed memory score before and three months after surgery.
| Pre-Surgery | Post-Surgery | Change value | t | P | |
|---|---|---|---|---|---|
| MoCA scale score | 25.5 ± 0.97 | 28.5 ± 0.64 | 2.9 ± 0.88 | 20.86 | <0.05 |
| Immediate memory score | 4.8 ± 0.35 | 6.1 ± 0.35 | 1.3 ± 0.42 | 19.96 | <0.05 |
| Delayed memory score | 3.1 ± 0.23 | 3.5 ± 0.24 | 0.4 ± 0.19 | 14.26 | <0.05 |
MoCA scale score: Montreal Cognitive Assessment scale score.
Analysis of variance showed that the immediate and delayed memory scores improved after LSG. The differences in immediate and delayed memory scores pre- and post-operatively were statistically significant (P < 0.05; Table 2).
3.3. Changes in gut microbiota and cytokines following LSG
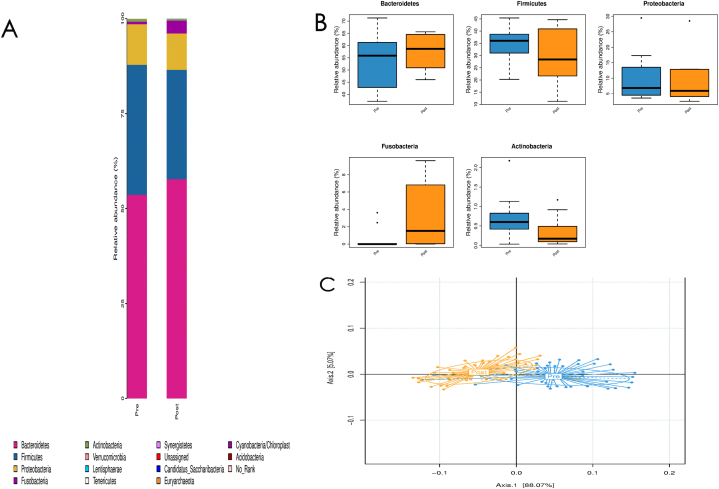
Surgery induced changes in gut microbiome abundance, diversity, and composition. After removing low abundance species (<1%), there was a trend toward an increase of bacterial alpha diversity 3 months postoperatively (3.62 ± 0.46 [pre-operatively] vs. 3.92 ± 0.52 [post-operatively], P = 0.12; Fig. 1A). The analysis of phylum changes showed that compared to pre-surgery, the relative abundance of Bacteroidetes and Fusobacteria had showed an increasing tendency without significantly following LSG. While the relative abundance of Firmicutes, Proteobacteria, and Actinobacteria had revealed a decreasing tendency without significantly (Fig. 1B). There was a significant increase in beta diversity observed after surgery as compared to before surgery (P = 0.04 [analysis of similarities]; Fig. 1C).
Fig. 1.
Changes of alpha and beta diversity pre and post bariatric surgery (A: alpha diversity of pre- and 3 months post-surgery (3.62 ± 0.46 vs. 3.92 ± 0.52, P = 0.12); B: phylum changes of pre- and 3 months post-surgery; C: beta diversity of pre- and 3 months post-surgery (P = 0.04, analysis of similarities)).
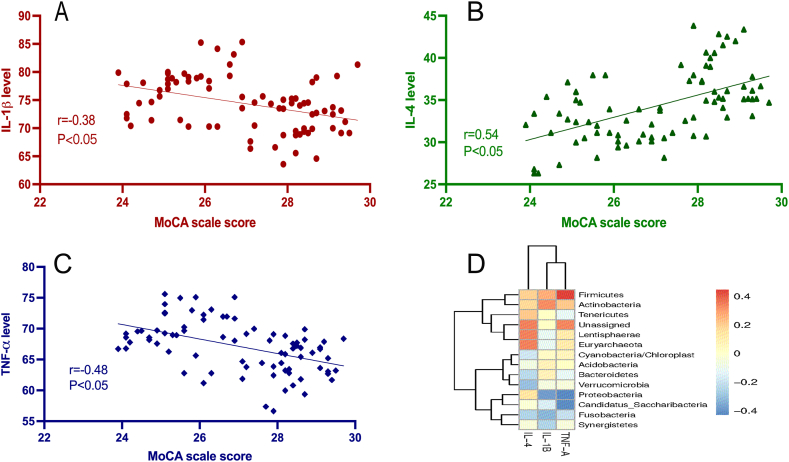
The levels of TNF-α (t = 18.85, P < 0.05) and IL-1β (t = 19.61, P < 0.05) were observed to be significantly reduced after the surgery in comparison to their levels before surgery, while IL-4 (t = −19.32, P < 0.05) was increased significantly after surgery (Table 3). Questionary score was significant positive correlated with inflammation cytokine levels, including IL-1β (r = −0.38, P < 0.0001; Fig. 2A), TNF-α (r = −0.48, P < 0.05; Fig. 2B), and IL-4 (r = 0.54, P < 0.05; Fig. 2C). In addition, the Spearman rank correlation analysis indicated that there was a significant correlation between gut microbiota and cytokines in all kinds of gut microbiota taxonomy, except for phylum (Supplementary Fig. 1). Firmicutes had a positive correlation with TNF-α and Actinobacteria had a positive correlation with IL-1β, while Fuscobacteria had a negative correlation with IL-1β. Lentisphaerae and Euryarchaeota were positively correlated with IL-4, Bacteroidetes was negatively correlated with IL-4, and Proteobacteria was negatively correlated with TNF-α and IL-1β (Fig. 2D).
Table 3.
Cytokine levels (IL-1β, TNF-α and IL-4) before and three months after surgery.
| Cytokine levels (pg/ml) | Pre-Surgery | Post-Surgery | Change value | t | P |
|---|---|---|---|---|---|
| IL-1β | 76.9 ± 4.167 | 71.8 ± 4.03 | 5.1 ± 1.65 | 19.61 | <0.05 |
| TNF-α | 69.6 ± 3.46 | 64.6 ± 3.17 | 4.9 ± 1.65 | 18.85 | <0.05 |
| IL-4 | 31.8 ± 2.99 | 36.9 ± 3.42 | 5.1 ± 1.66 | 19.32 | <0.05 |
Fig. 2.
Correlations between cognition and cytokine levels (A: correlation between cognition and IL-1β, r = −0.38, P < 0.0001; B: correlation between cognition and IL-4, r = 0.54, P < 0.05; C: correlation between cognition and TNF-α, r = -0.48, P < 0.05; D: Spearman correlation analysis of cytokine and microbiota, Firmicutes was positive correlation with TNF-α and Actinobacteria was positive correlation with IL-1β, Fuscobacteria and Proteobacteria were negative correlation with IL-1β. Lentisphaerae and Euryarchaeota were positively correlated with IL-4, Bacteroidetes was negatively correlated with IL-4, Proteobacteria was negatively correlated with TNF-α).
3.4. Correlation of gut microbiota with functional capacity
Changes in the functional capacity of gut microbiota, as indicated by KEGG and the MetaCyc pathway, were different pre- and post-operatively. There were 43 KEGG changes (Fig. 3A) and 48 MetaCyc changes (Fig. 3B) after LSG. Based on Faprotax analysis, there were 3 significant changes in the biogeochemical cycles (decreases in fermentation and chemoheterotrophs and increases in animal parasites or symbionts; Fig. 3C).
Fig. 3.
Significant changes in the relative functional abundance of gut microbiota between pre- and post-surgery (A: Forty-three KEGG significant pathways change in gut microbiota after LSG. B: Forty-eight MetaCyc significant pathways change in gut microbiota after LSG; C: Three Faprotax significant pathways change in gut microbiota after LSG).
4. Discussion
Our study is unique in that it explores the link between LSG and cognitive function through a comprehensive examination of the gut microbiome and cytokine activity. Although our sample size is small, our results indicate a strong association between LSG, enhanced cognitive performance, modifications in gut microbiome diversity and composition, and alterations in inflammation cytokine levels (including TNF-α, IL-1β, and IL-4).
Obesity is a major public health issue, with serious implications for overall health due to its association with chronic conditions such as hypertension, type 2 diabetes mellitus, and depression [14]. In addition, research suggests that obesity can negatively impact cognitive function, with up to 23.9% and 22.9% of severely obese patients experiencing learning and memory disorders, respectively [15]. In this study, we sought to investigate the impact of bariatric surgery on cognitive function by evaluating immediate and delayed memory as well as overall cognitive function in a group of 40 obese patients both before and after undergoing LSG. Our findings show that LSG significantly restored impaired cognitive function and memory, as demonstrated by questionnaire scores. Moving forward, we aim to further investigate the underlying mechanisms of neurocognitive recovery following bariatric surgery.
Recent research has highlighted the significant role of the gut microbiota in behavioral, physiological, and cognitive functions, with a growing focus in neuroscience on the interconnection between the gut microbiota and the brain [[16], [17], [18], [19]]. The gut microbiota is highly diverse, with approximately 1000 different bacteria, mainly composed of Bacteroidetes and Firmicutes phyla [[20], [21], [22]]. Our study observed a trend towards an increase in bacterial diversity and composition post-surgery, with changes in five major phyla, including an increase in Bacteroidetes and Fusobacteria, and a decrease in Firmicutes, Proteobacteria, and Actinobacteria. Previous research has shown that in individuals with obesity, the proportion of Firmicutes phyla increases while the abundance of Bacteroidetes phyla decreases, leading to a state of dysbiosis [23,24]. A high percentage of Firmicutes is associated with metabolic degradation, leading to increased caloric absorption and weight gain [25]. To better understand the communication mechanism between the brain and gut microbiota, we assessed the inflammatory cytokine levels of 40 obese patients pre- and post-LSG, including IL-1β, TNF-α, and IL-4.
While it remains unclear exactly how the gut communicates with the brain, research shows that the gut microbiome can influence the brain through various systems such as the nervous, endocrine, immune, and metabolic systems. Pro-inflammatory cytokines are one of the key immune signals that link the gut and the brain, and they can travel from the gut via the bloodstream to the nerve nucleus [26]. Studies have shown that LSG can improve serum biomarkers and modify the quantity and diversity of fecal microbiota [27]. Although pro-inflammatory cytokines are unlikely to cross the blood-brain barrier (BBB) under normal physiological conditions, there is growing evidence to suggest that they may signal across the BBB and affect brain regions such as the BBB-deficient hypothalamus. Our study found a decrease in IL-1β and TNF-α and an increase in IL-4 after LSG, consistent with previous studies linking serum biomarkers or bacterial abundance with diabetes, obesity, and bulimia nervosa [28]. Several previous studies have highlighted the negative impact of elevated levels of IL-1β on neuronal cell death and cognitive function. Conversely, other research has indicated that IL-4 may offer protective benefits to cognitive function [[29], [30], [31], [32], [33]]. Despite the research findings, it is important to note that when cytokines excessively permeate the nervous system due to a compromised BBB, it can trigger a local immune response, causing oxidative stress and mitochondrial damage. This can lead to the production of neurotoxic metabolites that worsen neuron structure and function via the kynurenine pathway. Additionally, excessive inflammation cytokines can result in direct neurotoxic effects in the brain [34,35]. In addition, our analysis showed that there was a significant positive relationship between Firmicutes and levels of TNF-α, Actinobacteria and IL-1β, whereas Fuscobacteria showed a negative correlation with IL-1β. Furthermore, Lentisphaerae and Euryarchaeota were positively associated with IL-4, providing further evidence for the connection between gut microbiome composition and cytokine levels.
There are several limitations to the current study that need to be addressed in future research. To begin with, it should be noted that the study had a relatively small sample size and the duration of follow-up was relatively brief. In addition, It is worth noting that this study employed a cross-sectional design, which limits its ability to establish causality. To fully understand the mechanisms involved in the gut-microbiota-brain axis, further research is needed to explore the structural changes in brain regions following bariatric surgery and determine the impact of obesity on BBB integrity. Therefore, future studies should focus on evaluating these factors in a longitudinal design to provide more conclusive evidence. Furthermore, it is crucial to investigate the sustainability of changes in cognitive function and gut microbiome over an extended period. Additionally, it is essential to explore the impact of obesity on the integrity of the BBB and any consequent alterations in neuro-structures.
5. Conclusion
Recent research suggests that undergoing bariatric surgery may enhance bacterial diversity and cognitive function in individuals with obesity. What's particularly noteworthy is that this is the first study to show a link between changes in gut microbiota and improved cognitive function following LSG surgery. The study sheds light on the crucial function of cytokines, such as IL-1β, IL-4, and TNF-α, in facilitating the communication between the gut, microbiota, and brain, ultimately resulting in improved cognitive abilities. It is essential to acknowledge that the outcomes of this study need to be confirmed by conducting more extensive research involving a greater number of participants. Furthermore, exploring the effects of bariatric surgery on brain structure and blood-brain barrier integrity among patients could provide added insight into the workings of the gut-microbiota-brain axis.
Funding
This work was supported by the Scientific Research Foundation of Higher Education Institutions of Anhui Province, China (grant number 2022AH050726 and 2022AH050728) and Key Research and Development Program of Anhui Province (grant number 2022e07020049).
Author contribution statement
Wanjing Chen and Jiahong Song: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yunsheng Cheng: Contributed reagents, materials, analysis tools or data.
Benli Jia and Liang Yu: Analyzed and interpreted the data.
Yawei He: Performed the experiments.
Gang Yu and Yong Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors thank Yanyan Xv, Gui Wang and Xin Gong for their critical reading of our manuscript and informative suggests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19245.
Contributor Information
Gang Yu, Email: yugang1189@sina.cn.
Yong Wang, Email: yongwangefy@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kim R., Lee D.H., Subramanian S.V. Understanding the obesity epidemic. BMJ. 2019;366:l4409. doi: 10.1136/bmj.l4409. [DOI] [PubMed] [Google Scholar]
- 2.Song X., Jousilahti P., Stehouwer C.D., Soderberg S., Onat A., Laatikainen T., Yudkin J.S., Dankner R., Morris R., Tuomilehto J., et al. Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in Europeans. Nutr. Metabol. Cardiovasc. Dis. 2015;25(3):295–304. doi: 10.1016/j.numecd.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Elias M.F., Elias P.K., Sullivan L.M., Wolf P.A., D'Agostino R.B. Obesity, diabetes and cognitive deficit: the framingham heart study. Neurobiol. Aging. 2005;26(Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Lars Sjöström M.P., Jacobson Peter, Sjöström C David, Karason Kristjan, Wedel Hans, Ahlin Sofie, Anveden Åsa, Bengtsson Calle, Bergmark Gerd, Bouchard Claude, Carlsson Björn, Dahlgren Sven, Karlsson Jan, Anna-Karin Lindroos, Lönroth Hans, Narbro Kristina, Näslund Ingmar, Olbers Torsten, Per-Arne Svensson. Lena M S carlsson: bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 5.Gareau M.G. Cognitive function and the microbiome. Int. Rev. Neurobiol. 2016;131:227–246. doi: 10.1016/bs.irn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Dinan T.G., Stilling R.M., Stanton C., Cryan J.F. Collective unconscious: how gut microbes shape human behavior. J. Psychiatr. Res. 2015;63:1–9. doi: 10.1016/j.jpsychires.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D., et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. 609 e591-593. [DOI] [PubMed] [Google Scholar]
- 8.Marilia Carabotti A.S., Antonietta Maselli Maria, Severi Carola. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 9.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matam Vijay-Kumar J.D.A., Carvalho Frederic A., Cullender Tyler C., Simon Mwangi, Srinivasan Shanthi, Shanthi V., Sitaraman, Knight Rob, Ley Ruth E., Gewirtz Andrew T. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science. 2010:328. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furet J.P., Kong L.C., Tap J., Poitou C., Basdevant A., Bouillot J.L., Mariat D., Corthier G., Dore J., Henegar C., et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholtz S., Miras A.D., Chhina N., Prechtl C.G., Sleeth M.L., Daud N.M., Ismail N.A., Durighel G., Ahmed A.R., Olbers T., et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891–902. doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith Tasha, Gildeh Nadia, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can. J. Psychiatr. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 14.Huang H., Yan Z., Chen Y., Liu F. A social contagious model of the obesity epidemic. Sci. Rep. 2016;6(1) doi: 10.1038/srep37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alosco M.L., Spitznagel M.B., Strain G., Devlin M., Cohen R., Paul R., Crosby R.D., Mitchell J.E., Gunstad J. Improved memory function two years after bariatric surgery. Obesity. 2014;22(1):32–38. doi: 10.1002/oby.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins T.A., Nguyen J.C., Polglaze K.E., Bertrand P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain Axis. Nutrients. 2016;8(1) doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith P.A. The tantalizing links between gut microbes and the brain. Nature. 2015;526:312–314. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 18.C S: mental health: thinking from the gut. Nature. 2015;518(12–15) doi: 10.1038/518S13a. [DOI] [PubMed] [Google Scholar]
- 19.Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014;34(46):15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lay C., Sutren M., Rochet V., Saunier K., Dore J., Rigottier-Gois L. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 2005;7(7):933–946. doi: 10.1111/j.1462-2920.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 21.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamant M., Blaak E.E., de Vos W.M. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 2011;12(4):272–281. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 23.J Samuel, Kallus L.J.B. The intestinal microbiota and obesity. J. Clin. Gastroenterol. 2012;46:16–24. doi: 10.1097/MCG.0b013e31823711fd. [DOI] [PubMed] [Google Scholar]
- 24.Campisciano G., Palmisano S., Cason C., Giuricin M., Silvestri M., Guerra M., Macor D., De Manzini N., Croce L.S., Comar M. Gut microbiota characterisation in obese patients before and after bariatric surgery. Benef. Microbes. 2018;9(3):367–373. doi: 10.3920/BM2017.0152. [DOI] [PubMed] [Google Scholar]
- 25.Semova I., Carten J.D., Stombaugh J., Mackey L.C., Knight R., Farber S.A., Rawls J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12(3):277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabasi M., Eybpoosh S., Siadat S.D., Elyasinia F., Soroush A., Bouzari S. Modulation of the gut microbiota and serum biomarkers after laparoscopic sleeve gastrectomy: a 1-year follow-up study. Obes. Surg. 2021;31(5):1949–1956. doi: 10.1007/s11695-020-05139-2. [DOI] [PubMed] [Google Scholar]
- 28.Tabasi M., Eybpoosh S., Sadeghpour Heravi F., Siadat S.D., Mousavian G., Elyasinia F., Soroush A., Bouzari S. Gut microbiota and serum biomarker analyses in obese patients diagnosed with diabetes and hypothyroid disorder. Metab. Syndr. Relat. Disord. 2021;19(3):144–151. doi: 10.1089/met.2020.0119. [DOI] [PubMed] [Google Scholar]
- 29.Samatra D., Pratiwi N.M.D., Widyadharma I.P.E. High il-1beta serum as a predictor of decreased cognitive function in mild traumatic brain injury patients. Open Access Maced J. Med. Sci. 2018;6(9):1674–1677. doi: 10.3889/oamjms.2018.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding H.G., Deng Y.Y., Yang R.Q., Wang Q.S., Jiang W.Q., Han Y.L., Huang L.Q., Wen M.Y., Zhong W.H., Li X.S., et al. Hypercapnia induces IL-1beta overproduction via activation of NLRP3 inflammasome: implication in cognitive impairment in hypoxemic adult rats. J. Neuroinflammation. 2018;15(1):4. doi: 10.1186/s12974-017-1051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baratz R., Tweedie D., Wang J.Y., Rubovitch V., Luo W., Hoffer B.J., Greig N.H., Pick C.G. Transiently lowering tumor necrosis factor-alpha synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice. J. Neuroinflammation. 2015;12:45. doi: 10.1186/s12974-015-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boccardi V., Westman E., Pelini L., Lindberg O., Muehlboeck J.S., Simmons A., Tarducci R., Floridi P., Chiarini P., Soininen H., et al. Differential associations of IL-4 with hippocampal subfields in mild cognitive impairment and alzheimer's disease. Front. Aging Neurosci. 2018;10:439. doi: 10.3389/fnagi.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derecki N.C., Cardani A.N., Yang C.H., Quinnies K.M., Crihfield A., Lynch K.R., Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207(5):1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaylia Jean Harry A.D.K. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expet Opin. Drug Metabol. Toxicol. 2008:1265–1277. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y.K., Won E. The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behav. Brain Res. 2017;329:6–11. doi: 10.1016/j.bbr.2017.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.