Abstract
Objectives
Several researchers performed case-control studies to explore the relationship between Hashimoto's thyroiditis(HT) and ovarian reserve using anti-Müllerian hormone(AMH) in adolescent girls and women. But the results among these studies are inconsistent and the relationship between HT and ovarian reserve is still controversial. The study aimed to conduct the meta analysis of case-control studies to confirm the relationship between HT and ovarian reserve using AMH.
Methods
6 electronic databases including PubMed, EMBASE, the Cochrane Library, China National Knowledge Internet(CNKI), SinoMed and Wanfang were searched from inception to December 2021. Endnote X7.0 software was applied to managing all the relevant records. Then data extraction and evaluation of methodological quality of included studies were conducted after two-step selection.Review manager 5.4 version software and Stata 12.0 version software were used to perform all statistical analyses.
Results
10 case-control studies involving 1202 individuals were included in the present study. The preliminary results revealed AMH values were significantly higher in adolescent girls with euthyroid HT compared with healthy adolescent girls(MD = 1.97; 95%CI, 1.43–2.51; P < 0.001; I2 = 0%). The pooled results in the subgroup of female adults with euthyroid HT showed AMH values were not significantly different between patients with HT and healthy women(MD = −0.21; 95%CI, −0.51-0.09; P = 0.18; I2 = 38%). The pooled results in the two subgroups of female adults with subclinical hypothyroidism and overt hypothyroidism both showed AMH values were significantly lower in the HT group compared with healthy women [(MD = −0.60; 95%CI, −0.86 to −0.34; P < 0.001; I2 = 0%), (MD = −1.34; 95%CI, −1.94 to −0.74; P < 0.001; I2 = 65%)].
Conclusions
Ovarian reserve evaluated by serum AMH concentration is affected by female adults with subclinical hypothyroidism and overt hypothyroidism. The AMH level was significantly higher in euthyroid adolescent girls.
Keywords: Hashimoto's thyroiditis, anti-Müllerian hormone, Ovarian reserve, Meta analysis
1. Introduction Background
Hashimoto's thyroiditis (HT), also known as autoimmune or chronic lymphocytic thyroiditis, is one of the most frequent thyroid diseases in adolescent girls and women, which diagnosed by three aspects including antibodies specific to thyroid antigens, thyroid volume, and lymphocyte infiltration of parenchyma [[1], [2], [3]]. The incidence of HT has a rapid growth in the last 3 decades and is 0.3–1.5 persons per 1000 individuals [4,5]. HT could be divided into different types including euthyroid HT, subclinical hypothyroidism, overt hypothyroidism, or hyperthyroidism based on the thyroid function [6,7].
Secreted from granulose cells of preantral follicles (PAF) and early antral follicles (EAF), anti-Müllerian hormone(AMH) is a glycoprotein which could regulate the follicle growth and inhibit the accumulation of primordial follicle [8]. AMH has an initial increase during early adulthood and then has a slow decrease with age until it couldn't be detected when the primordial follicles is depleted [9]. It is well known that serum AMH concentration accurately reflects the reserve of antral follicles, and so it is the best available marker of functional ovarian reserve at present compared with follicle-stimulating hormone (FSH), antral follicle count(AFC) and inhibin B [10,11].
Because thyroid hormones(TH) are involved in regulating the menstrual cycle and oocytes possess triiodothyronine receptors and TH receptors on the cells’ surface whichaffecting the secretion of follicle-stimulating hormone(FSH) and luteinizing hormone, the incidence of the disturbances of menstrual cycle and even the premature ovarian failure(POF) is increased in patients with HT [12,13]. Some published data indicated the disturbances of the menstrual cycle are increased as much as 3-fold in HT [14]. In order to further elucidate the impact of HT on ovarian reserve, several researchers performed case-control studies to explore the relationship between HT and ovarian reserve using AMH in adolescent girls and women. Some studies showed serum AMH values were affirmed to be significantly lower in the HT group [[15], [16], [17], [18]]. In contrary, some studies showed AMH values were not significantly different between patients with HT and healthy women [[19], [20], [21], [22]]. Two studies offered the evidence that AMH values were significantly higher in patients with HT [23,24]. The relationship between HT and ovarian reserve is still controversial, so we performed the first meta analysis of the published studies investigating the association between HT and ovarian reserve.
2. Methods
2.1. Searching strategy
The present meta analysis was rigorously performed based on the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA), and the protocol of the present meta analysis was not prepared [25]. Using the combination between Medical Subject Headings(MeSH) and key words, the six electronic databases including PubMed, EMBASE, the Cochrane Library, China National Knowledge Internet(CNKI), SinoMed and Wanfang were independently searched by two investigators(CJS and TRS) from inception to December 2021. The key words were consisted of ‘Hashimoto's thyroiditis’ and ‘anti-Müllerian hormone. Meanwhile, the manual searching was completed by finding the references of all the included studies and related-topic reviews. The language of published studies was restricted in Chinese and English. We used Endnote X7.0 software to manage all the literatures retrieved. The search strategy for PubMed was provided in Supplemental Table 1.
2.2. Selecting criteria
Firstly the selecting criteria were constructed according to the four aspects including patients, comparison, outcome measures and study design(PCOS) before performing the search. The inclusion criteria were shown as follow:
-
(P)
: All the patients were aged 12 and above, and the gender was female. The patients were clearly diagnosed with HT.
-
(C)
: Adolescent girls or women with normal levels of thyroidhormones and negative for anti-thyroperoxidase antibodies (anti-TPO) antibodies.
-
(O)
: Serum AMH values.
-
(S)
: Case-control studies or cohort studies.
The exclusion criteria were shown as follow:
-
(P)
: Patients with polycystic ovary syndrome(PCOS).
-
(S)
: Animal experiment or studies without sufficient information.
2.3. Study selection
After comprehensive searching, two investigators(CJS and XDZ) were independently assigned to review the title and abstract of all the relevant records to assess the eligibility. After that, full-text records were downloaded to further review to evaluate its eligibility.
2.4. Data extraction
After including the eligible studies, two investigators(CJS and TRS) independently extracted the basic materials including the first author, publication year, country, age, inclusion criteria, sampling time of blood, assay method, the sensitivity of assay, and AMH values. If there was any inconsistent agreement between the two investigators, the third investigator(BW) would solve the discrepancy.
2.5. Evaluation of methodological quality of included studies
Two investigators(CJS and XDZ) independently assessed the methodological quality of case-control studies or cohort studies based on the Cochrane's Newcastle-Ottawa Scale(NOS).Three quality parameters of the NOS including selection, comparability, and outcome were evaluated from 8 items. Each item is scored from 1 point, except for comparability, which could be adapted to scoring up to 2 points.When the scores were 6 and above, themethodological quality of included studies was rated as high and the total scores was 9 [26,27].The third investigator(BW) would solve thediscrepancy.
2.6. Statistical analysis
The continuous data were described with mean difference(MD) with 95% confidence interval(CI). Owing tothe heterogeneity within and across trials, random-effect model was used to perform statistical analysis of AMH values in the present meta analysis [28]. The AMH values were analyzed through the inverse variance method, with MD effect measure with 95% CI in two groups.In order to express the heterogeneity, chi-square test was used to perform the qualitative description and I2 statistic to perform the quantitative description. Whenthe value of I2 was 25–50%, 50–75%, and> 75%, it represents low, moderate, and significant heterogeneity [29]. Subgroupanalyses were conducted to assess between-group differences among the studies according to type of HT and age,andthe wald test was used for interaction for subgroup analyses.Sensitivity analysis was conducted by excluding studies one at a time in order to evaluate the robustness of pooled results about the direction and the size of adjusted effect estimates of the metaanalysis [30]. When a Pvalue is lower than0.05, it was considered statistically significant.Potential publication bias was evaluated by funnel plot, Egger's regression and Begg's rank tests (P < 0.1 was considered to be publication bias). Review manager 5.4 version softwareand Stata 12.0 version software were used to perform all statistical analyses.
3. Results
3.1. Results of searching and selecting
A total of 64 articles were captured from six electronic database. After two-step selection, 10 studies involving 1202 individuals were included in the present study [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. The flow diagram of searching and selecting of records was presented in Fig. 1.
Fig. 1.
The flow diagram of searching and selecting of records.
3.2. The basic characteristics of 10 included studies
As shown in Table 1, we concluded the basic characteristics of 10 included studies. For the 10 included studies in the meta analysis, the sample size of every study in HT group ranged from 21 to 108. The countries of publication of 10 studies were located in China, Turkey, Mexico and Poland. Three studies [19,20,24] only included the adolescent girls and the other studies enrolled female adults. Seven studies took the blood sample in the early follicular phase and three studies [15,20,21] didn't report the method of sampling. The scores of NOS of all the included studies were 6 and above, which indicating the overall methodological quality of included studies was rated as high (see Table 2).
Table 1.
The basic characteristics of all the included studies.
| Study ID | Country | HT/C (N) | Age of Patients HT/C(years) | Inclusion criteria |
sampling time of blood | Assay method | sensitivity of assay(ng/ml) | AMH HT/C (ng/mL) |
NOS Scores |
|
|---|---|---|---|---|---|---|---|---|---|---|
| HT | C | |||||||||
| Shou 2021 |
China | 63/48/45/53 | 29.71 ± 4.14/28.73 ± 3.62/28.62 ± 4.11/29.45 ± 3.84 | 3 groups: euthyroid; subclinical hypothyroidism; overt hypothyroidism |
healthy fertile women | in the early follicular phase (days 2–4) | ECLIA | – | 2.86 ± 1.05/2.40 ± 1.02/1.31 ± 1.27/3.01 ± 0.78 | 7 |
| Shou (1) 2021 |
China | 86/34/29/45 | 30.6(R:18–40)/— | 3 groups from rural women: euthyroid; subclinical hypothyroidism; overthypothyroidism |
healthy rural fertile women | – | ECLIA | – | 2.81 ± 1.02/2.21 ± 1.04/1.29 ± 1.21/2.79 ± 1.01 | 6 |
| Tuten 2014 |
Turkey | 32/49 | 34.93 ± 8.87/34.16 ± 4.74 | premenopausal women with HT under replacement therapy with thyroxine | healty women | in the early follicular phase | ELISA | 0.006 | 4.54 ± 4.59/2.45 ± 1.27 | 8 |
| Pirgon 2015 |
Turkey | 30/30 | 15.1 ± 1.4/15.2 ± 1.4 | non-obese HT, adolescents, euthyroid | healthy adolescents | in the early follicular phase | ELISA | 0.09 | 10.6 ± 10.4/7.5 ± 7.3 | 9 |
| Ünsal 2021 |
Turkey | 108/172 | 32(IQR:27.3–38)/31 (25–37) | euthyroid HT | healthy women | in the early follicular phase |
ECLIA | – | 1.99 ± 2.13/2.41 ± 2.02 | 7 |
| Akin 2018 |
Turkey | 30/30 | 14.4 ± 1.85/14.4 ± 1.85 | adolescent HT, either euthyroid or subclinical hypothyroidism | euthyroid and auto-antibody negative adolescents | – | ELISA | 0.08 | 1.96 ± 1.13/2.06 ± 1.28 | 8 |
| Felipe 2021 |
Mexico | 27/25 | 27.70 ± 4.71/26.2 ± 3.02 | primary HT | normal levels of thyroid hormones and negative for anti-TPO antibodies |
– | ELISA | 0.35 | 2.42 ± 1.86/2.77 ± 2.50 | 8 |
| Kucukler 2018 |
Turkey | 21/21/32 | 35.4 ± 5.9/34.2 ± 4.7/32.0 ± 5.1 | subclinical HT; overt HT |
healthy women | in the early follicular phase (days 2–4) | ECLIA | – | 1.5 ± 1.3/1.8 ± 2.2/2.1 ± 1.4 | 8 |
| Erol 2016 |
Turkey | 57/50 | 15.4 ± 1.4/15.1 ± 1.6 | euthyroid adolescent HT | healthy adolescents | in the early follicular phase (days 3–5) | ELISA | 0.028 | 4.68 ± 1.61/2.73 ± 1.25 | 8 |
| Adamska 2021 |
Poland | 39/46 | 26.8(IQR:24–29)/26 (25–27) | euthyroid HT | healthy women | in the early follicular phase (days 3–5) | ELISA | 0.08 | 5.09 ± 3.46/6.48 ± 3.14 | 8 |
Notes: ‘—’: No report, HT: Hashimoto's thyroiditis, C:control group, AMH: anti-Müllerian hormone, R: range from minimum to maximum, IQR: interquartile range, ECLIA: electrochemiluminescence immunoassay, ELISA: enzyme-linked immunosorbent assay.
Table 2.
Results of sensitivity analysis of all the included studies.
| Studies included | Random-effects summary MD(95% CI) | P | I2(%) | |
|---|---|---|---|---|
| adolescent girls | All studies | 1.19 [-0.66, 3.05] | 0.21 | 92 |
| Without Akin et al. | 1.97 [1.43, 2.51] | <0.001 | 0 | |
| Without Erol et al. | 0.67 [-2.01, 3.36] | 0.62 | 46 | |
| Without Pirgon et al. | 0.93 [-1.08, 2.94] | 0.36 | 96 | |
| Female adults | All studies | −0.56 [-0.96, −0.16] | 0.006 | 84 |
| Without Adamska et al. | −0.52 [-0.93, −0.11] | 0.01 | 85 | |
| Without Felipe et al. | −0.58 [-0.99, −0.16] | 0.007 | 85 | |
| Without Kucukler1 et al. | −0.56 [-0.99, −0.13] | 0.01 | 85 | |
| Without Kucukler2 et al. | −0.58 [-1.00, −0.16] | 0.007 | 85 | |
| Without Shou(1)1 et al. | −0.63 [-1.05, −0.21] | 0.003 | 82 | |
| Without Shou(1)2 et al. | −0.56 [-1.00, −0.11] | 0.01 | 85 | |
| Without Shou(1)3 et al. | −0.47 [-0.87, −0.07] | 0.02 | 82 | |
| Without Shou1 et al. | −0.61 [-1.05, −0.17] | 0.007 | 83 | |
| Without Shou2 et al. | −0.55 [-1.01, −0.09] | 0.02 | 85 | |
| Without Shou3 et al. | −0.45 [-0.79, −0.11] | 0.009 | 73 | |
| Without Tuten et al. | −0.67 [-1.05, −0.29] | 0.001 | 82 | |
| Without Ünsal et al. | −0.57 [-1.02, −0.13] | 0.01 | 85 | |
3.3. The meta analysis of AMH level
Three studies were included in the subgroup of adolescent girls and the pooled results showed AMH values were not significantly different between patients with HT and healthy adolescent girls(MD = 1.19; 95%CI, −0.66-3.05; P = 0.21; I2 = 92%) [19,20,24]. Seven studies were included in the subgroup of female adults and the pooled results revealed AMH values were significantly lower in the HT group compared with healthy women(MD = −0.56; 95%CI, −0.96 to −0.16; P = 0.006; I2 = 84%) [[15], [16], [17], [18],[21], [22], [23]].There was no significant subgroup difference (P = 0.07) between them. The results were presented in Fig. 2.
Fig. 2.
Meta analysis of AMH level.
3.4. Subgroup analysis based on types of HT
In order to further explore the association between different types of HT and AMH level, subgroup analysis was conducted according to types of HT. The pooled results of two studies including adolescent girls with euthyroid HT suggested AMH values were significantly higher in patients with HT compared with healthy adolescent girls(MD = 1.97; 95%CI, 1.43–2.51; P < 0.001; I2 = 0%; presented in Fig. 3) [19,24].
Fig. 3.
Meta analysis of AMH level in subgroup of adolescent girls.
The pooled results in the subgroup of female adults with euthyroid HT showed AMH values were not significantly different between patients with HT and healthy women(MD = −0.21; 95%CI, −0.51-0.09; P = 0.18; I2 = 38%) [[15], [16], [17], [18]]. The pooled results in the two subgroups of female adults with subclinical hypothyroidism and overt hypothyroidism both showed AMH values were significantly lower in the HT group compared with healthy women [(MD = −0.60; 95%CI, −0.86 to −0.34; P < 0.001; I2 = 0%), (MD = −1.34; 95%CI, −1.94 to −0.74; P < 0.001; I2 = 65%)] [15,16,22]. There was significant subgroup difference (P = 0.003) between them and it indicated the type of HT may be one of the reasons for heterogeneity.The results were displayed in Fig. 4.
Fig. 4.
Meta analysis of AMH level in subgroup of female adults.
3.5. Sensitivity analysis
The results of sensitivity analysis showed the direction and size of adjusted summary MD changed meaningfully and I2 was 0 when excluding one study performed by Akin et al. in the subgroup of adolescent girls [20]. Thus, only two studies were retained in the final meta-analysis of subgroup of adolescent girls [19,24].
The direction and magnitude of adjusted summary MD did not change meaningfully in the subgroup of female adults, which revealing the stability of the meta-analysis of subgroup of female adults.
3.6. Publication bias
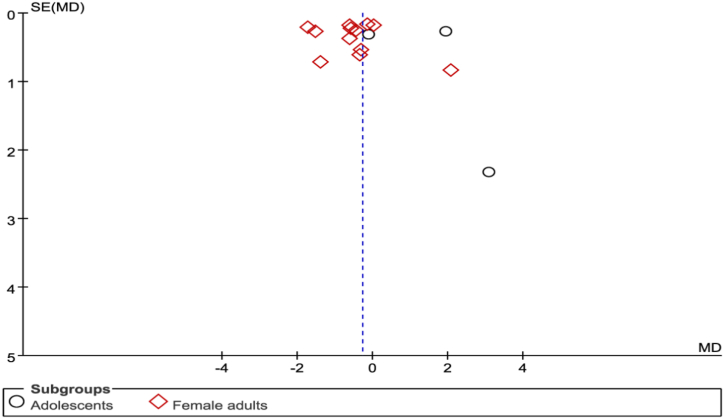
Although the number of the included studies in each subgroup was less 10, we performed the assessment of publication bias of all the included studies by funnel plot. The funnel plot showed little evidence of asymmetry, which revealing little evidence of publication bias(seen in Fig. 5).The results of Begg's (P = 0.488) and Egger's(P = 0.594) also didn't show evidence of publicationbias.
Fig. 5.
The funnel plot of 10 studies.
4. Discussion
Ovarian reserve plays an important role in pregnancy and keeping menstrual regularity and AMH is considered as the most sensitive marker to predict the ovarian reserve in clinical practice [31].In recent years, plenty of researchers explored the association between ovarian reserve and HT using AMH level. Some studies showed HT was associated with decreased AMH level [[15], [16], [17], [18]]. However, some studies showed HT was not related with AMH level [[19], [20], [21], [22]]. The relationship between HT and ovarian reserve is still controversial. No any previous meta-analysis addressing similar question was available for relative appraisal. The present study is the first meta analysis to confirm the relationship between HT and AMH level and the results of the present study could offer the evidence for clinical staffs to evaluate the ovarian reserve in women and adolescent girls with HT by serum AMH level.
In the meta analysis, the pooled analysis in subgroup of adolescent girls with HT revealed AMH values were not significantly different between patients with HT and healthy adolescent girls [19,20,24]. However, inthe subgroup of female adultsAMH values were significantly lower in the HT group compared with healthy women [[15], [16], [17], [18],[21], [22], [23]].
The results of sensitivity analysis in subgroup of adolescent girls showed the direction and size of adjusted summary MD changed meaningfully and I2 was 0 when excluding one study performed by Akin et al. , which indicating the heterogeneity probably came from types of HT. Hence, excluding the study performed by Akin et al., the subgroup analysis on the basis of types of HT was conducted. The preliminary results from two studies revealed AMH values were significantly higher in adolescent patients with euthyroid HT compared with healthy adolescent girls. Serum total testosterone and dehydroepiandrosterone sulfate(DHEAS) levelswere significantly higher in adolescents with HT than in controls.Therefore, some researchers thought higher AMH levels in adolescent girls with HT may result from hyperandrogenaemia [32]. Furthermore, impaired systemic antioxidant capacitymay causethe oxidative environment in the follicles, which inturn increasing the serum AMH level, indicating a systemicoxidant–antioxidant imbalance [33].
The pooled results in the subgroup of female adults with euthyroid HT showed AMH values were not significantly different between patients with HT and healthy women [[15], [16], [17], [18]]. The pooled results in the two subgroups of female adults with subclinical hypothyroidism and overt hypothyroidism both showed AMH values were significantly lower in the HT group compared with healthy women [15,16,22]. Thyroid hormones(TH) are involved in regulating the menstrual cycle and oocytes possess triiodothyronine receptors and TH receptors on the cells' surface whichaffecting the secretion of follicle-stimulating hormone(FSH) and luteinizing hormone. Hence, the incidence of the disturbances of menstrual cycle and even the premature ovarian failure(POF) is increased in patients with thyroid dysfunction [34]. The thyroid function of the patients with euthyroid HT is still normal, so euthyroid HT didn't have impact on ovarian reserve.
It is obliged to admit the fact that there are still some limitations in the meta analysis including 10 studies involving 1202 individuals. Eventhough the results of Begg's (P = 0.488) and Egger's(P = 0.594) also didn't show evidence of publicationbias, we did not try to uncover unpublished observations and not include studies with insufficient information to estimate an adjusted MD, which still could bring publication bias. Moreover, I2 was larger than 50% for the subgroup of overt HT in female adults, but we couldn't have a further exploration with the limited numbers of included studies and the heterogeneity didn't have an obvious impact on the pooled results.Finaly, the sample size is not adequately large, especially in subgroup of adolescent girls, which limited its strengths of evidence. More multi-center case-control studies are still required.
5. Conclusions
With the present findings, AMH values were not significantly different between patients with euthyroid HT and healthy women. Ovarian reserve evaluated by serum AMH concentration is affected by adult patients with subclinical hypothyroidism and overt hypothyroidism. The AMH level was significantly higher in euthyroid adolescent girls. However, more large multi-center studies with more rigorous methodology are required to improve the quality of evidence before determining clinical decisions owing to the presence of limitations.
Author contribution statement
Juan Cui Shi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rui Tian Shao; Dong Xu Zhao: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Bin Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19204.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weetman A.P. An update on the pathogenesis of Hashimoto's thyroiditis. J. Endocrinol. Invest. 2021;44(5):883–890. doi: 10.1007/s40618-020-01477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralli M., Angeletti D., Fiore M., D'Aguanno V., Lambiase A., Artico M., de Vincentiis M., Greco A. Hashimoto's thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020;19(10) doi: 10.1016/j.autrev.2020.102649. [DOI] [PubMed] [Google Scholar]
- 3.Ragusa F., Fallahi P., Elia G., Gonnella D., Paparo S.R., Giusti C., Churilov L.P., Ferrari S.M., Antonelli A. Hashimotos' thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019;33(6) doi: 10.1016/j.beem.2019.101367. [DOI] [PubMed] [Google Scholar]
- 4.Ott J., Meusel M., Schultheis A., Promberger R., Pallikunnel S.J., Neuhold N., Hermann M. The incidence of lymphocytic thyroid infiltration and Hashimoto's thyroiditis increased in patients operated for benign goiter over a 31-year period. Virchows Arch. 2011;459(3):277–281. doi: 10.1007/s00428-011-1130-x. [DOI] [PubMed] [Google Scholar]
- 5.Caturegli P., De Remigis A., Chuang K., Dembele M., Iwama A., Iwama S. Hashimoto's thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid. 2013;23(2):142–150. doi: 10.1089/thy.2012.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajamanickam R., Shanmugavelu L., Subramanian S., Prasad H.K., Krishnamoorthy N. Hashimoto's thyroiditis in south Indian centre. Indian J. Pediatr. 2016;83(11):1227–1231. doi: 10.1007/s12098-016-2099-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee H.S., Hwang J.S. The natural course of Hashimoto's thyroiditis in children and adolescents. J. Pediatr. Endocrinol. Metab. 2014;27(9–10):807–812. doi: 10.1515/jpem-2013-0373. [DOI] [PubMed] [Google Scholar]
- 8.Pellatt L., Rice S., Dilaver N., Heshri A., Galea R., Brincat M., Brown K., Simpson E.R., Mason H.D. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011;96(5):1246–12451.e1. doi: 10.1016/j.fertnstert.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 9.deVet A., Laven J.S., de Jong F.H., Themmen A.P., Fauser B.C. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil. Steril. 2002;77(2):357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 10.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil. Steril. 2020;114(6):1151–1157. doi: 10.1016/j.fertnstert.2020.09.134. [DOI] [PubMed] [Google Scholar]
- 11.Broer S.L., Broekmans F.J., Laven J.S., Fauser B.C. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum. Reprod. Update. 2014;20(5):688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 12.Deroux A., Dumestre-Perard C., Dunand-Faure C., Bouillet L., Hoffmann P. Female infertility and serum auto-antibodies: a systematic review. Clin. Rev. Allergy Immunol. 2017;53(1):78–86. doi: 10.1007/s12016-016-8586-z. [DOI] [PubMed] [Google Scholar]
- 13.Košir Pogačnik R., Meden Vrtovec H., Vizjak A., Uršula Levičnik A., Slabe N., Ihan A. Possible role of autoimmunity in patients with premature ovarian insufficiency. Int J Fertil Steril. 2014;7(4):281–290. [PMC free article] [PubMed] [Google Scholar]
- 14.Krassas G.E., Poppe K., Glinoer D. Thyroid function and human reproductive health. Endocr. Rev. 2010;31(5):702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 15.Shou Y.N., Jin X.P., Shou H.Q., Zhang Z.L. Anti-mullerian hormone screening analysis in rural women in reproductive age with Hashimoto's thyroiditis. Maternal and Child Health Care of China. 2021;36(6):1272–1274. doi: 10.19829/j.zgfybj.issn.1001-4411.2021.06.019. [DOI] [Google Scholar]
- 16.Shou Y.N., Jin X.P., Shou H.Q., Zhang Z.L. Correction between AMH levels and thyroid indicators in fertile women with Hashimoto's thyroiditis. Zhejiang Medicine. 2021;43(11):1213–1216. doi: 10.12056/j.issn.1006-2785.2021.43.11.2019-3248. [DOI] [Google Scholar]
- 17.Öztürk Ünsal İ., Hepşen S., Akhanlı P., Çalapkulu M., Sencar M.E., Yalçındağ A., Çakal E. Evaluation of serum anti-Müllerian hormone levels in women with Hashimoto thyroiditis in the reproductive age. Turk. J. Med. Sci. 2021 Apr 30;51(2):716–721. doi: 10.3906/sag-2012-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamska A., Popławska-Kita A., Siewko K., Łebkowska A., Krentowska A., Buczyńska A., Ł Popławski, Szumowski P., Szelachowska M., Krętowski A.J., Kowalska I. Body composition and serum anti-müllerian hormone levels in euthyroid caucasian women with Hashimoto thyroiditis. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.657752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirgon O., Sivrice C., Demirtas H., Dundar B. Assessment of ovarian reserve in euthyroid adolescents with Hashimoto thyroiditis. Gynecol. Endocrinol. 2016;32(4):306–310. doi: 10.3109/09513590.2015.1116510. [DOI] [PubMed] [Google Scholar]
- 20.Özalp Akın E., Aycan Z. Evaluation of the ovarian reserve in adolescents with Hashimoto's thyroiditis using serum anti-müllerian hormone levels. J Clin Res Pediatr Endocrinol. 2018;10(4):331–335. doi: 10.4274/jcrpe.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales-Martínez F.A., Sordia-Hernández L.H., Ruiz M.M., Garcia-Luna S., Valdés-Martínez O.H., Vidal-Gutierez O. Association between thyroid autoimmunity and ovarian reserve in women with hypothyroidism. Thyroid Res. 2021;14(1):6. doi: 10.1186/s13044-021-00095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucukler F.K., Gorkem U., Simsek Y., Kocabas R., Guler S. Evaluation of ovarian reserve in women with overt or subclinical hypothyroidism. Arch. Med. Sci. 2018;14(3):521–526. doi: 10.5114/aoms.2016.58621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuten A., Hatipoglu E., Oncul M., Imamoglu M., Acikgoz A.S., Yilmaz N., Ozcil M.D., Kaya B., Misirlioglu A.M., Sahmay S. Evaluation of ovarian reserve in Hashimoto's thyroiditis. Gynecol. Endocrinol. 2014;30(10):708–711. doi: 10.3109/09513590.2014.926324. [DOI] [PubMed] [Google Scholar]
- 24.Erol O., Parlak M., Ellidağ H.Y., Parlak A.E., Derbent A.U., Eren E., Yılmaz N. Serum anti-Müllerian hormone levels in euthyroid adolescent girls with Hashimoto's thyroiditis: relationship to antioxidant status. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;203:204–209. doi: 10.1016/j.ejogrb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., McKenzie J.E. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 27.Hartling L., Milne A., Hamm M.P., Vandermeer B., Ansari M., Tsertsvadze A., Dryden D.M. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013;66(9):982–993. doi: 10.1016/j.jclinepi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Jackson D., Turner R. Power analysis for random-effects meta-analysis. Res. Synth. Methods. 2017;8(3):290–302. doi: 10.1002/jrsm.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patsopoulos N.A., Evangelou E., Ioannidis J.P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turan V., Sonmezer M., Sonmezer M. Ongoing pregnancy and healthy live births following very short ovarian stimulation of incidentally observed big antral follicles in oligoamenorrheic patients with extremely decreased ovarian reserve. JBRA Assist Reprod. 2021;25(2):324–327. doi: 10.5935/1518-0557.20200095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen C.Y., Lossl K. Increased intrafollicular androgen levels affect human granulosa cell secretion of anti-Müllerian hormone and inhibin-B. Fertil. Steril. 2008;89(6):1760–1765. doi: 10.1016/j.fertnstert.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Diamanti-Kandarakis E., Piouka A., Livadas S., Piperi C., Katsikis I., Papavassiliou A.G., Panidis D. Anti-mullerian hormone is associated with advanced glycosylated end products in lean women with polycystic ovary syndrome. Eur. J. Endocrinol. 2009;160(5):847–853. doi: 10.1530/EJE-08-0510. [DOI] [PubMed] [Google Scholar]
- 34.Serin A.N., Birge Ö., Uysal A., Görar S., Tekeli F. Hashimoto's thyroiditis worsens ovaries in polycystic ovary syndrome patients compared to Anti-Müllerian hormone levels. BMC Endocr. Disord. 2021;21(1):44. doi: 10.1186/s12902-021-00706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.