Abstract
Ethnopharmacological relevance
Viticis Fructus (called Manjingzi in China) is the dried ripe fruits of the plant species Vitex trifolia subsp. litoralis Steenis and Vitex trifolia L. in the family Lamiaceae. Viticis Fructus has been used as a traditional Chinese medicine for thousands of years to treat illness such as colds, headache, vertigo, anesthesia, and hyperkinesias. More chemical constituents and medicinal effects have been discovered in Viticis Fructus with the development of modern technology.
The aim of the review: This review aims to analyze the research progress of Viticis Fructus from the aspects of botany, ethnopharmacology, phytochemistry, and pharmacological activity, as well as to provide an outlook on the research and use prospects of Viticis Fructus.
Material and methods
A comprehensive literature search using online databases such Science Direct, CNKI, Wiley online library, Spring Link, Web of Science, PubMed, Wanfang Data and SCI-Finder. In addition, information was obtained from local and foreign books on ethnobotany and ethnomedicine.
Results
The application of Viticis Fructus as a medicine can be traced back to around 480 AD. So far, more than 190 compounds have been isolated from Viticis Fructus, including flavonoids, sterols, cyclic enol ether terpenoids, and diterpenoids. Modern pharmacological studies have shown that the extracts of Viticis Fructus have various pharmacological effects, such as anti-allergic, antioxidant, anti-inflammatory, anti-cancer, and anti-bacterial effects.
Conclusion
As a widely used traditional medicine, Viticis Fructus is rich in chemical compositions and has an obvious biological activity. However, the application and pharmacological activity of Viticis Fructus have not been scientifically evaluated or convincing due to poor methodology, unclear results and lack of clinical data. Systematic and comprehensive research evaluations are needed to verify its pharmaceutical activity, clinical therapeutic efficacy and safety. As an important herbal medicine, it should be further explored to facilitate the development of new medicines and treatments for a variety of diseases.
Keywords: Viticis fructus, Traditional applications, Anti-allergic, Anti-cancer
1. Introduction
Viticis Fructus is the dried fruits of two perennial plants Vitex. trifolia subsp. litoralis Steenis and Vitex. trifolia L. (Lamiaceae), native to China on the coast. These species are also distributed in Korea, Japan, Australia, etc [1,2] (Fig. 1(a–c)). Viticis Fructus is known as “Manjingzi” in China, “Man Hyung Ja” in Korea, “Man keishi” in Japan which is predominantly regarded as a folk remedy. It is used to treat anemopyretic cold, headache, swelling and pain of eyes, tinnitus and deafness, in traditional Chinese medicine (TCM). In the traditional Indian medical system, tribal and local doctors use Viticis Fructus to treat a variety of ailments, including liver disease, tumors, rheumatic pain, inflammation, sprains, fever, and tuberculosis. In Korea, Viticis Fructus was used to treat several allergic diseases and upper respiratory tract infections [3].
Fig. 1.
The picture of Viticis Fructus (a: Viticis Fructus; b: V. trifolia subsp. litoralis; c: V. trifolia;d: the worldwide distribution of Viticis Fructus, red represents V. trifolia subsp. litoralis, blue represents V. trifolia, and yellow indicates the presence of both).
Because these plants have long been used to treat various diseases, a series of studies have been conducted on them, and more than 190 chemical components have been isolated from them, including flavonoids, triterpenoids, diterpenoids, iridois, phenolis, and ligans. Among them, flavonoids are considered to be the main bioactive components, most likely responsible for most of the activity of both plants. In addition, in vivo or in vitro experiments have shown that Viticis Fructus extracts have a wide range of pharmacological properties, including the following activities: anti-oxidative activities [[4], [5], [6]], anti-inflammatory activities [7,8], anticancer activities [9], Larvicidal activity [10], hepatoprotective activity [11,12], analgesic activity [13], antibacterial activity [14], antifungal activity [15], anti-feeding activity [15] and so on.
Due to the assorted medical specialty properties and complicated chemical composition of Viticis Fructus, a scientific and significant analysis of future analysis directions during this field and its applications is critical for Viticis Fructus. Therefore, the manuscript is concentrated on finding out the standard medical uses of Viticis Fructus, exploring its basal plant species, and summarizing its bioactive components as well as its pharmacological effects.
The present review aims to provide a critical and updated review of Viticis Fructus with regard to its traditional use, phytochemistry and pharmacology means to support the further research and therapeutic potential of this folk medicine.
2. Botanical description
-
V.
trifolia subsp. litoralis is perennial shrubs, prostrate to creeping, rooting at nodes; branchlets silky tomentose when young. Leaves mostly 1 (−3)-foliolate, sessile or short petiolate; blade obovate-spatulate, ovate-elliptic, broadly oblong-elliptic, or circular, 2.5–5 × 1.5–3 cm, abaxially velvety to minutely silky tomentose, adaxially usually pale dull green and pubescent, base attenuate to rounded, margin entire, apex abruptly subacuminate to rounded. Inflorescences terminal thyrses, 3–10 × 1–2.5 cm. Calyx cup-shaped, 4–5 mm, slightly 2-lipped, 5-denticulate, outside minutely silky tomentose and glandular, inside glabrous. Corolla purplish mauve to lilac blue, salverform, outside minutely silky tomentose and glandular, villous in tube and inside on lower half of large anterior lobe of lower lip. Stamens and style exserted. Ovary globose, glabrous, densely glandular. The plants flowers in month of July to September, and it produces the fruits in the month of September to November. The herb grows on the open sandy areas, usually distributed along the Chinese coastline from high latitude to low latitude, as well as in Japan, SE Asia, Pacific Islands [1].
-
V.
trifolia is shrubs or small trees, 1.5–5 m tall, erect. Branchlets densely pubescent. Leaves 1–3(-5)-foliolate; petiole 1–3 cm; leaflets sessile, oblong, lanceolate, or obovate, abaxially densely gray tomentose, adaxially green and glabrous or subglabrous, base cuneate, margin entire, apex obtuse, veins circa 8 pairs and slightly prominent on both surfaces; central or single leaflet 2.5–9 × 1.7–3 cm. Panicles 3–15 cm; peduncle densely gray tomentose. Calyx slightly 5-dentate, outside gray pubescent, inside glabrous. Corolla purplish to bluish purple, 6–10 mm, outside scaly white, pubescent at filament bases and on inside of lower lobe. Stamens exserted. Ovary glabrous, with or without glands. Style glabrous. The plant flowers in the month of April to August, and it produces the fruits in the month of August to November [1].
The herb grows in Fujian, Guangdong, Hainan, Yunnan, Guangxi, etc., and also grows in S and SE Asia, Australia, Pacific Islands.
The distribution of these two plants is very similar, as shown in Fig. 1(d) [16]. However, the classification of two plant sources of Vitex Fructus has always been controversial. In the 2020 edition of the Pharmacopoeia of the People's Republic of China, V. trifolia subsp. litoralis is considered as a variant of V. trifolia. In the newly published Flora of China, V. trifolia subsp. litoralis is identified as V. rotundifolia L. f., which is an independent species of Vitex, because it can be identified by its usually simple leaves.
3. Traditional use in TCM
Viticis Fructus has an ancient history as a medicine in Asian countries, particularly in China, and is universally used as herbal medicine in the herbal books of ancient times and in the Pharmacopoeia of the People's Republic of China [2]. According to the theory of TCM, it can drain the wind heat and clear the leader. According to the application of classical prescription XiangSuSan,Viticis Fructus has been used to treat cold, invigorating qi for strengthening superficies, and right evil spirits by ingesting it orally [17]. In the TCM theory, Viticis Fructus is also used to treat dizziness, headaches, swelling and pain of eye and tinnitus and deafness. Many studies support the use of headache, migraine and vertigo. In the clinic practice of TCM, it is reported to treat senile cataract, gastritis, acute mastitis and habitual constipation [18]. Traditionally, the treatments of various kinds of pain with headache as the main treatment of the Viticis Fructus. The clinical treatment is usually used Viticis Fructus to treat nasitis, supra-orbital neuralgia [19], vascular headache [20],migraine, douloureux tic [21], sciatica [22], nerve root type cervical vertigo, brain disease [23].
Viticis Fructus is commonly used in herbal formulations in TCM. There are many prescriptions containing Viticis Fructus with a wide range of pharmacological effects, and these have good therapeutic effects. We have collected traditional prescriptions for the application of Viticis Fructus, which are shown in Table 1.
Table 1.
Traditional prescriptions containing Viticis Fructus.
| Compound Preparation name | Main TCM | Traditional clinical use | References |
|---|---|---|---|
| Qi Wei Ke Teng Zi Wan | Entada phaseoloides(L.) Merr.; Croton tiglium L.; Ferula sinkiangensis K. M. Shen; Piper nigrum L.; Viticis Fructus; V. trifolia; Nigella glandulifera Freyn et Sint.; Eclipta prostrata (L.) L. | Dispelling summer heat, harmonizing the middle, relieving spasm and pain. Used for vomiting and diarrhea, abdominal pain, chest tightness, dysthymia, headache and fever. | [2] |
| Xiong Ju Shang Qing Wan | Ligusticum sinense 'Chuanxiong'; Chrysanthemum morifolium Ramat.; Scutellaria baicalensis Georgi; Gardenia jasminoides J. Ellis; Viticis Fructus; Coptis chinensis Franch.; Mentha canadensis L.; Forsythia suspensa (Thunb.) Vahl; Nepeta cataria L.; Hansenia weberbaueriana (Fedde ex H. Wolff) Pimenov & Kljuykov; Conioselinum anthriscoides (H. Boissieu) Pimenov & Kljuykov; Platycodon grandiflorus (Jacq.) A. DC.; Saposhnikovia divaricata (Turcz.) Schischk.; Glycyrrhiza uralensis Fisch.; Angelica dahurica (Fisch. ex Hoffm.) Benth. & Hook. f. ex Franch. & Sav. | Clearing heat and relieving symptoms, dispersing wind and relieving pain. Used for the symptoms of external wind, such as bad wind and body heat, migraine headache, runny nose, toothache and sore throat. | [2] |
| Fu Ke Yang Kun Wan | Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. & C. A. Mey.; Glycyrrhiza uralensis Fisch.; Ligusticum sinense 'Chuanxiong'; Angelica sinensis (Oliv.) Diels; Corydalis yanhusuo (Y. H. Chou & C. C. Hsu) W. T. Wang ex Z. Y. Su & C. Y. Wu; Scutellaria baicalensis Georgi; Curcuma aromatica Salisb.; Dolomiaea souliei (Franch.) C. Shih; Eucommia ulmoides Oliv.; Cyperus rotundus L.; Paeonia lactiflora Pall.; Viticis Fructus; Amomum villosum Lour. | Diversifying the liver and Qi, nourishing the blood and invigorating it. Used for irregular menses, amenorrhoea, dysmenorrhea and period headache due to blood weakness and depression of the liver. | [2] |
| Bo Yun Tui Yi Wan | Buddleja officinalis Maxim.; Tribulus terrestris L.; Chrysanthemum morifolium Ramat.; Equisetum hyemale L.; Snake slough; Cicada slough; Nepeta cataria L.; Viticis Fructus; Mentha canadensis L.; Angelica sinensis (Oliv.) Diels; Ligusticum sinense 'Chuanxiong'; Coptis chinensis Franch.; Lycium chinense Mill.; Zanthoxylum bungeanum Maxim.; Broussonetia papyrifera (L.) L'Hér. ex Vent.; Trichosanthes kirilowii Maxim.; Glycyrrhiza uralensis Fisch. | Dispersing wind and clearing heat, reducing opacity and brightening the eyes. Used for external obstruction of the eyes due to wind-heat upheaval, blurred vision, hidden pain and tears. | [2] |
| Huang Lian Shang Qing Pian | Coptis chinensis Franch.; Gardenia jasminoides J. Ellis; Forsythia suspensa (Thunb.) Vahl; Viticis Fructus; Saposhnikovia divaricata (Turcz.) Schischk.; Nepeta cataria L.; Angelica dahurica (Fisch. ex Hoffm.) Benth. & Hook. f. ex Franch. & Sav.; Scutellaria baicalensis Georgi; Chrysanthemum morifolium Ramat.; Mentha canadensis L.; Rheum palmatum L.; Phellodendron chinense C. K. Schneid.; Platycodon grandiflorus (Jacq.) A. DC.; Ligusticum sinense 'Chuanxiong'; gypsum; Inula japonica Thunb.; Glycyrrhiza uralensis Fisch. | Dispersing wind and clearing heat, relieving fire and pain. Used for dizziness and dizziness, violent fire eyes, tooth pain, sore mouth and tongue, sore throat, earache and tinnitus, constipation, short urine and red urine caused by wind-heat attack and heat in the lung and stomach. | [2] |
| Yi Qi Cong Ming Wan | Actaea cimicifuga L.; Pueraria montana (Lour.) Merr.; Phellodendron chinense C. K. Schneid.; Paeonia lactiflora Pall.; Viticis Fructus; Codonopsis pilosula (Franch.) Nannf.; Astragalus membranaceus var. mongholicus (Bunge) P. K. Hsiao; Glycyrrhiza uralensis Fisch. | Benefiting Qi, raising Yang, clearing the ears and brightening the eyes. For dimming of vision, deafness and tinnitus. | [2] |
| Ya Jiao Ha Dun San | Asparagus officinalis L.; Streptocaulon juventas (Lour.) Merr.; Benincasa hispida (Thunb.) Cogn.; Tacca chantrieri André; Duhaldea cappa (Buch.-Ham. ex DC.) Anderb.; Viticis Fructus | Clearing heat and removing toxins, relieving pain and stopping bleeding. Used for cold and fever, laryngitis, pain in the chest and abdomen, palpitations of false labor, menstrual disorders, postpartum bleeding. | [2] |
| Zhang Yan Ming Pian | Acorus calamus L.; Senna tora (L.) Roxb.; Cistanche deserticola Ma; Pueraria montana var. lobata (Ohwi) Maesen & S. M. Almeida; Celosia argentea L.; Codonopsis pilosula (Franch.) Nannf.; Viticis Fructus; Lycium chinense Mill.; Plantago asiatica L.; Paeonia lactiflora Pall.; Cornus officinalis Sieb. & Zucc.; Glycyrrhiza uralensis Fisch.; Cuscuta chinensis Lam.; Actaea cimicifuga L.; Prinsepia uniflora Batalin; Chrysanthemum morifolium Ramat.; Buddleja officinalis Maxim.; Ligusticum sinense 'Chuanxiong'; Polygonatum sibiricum Redouté; Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. & C. A. Mey.; Phellodendron chinense C. K. Schneid.; Astragalus membranaceus var. mongholicus (Bunge) P. K. Hsiao | Tonifying the liver and kidney, lightning cataracts and brightening the eyes. For dryness and discomfort, double vision in one eye, lumbar and knee weakness or mild loss of vision caused by deficiency of liver and kidney; for the above symptoms of cataract in early and middle age. | [2] |
4. Phytochemisty
In addition to the Viticis Fructus, the chemical constituents of V. trifolia subsp. litoralis and V. trifolia have been investigated in this review. Up to date, more than 190 compounds including diterpenes (104), triterpenes (11), flavonoids (19), iridoids (16), lignans (16), phenolics (22), steroids (3) etc. representing a wide spectrum of secondary metabolite classes, have been isolated and identified from V. trifolia subsp. litoralis and V. trifolia. The phytochemical investigations of both species showed that terpenes and flavonoids are their main metabolites. Table 2, Fig. 2 and Fig. 3 show the name and structures of the isolated compounds.
Table 2.
Compounds isolated from Viticis Fructus.
| Type | NO. | Chemical component | References |
|---|---|---|---|
| Diterpenes and diterpen glycosides | 1 | dihydrosolidagenone | [24] |
| 2 | vitetrifolin B | [24] | |
| 3 | rotundifuran | [24] | |
| 4 | abietatriene 3 b-ol | [24] | |
| 5 | ferruginol | [4] | |
| 6 | vitetrifolin A | [24] | |
| 7 | vitetrifolin C | [24] | |
| 8 | previtexilactone | [25] | |
| 9 | 6-acetoxy-9-hydroxy-13(14)-labden-16,15-olide | [26] | |
| 10 | vitexilactone | [26] | |
| 11 | viteagnusin I | [27] | |
| 12 | vitexifolin E | [28,26] | |
| 13 | vitrifolin A | [26] | |
| 14 | vitexifolin A | [28] | |
| 15 | vitexifolin B | [28] | |
| 16 | vitexifolin C | [28] | |
| 17 | vitexifolin D | [28] | |
| 18 | trisnor-γ-lactone | [28] | |
| 19 | isoambreinolide | [28] | |
| 20 | 13-hydroxy-5(10),14-halimadien-6-one | [6] | |
| 21 | vitetrifolin D | [29] | |
| 22 | vitetrifolin E | [29] | |
| 23 | vitetrifolin F | [29] | |
| 24 | vitetrifolinH | [29] | |
| 25 | vitetrifolin G | [29] | |
| 26 | monoacetate | [29] | |
| 27 | 9,13-epoxy-16-nor- labda-13E-en-15-al | [27] | |
| 28 | 13-epi-2-oxokolavelool | [27] | |
| 29 | isolophanthin A | [27] | |
| 30 | vitedoin B | [27] | |
| 31 | viteagnusin F | [27] | |
| 32 | viteagnusin G | [27] | |
| 33 | viterotulin A | [27] | |
| 34 | (rel 3S,5S,8R,9R,10S)-3,9-dihydroxy-13(14)- labden-16,15-olide | [27] | |
| 35 | viterotulin B | [27] | |
| 36 | vitexilactone B | [30] | |
| 37 | 6α,7α-diacetoxy-13-hydroxy-8(9),14-labdadien | [6] | |
| 38 | 9-hydroxy-13(14)-labden-16,15-olide | [6] | |
| 39 | deacetylvitexilactone | [30] | |
| 40 | viteoside A | [4] | |
| 41 | (rel 5S,6R,8R,9R,10S,13R,15R)-6-acetoxy-9,13; 15,16-diepoxy- 15-methoxylabdane. | [4] | |
| 42 | (rel 5S,6R,8R,9R,10S,13R,15S)-6-acetoxy-9,13; 15,16- diepoxy-15-methoxylabdane | [4] | |
| 43 | (rel 5S,6R,8R,9R,10S,13S,15S)-6-acetoxy-9,13; 15,16- diepoxy-15-methoxylabdane | [4] | |
| 44 | (rel 5S,6R,8R,9R,10S,13S,15R)-6-acetoxy-9,13; 15,16- diepoxy-15-methoxylabdane | [4] | |
| 45 | (rel 5S,6R,8R,9R,10S,13S,15S,16R)-6-acetoxy-9,13; 15,16-diepoxy-15,16-dimethoxylabdane | [4] | |
| 46 | (rel 5S,6R,8R,9R,10S,13S,15R,16R)-6-acetoxy-9,13; 15,16-diepoxy-15,16-dimethoxylabdane | [4] | |
| 47 | (rel 5S,6R,8R,9R,10S,13S,15S,16S)-6-acetoxy-9,13; 15,16-diepoxy-15,16-dimethoxylabdane | [4] | |
| 48 | (rel 5S,6R,8R,9R,10S,13S,15R,16S)-6-acetoxy-9,13; 15,16-diepoxy-15,16-dimethoxylabdane | [4] | |
| 49 | (rel 5S,6R,8R,9R,10S)-6-acetoxy-9-hydroxy-13(14)-labden-16,14-olide | [29] | |
| 50 | (rel 5S,6S,8R,9R,10S)-6-acetoxy-9-hydroxy-13(14)-labden-16,15-olide | [29] | |
| 51 | (rel 5S,6R,8R,9R,10S)-6-acetoxy-9-hydroxy-15- methoxy-13(14)-labdan-16,15-olide | [29] | |
| 52 | (rel 5S,6R,8R,9R,10S,13R,16S)-6-acetoxy-9,13-epoxy-16-methoxy-labdan-15,16-olide | [29] | |
| 53 | (rel 5S,6R,8R,9R,10S,13R)-6-acetoxy-9,13-epoxy-15-methoxy-labdan- 16,15-olide | [29] | |
| 54 | (rel 5S,6R,8R,9R,10S,13S,16S)-6-acetoxy-9,13-epoxy-16-methoxy-labdan-15,16-olide | [29] | |
| 55 | (rel 5S,6R,8R,9R,10S,13S)-6-acetoxy-9,13-epoxy-15-methoxy-labdan- 16,15-olide | [29] | |
| 56 | (rel 5S,8R,9R,10S,13S,15S,16R)-9,13; 15,16-diepoxy-15,16-dimethoxy-labdane | [29] | |
| 57 | (rel 5S,8R,9R,10S,13S,15R,16S)-9,13; 15,16-diepoxy-15,16- dimethoxylabdane | [29] | |
| 58 | (rel 5S,8R,9R,10S,13S,15R,16R)-9,13; 15,16-diepoxy-15,16- dimethoxylabdane | [29] | |
| 59 | (rel 5S,6R,8R,9R,10S)-6-acetoxy-9-hydroxy-13(14)- labden-16,15-olide | [31] | |
| 60 | vitetrifolin I | [32] | |
| 61 | vitextrifolin A | [30] | |
| 62 | vitextrifolin B | [30] | |
| 63 | vitextrifolin C | [30] | |
| 64 | vitextrifolin D | [30] | |
| 65 | vitextrifolin E | [30] | |
| 66 | vitextrifolin F | [30] | |
| 67 | vitextrifolin G | [30] | |
| 68 | negundol | [30] | |
| 69 | chastol | [33] | |
| 70 | epichastol | [33] | |
| 71 | vitextrifloxide A | [34] | |
| 72 | vitextrifloxide B | [34] | |
| 73 | prevetexilactone | [34] | |
| 74 | vitextrifloxide C | [34] | |
| 75 | vitextrifloxide D | [34] | |
| 76 | vitextrifloxide E | [34] | |
| 77 | vitextrifloxide F | [34] | |
| 78 | vitextrifloxide G | [34] | |
| 79 | vitextrifloxide H | [34] | |
| 80 | vitexfolin B | [34] | |
| 81 | vitextrifloxide I | [34] | |
| 82 | viterofolin A | [35] | |
| 83 | viterofolin B | [35] | |
| 84 | viterofolin C | [35] | |
| 85 | viterofolin D | [35] | |
| 86 | viterofolin E | [35] | |
| 87 | viterofolin F | [35] | |
| 88 | viterofolin G | [35] | |
| 89 | viterofolin H | [35] | |
| 90 | (3S,5S,6S,8R,9R,10S)-3,6,9-trihydroxy-13(14)-labdean-16,15-olide 3-O-β-D-glucopyranoside | [36] | |
| 91 | viteagnuside A | [29,36] | |
| 92 | viterotulin C | [37] | |
| 93 | vitexilactone D | [37] | |
| 94 | vitetrolins A | [38] | |
| 95 | vitetrolins B | [38] | |
| 96 | vitetrolins C | [38] | |
| 97 | vitetrolins D | [38] | |
| 98 | abietane 9 (11):12 (13) -di-a-epoxide | [24] | |
| 99 | (4aS,4bR,5aR,6S,7R,8aS,10aS)-7-isopropyl-6-methoxy-1,1,4a-trimethyldodecahydro-1H-phenanthro [4,4a-b]oxiren-7-ol | [24] | |
| 100 | ent-2R,15,16,19-tetrahydroxypimar-8(14)-ene | [24] | |
| 101 | abieta-9 (11),12-diene | [24] | |
| 102 | (E)-2-((2R,2′R,4a′S,8a′S)-2′,5′,5′,8a′-tetramethyldecahydro-2′H,5H-spiro[furan-2,1′-naphthalen]-5 ylidene)acetaldehyde | [26] | |
| 103 | (Z)-2-hydroxy-2-((2R,2′R,4a′S,8a′S)-2′,5′,5′,8a′-tetramethyldecahydro-2′H,5H-spiro[furan-2,1′-naphthalen]-5-ylidene)acetic acid | [26] | |
| 104 | (2R,2′R,4′R,4a′S,8a′S,E)-2′,5′,5′,8a′-tetramethyl-5-(2-oxoethylidene)decahydro-2′H,3H-spiro[furan-2,1′-naphthalen]-4′-yl acetate | [26] | |
| Triterpenes | 105 | α-amyrin | [6] |
| 106 | uvaol | [39] | |
| 107 | 3-epi-ursolic acid | [39] | |
| 108 | 2α,3β,24-trihydroxyolean-12-en-28-oic acid | [39] | |
| 109 | 2α,3α,24-trihydroxyurs-12-en-28-oic acid | [39] | |
| 110 | 2α,3α,24-trihydroxyolean-12-en-28-oic acid | [39] | |
| 111 | 2α,3α,24-trihydroxyolean-12-en-28-oic acid-28-O-β-D-glucopyranosyl ester | [39] | |
| 112 | ursolic acid | [6] | |
| 113 | 3β-acetyloxy-12-en-28-ursolic acid | [40] | |
| 114 | oleanolic acid | [41] | |
| 115 | 3β-hydroxy-30-al-urs-12-en-28-oic acid | [42] | |
| Flavones and flavone glycosides | 116 | vitexin | [6] |
| 117 | persicogenin | [31] | |
| 118 | luteolin | [43] | |
| 119 | vitexicarpin | [43] | |
| 120 | artemetin | [28] | |
| 121 | casticin | [28] | |
| 122 | centaureidin | [28] | |
| 123 | chrysosplenol-D | [31] | |
| 124 | 2′,3′,5-trihydroxy-3,6,7-trimethoxyflavone | [9] | |
| 125 | penduletin | [31] | |
| 126 | taxifolin | [4] | |
| 127 | emodin | [41] | |
| 128 | chrysophanol | [41] | |
| 129 | physcion | [41] | |
| 130 | 5,5′-dihydroxy-4′,6,7-trimethoxyflavanone | [43] | |
| 131 | agestricin D | [44] | |
| 132 | oroxylin A | [45] | |
| 133 | kaempferol | [46] | |
| 134 | quercetin | [46] | |
| Iridoids | 135 | rotundial | [47] |
| 136 | negundoside | [48] | |
| 137 | agnuside | [48] | |
| 138 | 6′-ρ-hydroxy benzoyl mussaenosidic acid | [48] | |
| 139 | viteoids I | [4] | |
| 140 | viteoids II | [4] | |
| 141 | eucommiol | [4] | |
| 142 | iridolactone | [4] | |
| 143 | pedicularislactone | [4] | |
| 144 | vr-i | [4] | |
| 145 | 1-oxo-eucommiol | [4] | |
| 146 | mussaenosidic acid | [6] | |
| 147 | (1S, 5S,6R,9R)-10-O-p-hydroxybenzoyl-5,6β-dihydroxy iridoid 1-O-β-D-glucopyranoside | [36] | |
| 148 | nishindaside | [36] | |
| 149 | 3-normal-butyl-nishindaside | [36] | |
| 150 | 3-normal-butyl-isonishindaside | [36] | |
| Lignans and lignan glycosides | 151 | detet rahydroconidendrin | [41] |
| 152 | vitedoamine A | [41] | |
| 153 | vitrofolal A | [49] | |
| 154 | vitrofolal B | [49] | |
| 155 | vitrofolal C | [49] | |
| 156 | vitrofolal D | [49] | |
| 157 | vitrofolal E | [49] | |
| 158 | vitrofolal F | [49] | |
| 159 | 4-(3,4-dimethoxyphenyl)-6-hydroxy-5-methoxynaphtho [2,3-c]furan-1(3H)-one | [49] | |
| 160 | 4-(3,4-dimethoxyphenyl)-6-hydroxy-7-methoxynaphtho [2,3-c]furan-1(3H)-one | [49] | |
| 161 | 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-7-methoxynaphtho [2,3-c]furan-1(3H)-one | [49] | |
| 162 | vitrifol A | [50] | |
| 163 | (+)-lariciresinol | [27] | |
| 164 | fiscusesquilignan A | [27] | |
| 165 | viterolignan A | [27] | |
| 166 | viterolignan B | [27] | |
| Phenolics and phenolic glycosides | 167 | vanillic acid | [4] |
| 168 | threo-guaiacyl glycerol | [4] | |
| 169 | erythro-guaiacyl glycerol | [4] | |
| 170 | dihydrodehydrodiconiferyl alcohol | [4] | |
| 171 | dihydrodehydrodiconiferyl alcohol-9- O-ρ-D-glucoside | [4] | |
| 172 | dihydrodehydrodiconiferyl alcohol-(4–8)-erythro-guaiacyl glycerol ether | [4] | |
| 173 | 4-hydroxybenzoic acid methyl ester | [43] | |
| 174 | vanillic acid methyl ester | [43] | |
| 175 | 4-hydroxy benzaldehyde | [43] | |
| 176 | 4-hydroxy benzoic acid | [43] | |
| 177 | ferulic acid | [43] | |
| 178 | vitexfolin A | [13] | |
| 179 | vitexfolin B | [13] | |
| 180 | ρ-methoxy benzoic acid | [6] | |
| 181 | 2-hydroxy,3- methoxy benzoic acid | [6] | |
| 182 | 2,3-dihydroxy benzoic acid | [6] | |
| 183 | 3,4-dihydroxybenzoic acid | [41] | |
| 184 | 4-hydroxy-3-methoxybenzoic acid | [41] | |
| 185 | caffeic acid | [41] | |
| 186 | protocatechuic acid | [46] | |
| 187 | coniferylaldehyde | [46] | |
| 188 | ficusa | [27] | |
| Steroids and glycosides | 189 | β-rosaterol palmitate | [39] |
| 190 | β-sitosterol | [39] | |
| 191 | β-daucosterol | [39] |
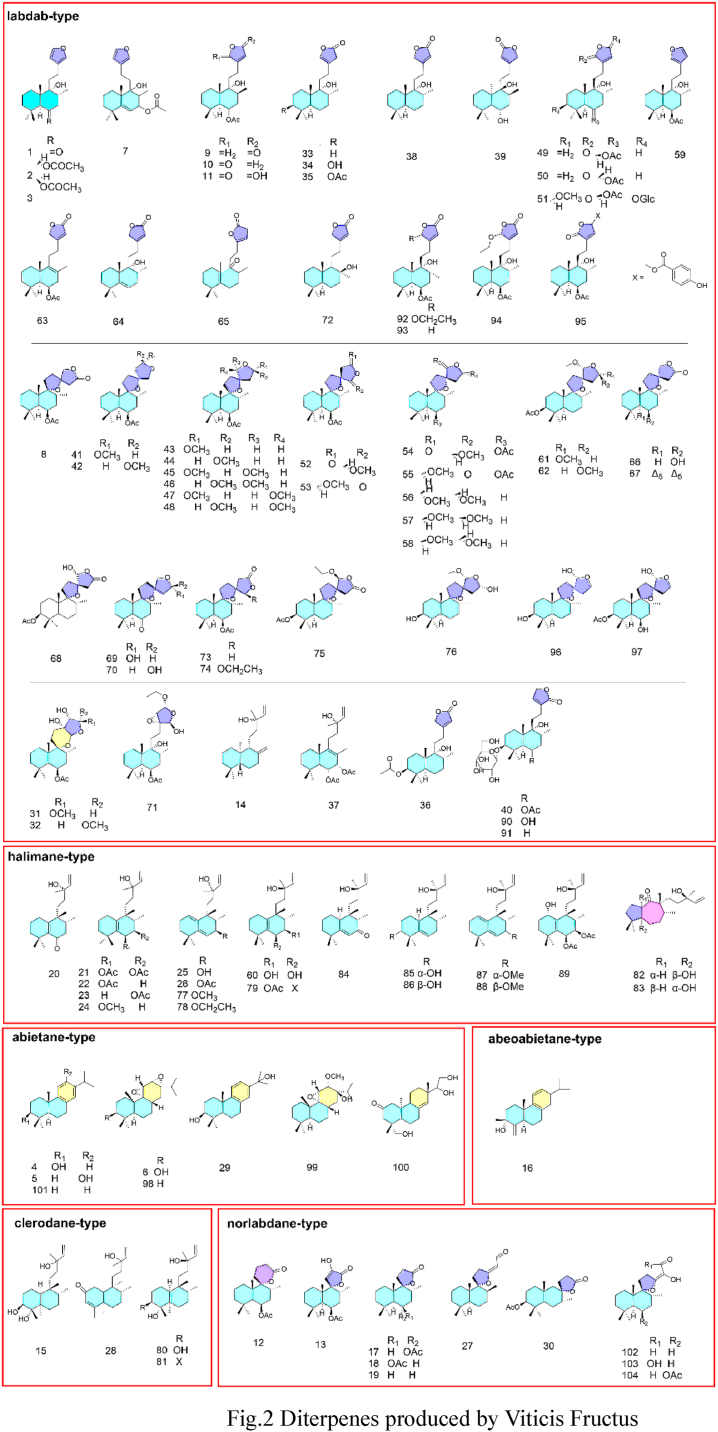
Fig. 2.
Diterpenes produced by Viticis Fructus.
Fig. 3.
Triterpenes, flavones, iridoids, lignans, phenolics and sterodis produced by Viticis Fructus.
The V. trifolia subsp. litoralis and V. trifolia are rich in diterpenes, the types of diterpenes including Ladane-Type (62) [[24], [27], [28], [29]], Halimane-Type (19) [6,29,27], Abietane-Type (8) [24], Norlabdane-Type (10) [28], Abeoabietane-Type (1) [28], Clerodane-Type (4) [28]. Besides the diterpenes, a range of terpenoids, steroides, lignans, flavonids, iridoids, phenolics and other type of compounds have been reported from these two species.
5. Pharmacological properties
5.1. Anti-allergic activity
To evaluate the antiallergic activity of the aqueous extract of Viticis Fructus, a model of anaphylaxis induced by compound 48/80 was used. The aqueous extract of V. trifolia subsp. litoralis fruit (VRFE) inhibited systemic allergic reactions induced by compound 48/80 at (10−4-1.0 g/kg) doses. When VRFE was used in the whole-body allergic reaction test, histamine levels in plasma were reduced in a dose dependent way. VRFE (5 × 10−1and 1.0 g/kg) inhibited passive skin allergic reactions activated by anti-dinitrophenyl (DNP) IgE. VRFE (10−3-1.0 mg/ml) also inhibited in a dose dependent manner compound 48/80 or anti-DNP IgE release of histamine from rat peritoneal mast cells (RPMC). In addition, VRFE (10−3 mg/ml) significantly inhibited the production of tumor necrosis factor-α from anti-DNP IgE-induced RPMC. These results indicate that VRFE may be useful in regulating an immediate type of allergic reactions [3].
Alcoholic extracts of V. trifolia leaves were shown to inhibit the IgE-dependent release of histamine from RBL-2H3 cells [51].
5.2. Antibacterial activity
Anti-bacterial activity of leaves of V. trifolia has been studied. Petrol extract (500 μg/disc) and EtOH extract (400 μg/disc) showed moderate activity against the majority of Gram-positive and Gram-negative bacteria tested [14]. Anti-bacterial activity was studied in vitro by using18 strains of methicillin-resistant Staphylococcus aureus (MRSA) andsix strains of methicillin-sensitive S. aureus (MSSA) with agar disk diffusion test, and the test showed that none of the compounds had effective activity against MSSA. However, in similar screening against MRSA, vitrofolal B, vitrofolal D and detetrahydroconidendrin showed anti-bacterial effect against eight out of 18 strains. The minimum inhibitory concentration (MIC) of these components was identified as less than 64 μg/ml [49]. Recent results have shown that methanolic extracts of the leaves of V. trifolia exhibit significant antibacterial activity against five Gram-positive strains and seven Gram-negative strains of human pathogenic bacteria [52]. In order to determine the fungicidal activity of the plant extracts, five fungi were tested: Penicillium sp., Aspergillus flavus, A. parasiticus, Trichoderma sp. and Fusarium sp. The results showed that hexanic leaf extract inhibited 100% of Fusarium sp. in 2 days of growth [15]. Different concentrations of crude extracts of V. trifolia (100 mg/ml, 50 mg/ml, 25 mg/ml) were used to test against selected bacterial pathogens namely Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes and Klebsiella pneumoniae. In vitro antimicrobial studies showed significant areas of suppression against the pathogenic bacteria tested [53].
5.3. Anticancer activity
5.3.1. Anti-angiogeneic activity
5,3′-Dihydroxy-6,7,4′-trimethoxyflavanone (DHTMF) is one of the ingredients of V. trifolia subsp. litoralis. It inhibits vasculogenesis and induces apoptosis via the Akt/mTOR pathway, and may induce pharmacological effects beneficial for the therapy of lung cancer [25]. Vitexicarpin (VIT) was isolated from the fruits of V. trifolia subsp. litoralis showing anti-angiogenic effects, and the results validate that VIT can be used as a novel angiogenesis inhibitor [54].
5.3.2. Anti-proliferative activity
Viticis Fructus considered as active antitumor candidate to inhibit cancer cells growth, such asK562 cells [31], tsFT210 cells [31], Human prostate cancer PC-3 cells [55], NCI–H446 cells [56], HL-60 cells [9]. Correlations among the pharmacological properties of Viticis Fructus and the active components indicated that flavonoids were the main bioactive components. For instance, vitexicarpin isolated from Viticis Fructus can induce caspase-mediated cell death in tsFT210 and K562 cells [31], and also can inhibit proliferation of the human adenocarcinoma PC-3 and HL-60 cells [55], persicogenin, artemetin, luteolin, penduletin and chrysosplenol-D from V. trifolia effectively evoked caspase-mediated cell death in tsFT210 and K562 cells [31]. Vitexilactone, (rel 5S,6R,8R, 10S)-6-acetoxy-9-hydroxy-13(14)-Labden-16,15-olide, rotundifuran, vitetrifolin D, and vitetrifolin E, significantly induced apoptosis in tsFT210 and K562 cells at higher concentrations (100 μg/ml) [31]. The studies also found that flavone extract can significantly reduce the volume of lung cancer and inhibited the function of both Sarcoma 180 cells and Hepatoma 22 cells [57].
2′,3′,5-trihydroxy-3,6,7-trimethoxyflavone and artemetin of V. trifolia subsp. litoralis decreased the proliferative activities in a dose-dependent manner in HL-60 cells with IC50 of 4.03 μM and 30.98 μM, respectively [9]. Anti-carcinogenic potential of ethanolic extracts of V. trifolia has been studied. Rat liver microsome degranulation is a short-term technology that has been used to detect potential chemical carcinogens in vitro, and the results showed that V. trifolia protected liver microsomes from attack by degranulation of the carcinogen EB, showing a significant decrease in proliferation of HCT-116 and A549 cancer cell lines [58]. Casticin, extracted from Viticis Fructus, is one of the flavonoid components that has been found to exert inhibitory effects on tumor cell proliferation through multiple modes of action. In this study, the effects of casticin on human colon cancer and its underlying mechanisms were studied. The results showed that casticin can cause the accumulation of reactive oxygen species (ROS) and increase the protein levels of apoptosis signal-regulated kinase 1 (ASK1), c-Jun N-terminal kinase (JNK), and B-cell lymphoma 2-interacting cell death mediator (BIM) in HT-29 cells, which can greatly induce apoptosis in HT29, HCT116, SW480, and Caco-2 cells [59]. It has also been found that casticin selectively inhibited the proliferation of 5–8F cells in vitro by participating in the regulation of GSDMD-dependent tumor cell death process [60]. Casticin induced G2/GM arrest and apoptosis by upregulating Bax/BCL2 expression, which could significantly inhibit the proliferation of nasopharyngeal carcinoma [61]. In addition, it has been shown that casticin inhibits breast cancer cell migration and invasion by inhibiting the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway [62]. DHTMF is one of the constituents of V. trifolia subsp. litoralis, it significantly inhibited the growth of lung cancer cells and induced apoptosis in a dose-dependent manner, as evidenced by decreased levels of Bcl-2 and increased levels of Bax and cleaved caspase-3 [25].
Persicogenin, artemetin, luteolin, penduletin, vitexicarpin and chrysosplenol-D, which have been isolated as new cell cycle inhibitors from V. trifolia. The inhibitory effects of these six flavonoids on the multiplication of mammalian cancer cells have been assessed by the sulforaphane B (SRB) method, and their effects on cell cycle and apoptosis were examined by flow cytometry and morphological observation under light microscopy and agarose gel electrophoresis for inter-ribosomal DNA fragments. These six compounds can mainly inhibit the proliferation of mouse tsFT210 cancer cells [31]. Vitetrifolin H, vitetrifolin I and vitexoid isolated from V. trifolia fruits were identified by an extensive spectroscopic approach and all these compounds demonstrated suppression of HeLa cell proliferation with IC50 values ranging from 4 to 28 μM [32]. Rotundifuran, a natural product isolated from V. trifolia L. inhibits HeLa cell proliferation by inducing mitochondria-dependent apoptosis through reactive oxygen species (ROS) action on MAPK and PI3K/Akt signaling pathways [63]. In vitro lymphocyte proliferation inhibitory activity of vitexicarpin was examined. Vitexicarpin revealed an inhibitory activity of >0.1 pM against Con A or LPS-induced lymphocyte proliferation [64].
5.3.3. Cytotoxic activity
The extracts of hexane and dichloromethane (DCM) from the stems and leaves of V. trifolia have been shown to be highly toxic to several cancer cell lines in culture (SQC-1 UISO, OVCAR-5, HCT-15 COLADCAR and KB) [15]. The cytotoxic activity of ethanol extract isolated from V. trifolia subsp. litoralis was analyzed by MTT method, the ethanol extract can inhibit the proliferation of human liver cancer cell line HepG2 in vitro showing that the ethanol extract has a certain cytotoxic activity [56]. Casticin is a polymethyl flavonoid from Fructus viticis, sub-cytotoxic concentrations of it could inhibit the stemness characteristics in hepatocellular carcinoma cells, as demonstrated by the expression of stemness biomarkers (CD44, EpCAM, Bmi1, Nanog, and Oct4). Casticin could inhibit stemness characteristics in hepatocellular carcinoma cells by interruption of the reciprocal negative regulation between DNA methyltransferase 1 and miR-148a-3p [65].
5.4. Anti-feeding activity
The anti-feeding activities have been studies in the early 1974s. The survey of ether extract of V. trifolia against the larvae of Spodoptera litura F. was examined. The tests have showed that V. trifolia can against S. litura F. at the concentration of 10, 5, 2.5, and 1% [66].
Hexane and DCM extracts of V. trifolia are tried to equally have anti-feeding activities. The hexanic extract of the leaves utterly inhibited the expansion of the phytopathogenic fungi Fusarium sp. throughout the primary a pair of days of the experiment [15].
5.5. Anti-hyperprolactinemia activity
The ethanol extract Viticis Fructus (the fruit of V. trifolia subsp. litoralis) have been studied. The test using acetic-acid-induced writhing and metodopramide-dihydrochloride induced in mice. In vivo experiment proved that the optimum concentration at the dose of 50 mg/kg [67]. The anti-hyperprolactinemia activity of casticin which is isolated from V. trifolia subsp. litoralis have been reported. In vivo, hyperprolactinemia (HP) was evoked by administering metoclopramide hydrochloride (50 mg/kg, tid, ip, for 10 days) to SD rats. Serum prolactin levels were 2.1-fold higher in the hyperprolactin model group than in the untreated control group (P < 0.01). Casticin (10, 20 and 40 mg/kg, ip, for 7 days) decreased serum levels of prolactin by 33.9%, 54.3% and 64.7%, respectively (P < 0.01). In vitro experiments further demonstrated that casticin suppressed E2-stimulated prolactin release from pituitary cells [68].
5.6. Anti-inflammatory activity
The anti-inflammatory activity of aqueous extract of V. trifolia subsp. litoralis and V. trifolia were tested. The aqueous extract reduced the induction of IL-1β, IL-6 and iNOS mRNA by LPS and inhibited the expression of various LPS-induced inflammatory genes in RAW 264.7 cells [7,69]. The effect of V. trifolia subsp. litoralis extracts on stimulated A549 cells was then assessed by analyzing Eotaxin secretion and eosinophil migration, showing that V. trifolia subsp. litoralis -treated A549 cells significantly inhibited Eotaxin secretion and eosinophil migration in a dose-dependent way. Casticin, an active compound isolated from V. trifolia subsp. litoralis has been found to show anti-inflammatory properties in vivo and vitro. In vivo, casticin significantly inhibited xylene-induced edema in mice, egg albumen-induced paw edema in rats, and acetic acid-induced vascular permeability in mice [70,71]. Casticin alleviated dextran sulfate sodium-induced ulcerative colitis by increasing the expression of the antioxidant enzymes peroxidase 3 and MnSOD and by reducing the production of pro-inflammatory chemokines through inhibition of AKT signaling [72]. In addition, casticin had a significant effect on cigarette smoke-induced lung inflammation in a mouse model [73]. The anti-inflammatory activity of casticin was studied in vitro in A549 human type II epithelial lung cells using an eotaxin inhibition assay, which showed that casticin can inhibit eosinophil migration and the activity of chemokines and adherence molecules participating in the process of asthma inflammation by inhibiting the NF-κB pathway [8]. Casticin alleviated monoiodoacetic acid-induced knee osteoarthritis by inhibiting of HIF-1α/NLRP3 inflammasome activation [74]. In addition, in LPS-induced inflammation, casticin significantly inhibited the NF-κB subunit p65 protein in the nucleus and reduced the activation of Akt and MAPK [75].
5.7. Antioxidant activity
Themethanol extract of Viticis Fructus and ferruginol isolated from V. trifolia subsp. litoralis showed a stronger antioxidant activity by using DPPH radical and ferric thiocyanate methods [4]. Negundoside (NS), agnuside (AS) and 6′-p-hydroxy benzoyl mussaenosidic acid (HMA) are known bioactive metabolites in V. trifolia and this study demonstrated a significantly active in DPPH and NO radical removal assays [48]. The aqueous extract of V. trifolia has also been found antioxidant activity by using DPPH assay [5].
5.8. Larvicidal activity
The larvicidal efficacy of V. altissima, V. negundo and V. trifolia fatty acid methyl ester (FAME) extracts against early fourth-instar Culex quinquefasciatus larvae was investigated. With an LC50 value of 9.25 ppm, the V. trifolia FAME extract had the highest larvicidal activity, followed by the V. altissima (14.82 ppm), and the V. negundo (18.64 ppm) [76]. The extracted substance methyl-p-hydroxybenzoate was tested for its larvicidal efficacy against early 4th instar larvae of Culex quinquefasciatus and Aedes aegypti. At a concentration of 20 ppm, the substance completely killed both mosquito larvae, with LC50 values of 5.77 and 4.74 ppm against C. quinquefasciatus and A. aegypti, respectively [77]. Anopheles gambiae Giless. s. larvae (Diptera: Culicidae) were tested for their ability to be controlled by methanol extract of V. trifolia leaves; it can result in 100% death at 100 ppm in 72 h [10]. Essential oil from V. trifolia revealed potential larvicidal properties, LC50 and LC90 for V. trifolia essential oils against Ae. aegypti and C. quinquefasciatus were 57.7 ± 0.4, 77.9 ± 0.9 ppm and 55.17 ± 3.14, respectively [78].
5.9. Hepatoprotective activity
The protective activity of aqueous and ethanolic extracts of V. trifolia leaves against carbon tetrachloride-induced liver damage was studied. The results of serum biochemical evaluation showed a significant decrease in total bilirubin and serum marker enzymes and an increase in total protein in animals treated with ethanol and aqueous extracts [79]. The protective effect of ethanolic extract of V. trifolia flowers on CCI4-induced liver damage in albino rats was investigated. The plant extract showed significant hepatoprotective activity at a dose of 200 mg/kg [11]. Casticin is one of the flavonoids extracted from Viticis Fructus, which can reduce the expression of matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 resulting from blocking TGF-β1/Smad signaling, as well as increased the apoptosis of hepatic stellate cells. The results suggest that casticin has potential benefits in the attenuation and treatment of liver fibrosis [80].
5.10. Estrogenic activity
The estrogen-like biological activity of the volatile components of the essential oil isolated from the fruit of V. trifolia subsp. litoralis was investigated in human breast cancer cells, and their experimental results showed that it was significantly inhibited by ICI 182,780, a specific estrogen receptor antagonist [67]. The ethanolic extract of V. trifolia subsp. litoralis fruit was tested for estrogen-like activity, as well as estrogen receptor (ERa), estrogen receptor-regulated progesterone receptor and pS2 mRNA expression in MCF-7 cells using a modified cell proliferation assay (E-SCREEN evaluation system). The results showed that VRE has estrogen-like activity [67].
5.11. Other activities
The ether extract of V. trifolia subsp. litoralis fruit exhibited effective inhibition of rat lens aldose reductase (RLAR) activity in vitro. It demonstrated an IC of 7.4 × 10−7 M against DL-glyceraldehyde as a substrate [81]. Extracts of Viticis Fructus appeared to have analgesic effects and activity-guided isolation was performed using acetic acid-induced peristalsis in mice. Vitexfolin A, agnuside, 10-O-vanilloylaucubin, revealed significant sprain inhibition after oral administration at doses of 15, 50, 25 and 50 mg/kg [13]. Cerebral protective and cognitive enhancing activities of aqueous extracts of V. trifolia leaves against scopolamine-induced amnesia and normal rats. In passive avoidance (PA) and T-maze (TM) models, higher doses (20 mg/kg) of the plant extract exhibited significant antiamnesic activity compared to controls (P < 0.01) [82]. A new natural compound, 1H,8H-Pyrano [3,4-c]Pyran-1,8-dione (PPY), was isolated from V. trifolia subsp. litoralis, and the mechanism of the anti-asthmatic response to PPY in vitro was elucidated. Stimulation of lung epithelial cells (A549 cells) with TNF-α, IL-4 and IL-1β. The expression of chemokines and adhesion molecules involved in eosinophil chemotaxis was induced. Eotaxin, IL-8, IL-16 and VCAM-1 mRNA expression were significantly reduced by PPY treatments [83]. Casticin, a flavonoid isolated from Viticis Fructus, significantly inhibited the growth of KB cells (IC50 ¼ 0.23 mM). On the contrary, there was no inhibition of proliferation of A431 cells by casticin, similar to normal cell lines, 3T3 Swiss Albino and TIG-103. Flow cytometric analysis showed that KB cells exposed to casticin resulted in a significant arrest at the G2-M phase. Casticin disrupted the mitotic spindle in immunostaining of KB cells. These results indicate that the blockade of G2-M by casticin may have anti mitogenic activity [84]. The flavone-enriched fraction of Viticis Fructus showed anti-nociceptive activity in a dose-dependent manner (10–50 mg/kg body wt., i. g.) [67]. The water extract of V. trifolia (aerial part) used in the treatment of AIDS was tested for HIV type 1 reverse transcriptase inhibitory activity, and the results revealed that the rate of HIV-1 RT inhibition (% IR) was higher than 90% at a concentration of 200 μg/ml [85]. The essential oil of V. trifolia was assessed on the spilosoma obliqua larvae and when topically applied to the dorsal side of the mesothoracic region, it had a regulating effect on the growth of the insects. It decreased the emergence of adults by increasing the mortality of larvae and the deformity of adults, and decreased the fertilization rate of females and the fertilization rate of eggs [86]. The isolation of a new natural mosquito repellent from the fresh leaves of V. trifolia subsp. litoralis. This compound exhibited potent repellent activity against Aedes aegypti [47]. The conventional treatments for Ciguatera fish poisoning (CFP) and the potential for application in the treatment of illnesses associated with excessive NO generation were both supported by the inhibitory action of the aqueous extract of V. trifolia [5]. 4-hydroxybenzoic acid, ferulic acid and vanillic acidwere isolated from fruits and leaves of V. trifolia subsp. litoralis. Using lettuce seedlings as a bioassay, these compounds' biological activities were investigated. The results of the investigation revealed that these compounds reduced the inhibitory action against root development [43]. Only vitexcarpin was active in a model utilizing sensitized guinea pig trachea activated by ovalbumin up to a minimum dosage of 1.3 × 10−5 M; Viteosin A and vitexcarpin isolated from V. trifolia may prevent spontaneous contraction of isolated male guinea pig trachea produced by histamine [87]. In excision, incision, and dead space wound models, the ability of ethanol leaf extracts of V. trifolia to promote wound healing was assessed. It was discovered to have considerable wound healing activity as demonstrated by a reduction in the time of epithelialization, an increase in the rate of wound contraction, skin breaking strength, granulation tissue dry weight, hydroxyproline content, and granulation tissue breaking strength [79].
6. Conclusion
We reviewed the existing traditional medical uses, phytochemical and pharmacological research on the Viticis Fructus, which is used in traditional Chinese medicine for the treatment of various diseases particularly colds, headaches, vertigo, and anesthesia with a long history. Currently, more than 190 compounds have been isolated, including the major ones such as diterpenes and flavones. These monomers compounds and crude extracts from V. trifolia subsp. litoralis and V. trifolia are screened for pharmacological activities in vivo and in vitro. Many experimental studies validated its traditional medicinal uses, and the chemical responsible.
By summarizing (Table 3), we can see that the research on the pharmacological effects of Viticis Fructus is not deep enough, such as antibacterial activity, larvicidal activity, anti-hyperprolactinemia activity, and other functions, but only at the level of pharmacological effects, without exploring the mechanism of action. Anticancer activity, anti-inflammatory activity is the popular direction for the study of Viticis Fructus, so the mechanism of action is also explored more deeply. For example, Casticin is one of the flavonoids extracted from Viticis Fructus, which is a structure with three phenyl rings, an ortho catechol moiety, an alkene group, two hydroxyl groups, and four methoxy groups. The C-30 and C-5 hydroxyl groups, as well as the C-3 and C-40 methoxy groups in the casticin molecule, consist of significant anti-proliferative activity, leading to unfavorable proliferation - a desirable feature of anti-cancer drugs [88]. Casticin induces mitochondria-dependent and ROS-mediated apoptosis, induces cell cycle arrest and inhibits invasive and migratory proliferation in breast, bladder, colon, lung, oral cavity and ovarian cancers. In addition, casticin affects multiple oncogenic pathways by regulating various proteins, such as MAPK, NF-κB and PI3K/Akt pathways; increases ROS production by enhancing Bax protein and decreasing Bcl protein, and induces cell cycle arrest by inhibiting the cell division cycle (cdc25c and cdc2) and cell cycle proteins (B1). These signaling pathways are the key pathways through which Viticis Fructus exerts its antitumor effects.
Table 3.
Pharmacological activities of natural products from Viticis Fructus.
| pharmacological activity | extracts/compounds | types | model | dose range | Activity concentration | effect | Reference |
|---|---|---|---|---|---|---|---|
| Anti-allergic activity | Aqueous extract | in vivo | The original stock of male Wistar rats | 0.001–1.0 g/kg | 1.0 g/kg | Inhibited sysivitytemic allergic reaction | [3] |
| Aqueous extract | in vitro | TNF-α | 0.01–10 mg/ml | 0.01 mg/ml | Inhibited TNF-α production | [3] | |
| Alcoholic extracts | in vitro | RBL-2H3 cells | 0.5 mg/ml | Inhibited histamine release | [51] | ||
| Anti-angiogeneic activity | 5,3′-Dihydroxy-6,7,5′-trimethoxyflavanon | in vitro | Human umbilical vascular endothelial cells | 0.5,2Μm | Inhibited tube formation and endothelial cells migration | [25] | |
| Vitexicarpin | in vitro | Human umbilical vascular endothelial cells | 0.1,1,2.5,5 μM | 5 μM | Inhibited endothelial cell proliferation and migration | [54] | |
| Antibacterial activity | Petroleum ether and Ethanol extracts | in vitro | Gram-positive and Gram-negative bacteria | 500 μg/disc 400 μg/disc |
Inhibited both gram-positive and gram-negative bacteria | [14] | |
| VitrofolalB, VitrofolalD, Detetrahydroconidendrin | in vitro | Methicillin-resistant Staphylococcus aureus methicillin-sensitive S. aureus | 64 μg/ml | Inhibited eight out of 18 strains of MRSA inactive against MSSA | [49] | ||
| Methanol extracts | in vitro | Five strains of Gram-positive and seven strains of Gram-negative human pathogenic bacterial strains | 8000.0, 4000.0, 2000.0, 1000.0, 500.0, 250.0and 62.5 μg/ml | 125.0 μg/ml | Inhibited both gram-positive and gram-negative bacteria | [52] | |
| Hexanic extract | in vitro | Pseudomonas aeruginosa, Staphylococcus aureus, Shigella sonei, Proteusmirabilis, Salmonella typhi, and Candida albicans | 10, 5,2.5, and 1.25 mg/ml | 10 mg/ml | Inhibited the growth of Gram-positive and Gram-negative bacteria | [15] | |
| Petroleum ether, Chloroform, Methanol and Hot Water extracts | in vitro | Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes and Klebsiella pneumoniae | 100 mg/ml, 50 mg/ml, 25 mg/ml | Inhibited the tested pathogenic bacteria | [53] | ||
| Anticancer activity | Vitexicarpin | in vitro | K562 cells | 1.0 μg/ml | 1.0 μg/ml | Induced apoptosis on human myeloid leukemia K562 cells | [89] |
| Vitexilactone,(rel 5S,6R,8R,9R,10S)-6-acetoxy-9-hydroxy-13(14)- Labden-16,15-olide,rotundifuran, Vitetrifolin D,Vitetrifolin E | in vitro | tsFT210 cells | 100 μg/ml | Induced apoptosis of the tsFT210 cells | [89] | ||
| Vitexicarpin | in vitro | Human prostate cancer PC-3 cells | 0–200 μM | 200 μM | Inhibited proliferation of the PC-3 cells | [55] | |
| Total flavonoids | in vitro | NCI–H446 cells | 0.5、1.0、2.0 μg/ml | Significantly reduce the volume of a sphere of lung cancer | [56] | ||
| Flavone extract | in vivo | The mice of Kunming species | 400 mg/kg、200 mg/kg、100 mg/kg | 400 mg/kg | Inhibited both Sarcoma 180 and Hepatoma 22 | [57] | |
| 2′,3′,5-trihydroxy-3,6,7-trimethoxyflavone Vitexicarpin Artemetin | in vitro | HL-60 cells | Inhibited the proliferation of HL-60 cells | [9] | |||
| Ethanol extract | in vitro | HCT 116 and A549 cells | 20, 40, 60 and 80 μg/ml | Reducedtheproliferation of the HCT 116 and A549 cancer cell lines | [58] | ||
| Casticin | in vitro | HT-29, HCT-116, SW480 and Caco-2 cells | 0,5,10,20 μmol/l | 10 μmol/l | Inhibited the proliferation colon cancer cell | [59] | |
| 5,3′-Dihydroxy-6,7,4′-trimethoxyflavanon | in vitro | H522, H23, H226 human lung cancer cells and IMR-90 cells | 0.1,0.5,1,2 μM | 1 μM | Inhibited the viability of lung cancer cells | [25] | |
| Anti-feeding activity | Hexane and DCM extract | in vitro | Spodoptera frugiperda | 10, 100and 1000 μg/ml | 1000 μg/ml | Larvae of S. frugiperda were weighed loss | [15] |
| Ether extract | in vitro | Spodoptera litura F. | 10, 5, 2.5, and 1% concentrations | Against Spodoptera litura F. | [66] | ||
| Anti-hyperprolactinemia activity | Ethanol extract | in vivo | Female ICR mice | 25 mg/kg 50 mg/kg | 50 mg/kg | Decreased the serum prolactin level | [67] |
| Casticin | in vitro | Primary pituitary cells were | 0.1, 1, and 10 μmol/l | Inhibited prolactin release from E2-stimulated pituitary cells | [68] | ||
| Casticin | in vivo | Female Sprague Dawley (SD) rats | 10, 20, and 40 mg/kg | Pituitary cell proliferation | [68] | ||
| Anti-inflammatory activity | Aqueous extract | in vitro | RAW 264.7 cells | 0, 25, 250, 2500 and 5000 μg/ml | 2500 μg/ml | Reduced the LPS-dependant induction of IL-1β, IL-6 and iNOS mRNA | [7] |
| Vitex trifolia subsp. litoralis extract | in vitro | A549 cells | 0.1, 1, or 10 μg/ml | 1 μg/ml | Inhibited Eosinophil Migration | [90] | |
| Casticin | in vitro | A549 cells | 0.001, 0.01, 0.1, 1, and 10 μg/ml | Ihhibited the eosinophil migration | [8] | ||
| Vitexicarpin | in vitro | Human umbilical vein endothelial cells (HUVEC) | 1–1000 nM | Inhibited the ROS–NF-κB pathway | [91] | ||
| Aqueous extract | in vitro | RAW 264.7 cells | 250,2500,5000 mg/ml | 5000 mg/ml | Inhibited the expression of various LPS-induced inflammatory genes in RAW 264.7 cells | [69] | |
| VR-extract/aqueous extract | in vivo | Male BALB/c mice | 100 mg kg–1 | Inhibited eosinophilia | [92] | ||
| Viteagnusin I,Vitetrifolin D,Viteagnusin F,Casticin,VitetrifolinH,Viterotulin A,Viterolignan A | in vitro | RAW264.7 cells | 11.3–24.5 μM | Inhibited nitric oxide production in RAW264.7 cells | [27] | ||
| Casticin | in vivo | Female C57BL/6 mice | 1,2, and10 mg/kg | Decreased numbers of total and inflammatory cells | [73] | ||
| Casticin | in vitro | RAW264.7 cells | 0.3–10 μM | Inhibit the inflammatory response | [75] | ||
| Casticin | in vivo | Male ICR mice | 5,10,20 mg/kg | Inhibited the acetic acid-induced increased vascular permeability | [70] | ||
| Extract from leaves of V. trifolia L. | in vivo | CA-induced rat paw edema model | 100–200 mg/kg | Inhibited the paw edema induced by CA (p < 0.05) and effectively reduced the inflammatory leukocyte infiltration | [93] | ||
| Antioxidant activity | Methanol extract | in vitro | DPPH radical | 1 ml | Inhibited DPPH | [4] | |
| Aqueous extract | in vitro | DPPH radical | 0.0025, 0.025, 0.25 and 2.5 g/l | Inhibited DPPH | [5] | ||
| Negundoside, agnuside and 6′-p-hydroxy benzoyl mussaenosidic acid | in vitro | DPPH radical | 9.96, 9.81 and 10.31 μg | Inhibited DPPH | [48] | ||
| Methanol extract | in vitro | Ferric thiocyanate method | 0.05 ml | [4] | |||
| Ferruginol | in vitro | DPPH radical | 1 ml | Inhibited DPPH | [24] | ||
| Essential Oil | in vitro | DPPH radical | 0.05–20 mg/ml | Inhibited DPPH | [94] | ||
| Anti-proliferative activity | Persicogenin, artemetin, luteolin, penduletin, vitexicarpin and chrysosplenol-D, | in vitro | tsFT210 cells | various concentrations | Inhibited the proliferation of tsFT210 cells | [31] | |
| vitetrifolin H vitetrifolin I and vitexoid | in vitro | Hela cells | 4–28 μM | Inhibited the proliferation of Hela cells | [32] | ||
| Vitexicarpin | in vitro | Spleens cell of male C57BL/6 mice | Inhibited activity on T-and B-cell proliferation | [64] | |||
| Larvicidal activity | Fatty acid methyl ester extracts | in vitro | Culex quinquefasciatus | 50, 25, 12.5, 6.25 and 3.125 ppm | Increased the larval mortality of C. quinquefasciatus | [76] | |
| Methyl-p-hydroxybenzoate | in vitro | Culex quinquefasciatus/Aedes aegypti | 20, 10, 5, 2.5 and 1.25 ppm | 20 ppm | Against C. quinquefasciatus and Ae. aegypti. | [77] | |
| Acetone and methanol extracts | in vitro | Anopheles gambiae Giles | 25, 50, 100, 250 and 500 ppm | Led to retardation and 100% mortality | [10] | ||
| Essential oils were extracted by steam distillation | in vitro | Aedes aegypti | 50, 75, 100, and 125 ppm | LC50 and LC90 For V. trifolia against Ae. aegypti and C. quinquefasciatus were 57.7 + 0.4, 77.9 + 0.9 ppm and 55.17 + 3.14, 78.28 + 2.23 ppm | [78] | ||
| Hepatoprotective activity | Aqueous and ethanol extracts | in vivo | Male Wistar rats | 20 and 30 mg/kg/day | Increase in the levels of total bilirubin, alanine transaminase, aspartate transaminase and alkaline phosphatase | [79] | |
| Ethanol extract | in vivo | Male Wister rats | 200 mg/kg/day | 200 mg/kg | Reduced the elevated levels of SGPT, SGOT, ALP, bilirubin and GGTP | [11] | |
| Estrogenic activity | Volatile | in vitro | MCF-7 cells | 5 mg/l to 500 mg/l | 50 mg/l | Stimulation of MCF-7 cell proliferation | [67] |
| Ethanol extract | in vitro | MCF-7 cells | 200 mg ml−1 | 200 mg ml−1 | Stimulate the proliferation of MCF-7 cells | [67] | |
| Cytotoxic activity | Hexane and DCM extract | in vitro | Carcinoma(SQC-1 UISO), ovarian cancer(OVCAR-5), colon carcinoma (HCT-15 COLADCAR),humannasopharyngeal carcinoma (KB)cells | 1, 10, 100 μg/ml | Hexanic and DCM extracts have shown interesting ED50 values | [15] | |
| Ethanol extract | in vitro | HepG2 cells | 50、25、12.5、6.25、3.125 μmol/l | Inhibited the HepG2 cells | [56] | ||
| Cell cycle inhibitory activitie | Persicogenin, artemetin, luteolin, penduletin, vitexicarpin | in vitro | tsFT210 cells | 100, 25, 6.25, 50, and 1.0 μg/ml | Accumulated at theG2/Mphase decreased | [89] | |
| Vitetrifolin I | in vitro | Hela cells | 5, 10, 25, and 50 μM | Accumulatied the cells in the G0/G1 phase | [32] | ||
| Other activities | |||||||
| Aldose reductase activity | Ether extract | in vitro | Enzyme reactions | 100 μl | Inhibited the enzyme activity | [81] | |
| Analgesic activity | Vitexfolin A,agnuside,10-O-vanilloylaucubin,dihydrodehydrodiconiferylalcohol-β-D(2′-Ο-Ρ-hydroxybenzoyl)glucoside | in vivo | Acetic acid-induced writhing method in mice | 15,50,25 and 50 mg/kg | 15,50,25 and 50 mg/kg | Inhibited the writhing symptoms | [13] |
| antiamnesic activity | Aqueous extract | in vivo | Wistar albino rats | 10 mg/kg, 20 mg/kg | 20 mg/kg | Improved in memory retention | [82] |
| antiasthmatic activity | 1H,8H-Pyrano [3,4-c]pyran-1,8-dione (PPY) | in vitro | A549 cells | 0.1,1,10 μg/ml | 10 μg/ml | Decreased eotaxin secretion and suppression of eosinophil migration | [83] |
| Antifungal activity | Hexane and DCM extract | in vitro | Penicillium sp., Aspergillus flavus, A. parasiticus, Trichoderma sp. and Fusarium sp. | Inhibited the growth of Fusarium sp. | [15] | ||
| Anti-mitotic activity | Casticin | in vitro | KB cells, A431 cells | 0.6 mM | Inhibited the growth of KB cells | [84] | |
| Anti-nociceptive activity | Ethanol extract | in vivo | Female ICR mice | 25 mg/kg 50 mg/kg | 50 mg/kg | Inhibited the writhing symptoms | [67] |
| HIV-1 reverse transcriptase inhibition activity | 80% ethanol extracts/aqueous extract | in vitro | HIV-1 reverse transcriptase | 200 μg/ml | 200 μg/ml | Inhibited HIV-1 reverse transcriptase | [85] |
| Insect growth regulatory activity | Essential oils | in vitro | Spilosoma obliqua | 0.5, 1.0, 1.5, 2.0 and 2.5 μl | 2.5 μl | Increase the larval mortality and larval duration of S. obliqua | [86] |
| Mosquito repelling activity | CHCI3 extract | in vitro | Aedes aegypti | [47] | |||
| NO inhibition activity | Aqueous extract | in vitro | RAW 264.7 cells | 0.0025, 0.025, 0.25 and 2.5 g/l | 2.5 g/l | Inhibited NO production | [5] |
| Plant growth activity | 4-hydroxybenzoic acid, ferulic acid, vanillic acid | in vitro | Lettuce seedlings | 10−3 M | Inhibited the root growth | [43] | |
| Tracheospasmolytic activity | ViteosinA, vitexcarpin | in vivo | Sensitized male guinea pigs | 1.3 × 10−4 M | 1.3 × 10−4 M | Inhibited the tracheal contraction | [87] |
| Wound healing activity | Ethanol extract | in vivo | Swiss Wistar strain rats | 20 mg/ml | Promoted the epithelialization of the wound area/increased the skin breaking strength | [79] |
Although studies have been made on some of V. trifolia subsp. litoralis and V. trifolia, there are issues still remaining to be resoved: 1. So far, no toxicological reports have been found on Viticis Fructus. More in-depth studies about the toxicity of Viticis Fructus should be carried out, which can provide a theoretical guidance for the application of Viticis Fructus. The number of in vivo studies conducted to date is small and the pharmacokinetic profile of Viticis Fructus (including dosing, bioavailability, distribution, etc.) is limited. the safety and efficacy of Viticis Fructus have not been reported, which poses a risk to the clinical evaluation of this drug. 2. In the 2020 edition of the Pharmacopoeia of the People's Republic of China, V. trifolia subsp. litoralis is seen as a variant of V. trifolia and the Latin name is V. trifolia L. var. simplicifolia Cham.(Committee, C·P., 2020). But in some monographs in the taxonomy, the V. trifolia subsp. litoralis has been regarded as an independent species of Vitex(Editorial Committee of the Flora of China, 2022). However, according to World Flora Online on the genus Vitex, V. trifolia subsp. litoralis is treated as a subspecies of V. trifolia [95]. So far, there is no consensus on this issue. Therefore, taxonomic studies should be strengthened. 3. In the Pharmacopoeia of the People’s Republic of China, the base sources of Viticis Fructus are V. trifolia L var. simplicifolia Cham. and V. trifolia. But in the process of the chemical composition and pharmacological effects of the chemical composition and pharmacological effects of the two in the phytochemistry and pharmacological effects are very different some differences in morphological. Otherwise, it has been reported that V. trifolia subsp. litoralis and V. trifolia have some differences in antipyretic, analgesic, sedative and other aspects. It should be aimed at strengthening of V. trifolia subsp. litoralis and V. trifolia comparative study, for the quality control of frucuts viticis and clinical application provides sufficient basis.
In conclusion, this review summarizes the traditional applications, botany, phytochemistry, and pharmacology of Viticis Fructus, and points out some shortcomings of the current research, providing a basis for further research and new product development.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by funds from the National Natural Science Foundation of China (Grant no. 82104326).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e19144.
Contributor Information
Ling Li, Email: 316262150@163.com.
Chang Liu, Email: mniliuchang@163.com.
Min Jia, Email: jm7.1@163.com.
Abbreviations
- iNOS
inducible Nitric Oxide Synthase
- JNK
c-Jun N-terminal kinase
- AIDS
Acquired Immune Deficiency Syndrome
- ASK1
apoptosis signal-regulating kinase 1
- LC50
Lethal Concentration resulting in 50% mortality
- AS
agnuside
- LPS
Lipopolysaccharide
- BIM
B-cell lymphoma 2 interacting mediator of cell death
- CFP
Ciguatera fish poisoning
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-sensitive S. aureus
- DCM
dichloromethanic
- NF-κB
nuclear factor kappa-B
- DHTMF
5,3′-Dihydroxy-6,7,4′-trimethoxyflavanone
- NS
Negundoside
- PA
passive avoidance
- DNP
dinitrophenyl
- PPY
1H,8H-Pyrano[3,4-c]pyran-1,8-dione
- DPPH
1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl
- ERa
estrogen receptor a
- RLAR
rat lens aldose reductase
- ROS
reactive oxygen species
- RPMC
rat peritoneal mast cells
- FAME
fatty acid methyl ester
- SRB
sulforhodamine B
- HMA
6′-p-hydroxy benzoyl mussaenosidic acid
- TCM
traditional Chinese medicine
- HP
Hyperprolactinemia
- TM
T-maze
- IC50
concentration that causes 50% inhibition
- TNF-α
tumor necrosis factor α
- IL-1β
interleukin 1β
- VCAM-1
vascular cell adhesion molecule-1
- IL-6
interleukin 6
- VIT
Vitexicarpin
- VRFE
extract of Vitex trifolia subsp. litoralis Steenis fruits
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.FOC Flora of China. http://www.iplant.cn/(20 Feb).
- 2.Commission C.P. China Medical Science Press; Beijing: 2020. Pharmacopoeia of the People’s Republic of China; pp. 379–380. [Google Scholar]
- 3.Shin T.Y., et al. Effect of Vitex rotundifolia on immediate-type allergic reaction. J. Ethnopharmacol. 2000;72(3):443–450. doi: 10.1016/s0378-8741(00)00258-0. [DOI] [PubMed] [Google Scholar]
- 4.Ono M., Masuoka C., Ito Y., Nohara T. Antioxidative constituents from viticis trifoliae fructus (fruit of Vitex rotundifolia L.) Food Sci. Tech. Int. Tokyo. 1998;4(1):9–13. [Google Scholar]
- 5.Kumar-Roiné S., et al. Ability of certain plant extracts traditionally used to treat ciguatera fish poisoning to inhibit nitric oxide production in RAW 264.7 macrophages. J. Ethnopharmacol. 2009;123(3):369–377. doi: 10.1016/j.jep.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari N., Thakur J., Saikia D., Gupta M.M. Antitubercular diterpenoids from Vitex trifolia. Phytomedicine. 2013;20(7):605–610. doi: 10.1016/j.phymed.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Matsui M., et al. Characterisation of the anti-inflammatory potential of Vitex trifolia L. (Labiatae), a multipurpose plant of the Pacific traditional medicine. J. Ethnopharmacol. 2009;126(3):427–433. doi: 10.1016/j.jep.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Koh D.J., et al. Inhibitory effects of casticin on migration of eosinophil and expression of chemokines and adhesion molecules in A549 lung epithelial cells via NF-κB inactivation. J. Ethnopharmacol. 2011;136(3):399–405. doi: 10.1016/j.jep.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Ko W.G., et al. Polymethoxyflavonoids from Vitex rotundifolia inhibit proliferation by inducing apoptosis in human myeloid leukemia cells. Food Chem. Toxicol. 2000;38(10):861–865. doi: 10.1016/s0278-6915(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 10.Nyamoita M.G., et al. Comparison of the effects of extracts from three Vitex plant species on Anopheles gambiae s.s. (Diptera: Culicidae) larvae. Acta Trop. 2013;127(3):199–203. doi: 10.1016/j.actatropica.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Anandan R., et al. Effect of ethanol extract of flowers of Vitex trifolia Linn. on CCL4 induced hepatic injury in rats. Pak. J. Pharm. Sci. 2009;22(4):391–394. [PubMed] [Google Scholar]
- 12.Manjunatha B.K., Vidya S.M. Hepatoprotective activity of Vitex trifolia against carbon tetrachloride-induced hepatic damage. Indian J. Pharmaceut. Sci. 2008;70(2):241–245. doi: 10.4103/0250-474X.41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuyama E., Fujimori S., Yamazaki M., Deyama T. Pharmacologically active components of viticis fructus (Vitex rotundifolia). II. The components having analgesic effects. Chem. Pharm. Bull. (Tokyo) 1998;46(4):655–662. doi: 10.1248/cpb.46.655. [DOI] [PubMed] [Google Scholar]
- 14.Hossain M.M., Paul N., Sohrab M.H., Rahman E., Rashid M.A. Antibacterial activity of Vitex trifolia. Fitoterapia. 2001;72(6):695–697. doi: 10.1016/s0367-326x(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 15.Hernández M.M., Heraso C., Villarreal M.L., Vargas-Arispuro I., Aranda E. Biological activities of crude plant extracts from Vitex trifolia L. (Verbenaceae) J. Ethnopharmacol. 1999;67(1):37–44. doi: 10.1016/s0378-8741(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 16.Global Biodiversity Information Facility. 2023. https://www.gbif.org/ 06.02. [Google Scholar]
- 17.Zhang C. 60 cases of cold caused by physical deficiency treated by modified xiangsu powder. Shaanxi J. Tradit. Chin. Med. 2009;30(4):405–406. [Google Scholar]
- 18.Dong J.F. Treatment of habitual constipation with viticis fructus. J. Tradit. Chin. Med. 2000;(12):713. [Google Scholar]
- 19.Li Y.K. Treatment of rhinitis and supraorbital neuralgia with Viticis Fructus. J. Yunnan Univ. Tradit. Chinese Medicine. 1998;(3):41. [Google Scholar]
- 20.Li K.R. Treatment of 93 cases of vascular headache with viticis fructus decoction. Chin. J. Med. 1992;(8):63. [Google Scholar]
- 21.Liu Y.Y. Viticis Fructus has good effect on trigeminal neuralgia. J. Tradit. Chin. Med. 2000;(12):712. [Google Scholar]
- 22.Wang S.G. Treatment of 56 cases of sciatica with viticis fructus. J. Hebei Tradit. Chinese Medicine Pharmacol. 2001;(4):24. [Google Scholar]
- 23.Wang W.T. Viticis Fructus treats tinnitus. New Chinese Medicine. 1997;(5):50. [Google Scholar]
- 24.Ono M., Sawamura H., Ito Y., Mizuki K., Nohara T. Diterpenoids from the fruits of Vitex trifolia. Phytochemistry. 2000;55(8):873–877. doi: 10.1016/s0031-9422(00)00214-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim K.M., et al. 5,3'-Dihydroxy-6,7,4'-trimethoxyflavanone exerts its anticancer and antiangiogenesis effects through regulation of the Akt/mTOR signaling pathway in human lung cancer cells. Chem. Biol. Interact. 2015;225:32–39. doi: 10.1016/j.cbi.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Kiuchi F., Matsuo K., Ito M., Qui T.K., Honda G. New norditerpenoids with trypanocidal activity from Vitex trifolia. Chem. Pharm. Bull. (Tokyo) 2004;52(12):1492–1494. doi: 10.1248/cpb.52.1492. [DOI] [PubMed] [Google Scholar]
- 27.Lee C., et al. Anti-inflammatory constituents from the fruits of Vitex rotundifolia. Bioorg. Med. Chem. Lett. 2013;23(21):6010–6014. doi: 10.1016/j.bmcl.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Ono M., Yanaka T., Yamamoto M., Ito Y., Nohara T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J. Nat. Prod. 2002;65(4):537–541. doi: 10.1021/np0105331. [DOI] [PubMed] [Google Scholar]
- 29.Ono M., Yamamoto M., Yanaka T., Ito Y., Nohara T. Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem. Pharm. Bull. (Tokyo) 2001;49(1):82–86. doi: 10.1248/cpb.49.82. [DOI] [PubMed] [Google Scholar]
- 30.Zheng C.J., et al. Labdane-type diterpenoids from the fruits of Vitex trifolia. J. Nat. Prod. 2013;76(2):287–291. doi: 10.1021/np300679x. [DOI] [PubMed] [Google Scholar]
- 31.Li W.X., Cui C.B., Cai B., Wang H.Y., Yao X.S. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J. Asian Nat. Prod. Res. 2005;7(4):615–626. doi: 10.1080/10286020310001625085. [DOI] [PubMed] [Google Scholar]
- 32.Wu J., Zhou T., Zhang S.W., Zhang X.H., Xuan L.J. Cytotoxic terpenoids from the fruits of Vitex trifolia L. Planta Med. 2009;75(4):367–370. doi: 10.1055/s-0028-1112211. [DOI] [PubMed] [Google Scholar]
- 33.Oshima N., et al. Identification of new diterpenes as putative marker compounds distinguishing agnus castus fruit (chaste tree) from shrub chaste tree fruit (viticis fructus) Planta Med. 2016;82(1–2):147–153. doi: 10.1055/s-0035-1558089. [DOI] [PubMed] [Google Scholar]
- 34.Luo P., et al. Diterpenoids with diverse scaffolds from Vitex trifolia as potential topoisomerase I inhibitor. Fitoterapia. 2017;120:108–116. doi: 10.1016/j.fitote.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Wang W.Q., Yin Y.P., Jun L., Xuan L.J. Halimane-type diterpenoids from Vitex rotundifolia and their anti-hyperlipidemia activities. Phytochemistry. 2018;146:56–62. doi: 10.1016/j.phytochem.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Bao F., et al. Terpenoids from Vitex trifolia and their anti-inflammatory activities. J. Nat. Med. 2018;72(2):570–575. doi: 10.1007/s11418-018-1178-x. [DOI] [PubMed] [Google Scholar]
- 37.Fang S.M., et al. Anti-inflammatory diterpenes from the fruits of Vitex trifolia L. var. simplicifolia Cham. J. Asian Nat. Prod. Res. 2019;21(10):985–991. doi: 10.1080/10286020.2018.1482881. [DOI] [PubMed] [Google Scholar]
- 38.Djimabi K., et al. Diterpenoids with α-glucosidase inhibitory activities from the fruits of Vitex trifolia Linn. Fitoterapia. 2022;161 doi: 10.1016/j.fitote.2022.105248. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q.Y., Chen Y.S., Wang F., Chen S.W., Zhang Y.H. Chemical of Vitex trifolia. Zhongguo Zhongyao Zazhi. 2014;39(11):2024–2028. [PubMed] [Google Scholar]
- 40.Yan L.H., Zhang Q.W., Wang Z.M., Xu L.Z., Yang S.L. Studies on chemical constituents of Vitex trifolia(Ⅱ) Chin. Tradit. Herb. Drugs. 2010;41(10):1622–1624. [Google Scholar]
- 41.Yan L.H., et al. Studies on chemical constituents of Vitex trifolia(Ⅰ) Chin. Tradit. Herb. Drugs. 2009;40(4):531–533. [Google Scholar]
- 42.Huang M., et al. A new ursane triterpenoid possessing cytotoxicity from the fruits of Vitex trifolia var. simplicifolia. Chem. Nat. Compd. 2016;52(4):660–663. [Google Scholar]
- 43.Yoshioka T., Inokuchi T., Fujioka S., Kimura Y. Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. Z. Naturforsch., C: J. Biosci. 2004;59(7–8):509–514. doi: 10.1515/znc-2004-7-810. [DOI] [PubMed] [Google Scholar]
- 44.Chen H.Y., Tu L.F., Xiao C.R., Luo Y.M. Chemical constituents from fruits of Vitex trifolia var. simplicifolia. Zhongguo Zhongyao Zazhi. 2018;43(18):3694–3700. doi: 10.19540/j.cnki.cjcmm.20180625.002. [DOI] [PubMed] [Google Scholar]
- 45.Xue G., Gang C. Isolation,identification and cytotoxic activity analysis of polymethoxylated flavonoids from Vitex rotundifolia fruit. J. Plant Resour. Environ. 2015;24(2):118–120. [Google Scholar]
- 46.Wu C., Zhang J., Yin Z.Q. Studies on chemical constituents of viticis fructus. Pharmaceut. Biotechnol. 2010;17(6):504–507+564. [Google Scholar]
- 47.Watanabe K., Takada Y., Matsuo N., Nishimura H. Rotundial, a new natural mosquito repellent from the leaves of Vitex rotundifolia. Biosci. Biotechnol. Biochem. 1995;59(10):1979–1980. doi: 10.1271/bbb.59.1979. [DOI] [PubMed] [Google Scholar]
- 48.Tiwari N., Luqman S., Masood N., Gupta M.M. Validated high performance thin layer chromatographic method for simultaneous quantification of major iridoids in Vitex trifolia and their antioxidant studies. J. Pharm. Biomed. Anal. 2012;61:207–214. doi: 10.1016/j.jpba.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Kawazoe K., et al. Phenylnaphthalene compounds from the subterranean part of Vitex rotundifolia and their antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2001;64(5):588–591. doi: 10.1021/np000307b. [DOI] [PubMed] [Google Scholar]
- 50.Gu Q., Zhang X.M., Zhou J., Qiu S.X., Chen J.J. One new dihydrobenzofuran lignan from Vitex trifolia. J. Asian Nat. Prod. Res. 2008;10(5–6):499–502. doi: 10.1080/10286020801967359. [DOI] [PubMed] [Google Scholar]
- 51.Ikawati Z., Wahyuono S., Maeyama K. Screening of several Indonesian medicinal plants for their inhibitory effect on histamine release from RBL-2H3 cells. J. Ethnopharmacol. 2001;75(2–3):249–256. doi: 10.1016/s0378-8741(01)00201-x. [DOI] [PubMed] [Google Scholar]
- 52.Kannathasan K., Senthilkumar A., Venkatesalu V. In vitro antibacterial potential of some Vitex species against human pathogenic bacteria. Asian Pac. J. Tropical Med. 2011;4(8):645–648. doi: 10.1016/S1995-7645(11)60164-8. [DOI] [PubMed] [Google Scholar]
- 53.Geetha V., Doss A., Doss A.P. Antimicrobial potential of Vitex trifolia linn. Ancient Sci. Life. 2004;23(4):30–32. [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B., et al. Vitexicarpin acts as a novel angiogenesis inhibitor and its target network. Evid Based Complement Alternat. Med. 2013;2013 doi: 10.1155/2013/278405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng F.M., et al. Vitexicarpin induces apoptosis in human prostate carcinoma PC-3 cells through G2/M phase arrest. Asian Pac. J. Cancer Prev. APJCP. 2012;13(12):6369–6374. doi: 10.7314/apjcp.2012.13.12.6369. [DOI] [PubMed] [Google Scholar]
- 56.Xiao-cheng C., Li-hong X., Qiao X., Xi-feng S. Inhibition of Viticis Fructus total flavonoids on self-renewal of lung cancer stem cells derived from NCI-H446 cell line. Chin. Tradit. Herb. Drugs. 2014;45(9):1284–1287. [Google Scholar]
- 57.Chen L., Li Y. Anti-cancer activity reserach on flavone extract from Fructus Vititcis. J. Anhui Agri. Sci. 2010;38(29):16234–16235+16238. [Google Scholar]
- 58.Mathankumar M., Tamizhselvi R., Manickam V., Purohit G. Assessment of anticarcinogenic potential of Vitex trifolia and Triticum aestivum linn by in vitro rat liver microsomal degranulation. Toxicol. Int. 2015;22(1):114–118. doi: 10.4103/0971-6580.172269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu L., et al. Activation of the apoptosis signal-regulating kinase 1/c-Jun N-terminal kinase pathway is involved in the casticin-induced apoptosis of colon cancer cells. Exp. Ther. Med. 2014;8(5):1494–1500. doi: 10.3892/etm.2014.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang C., Shi R., Chen B., Yan X., Tang G. Casticin elicits inflammasome-induced pyroptosis through activating PKR/JNK/NF-κB signal in 5-8F cells. Biomed. Pharmacother. 2020;123 doi: 10.1016/j.biopha.2019.109576. [DOI] [PubMed] [Google Scholar]
- 61.Liu J., et al. Casticin inhibits nasopharyngeal carcinoma growth by targeting phosphoinositide 3-kinase. Cancer Cell Int. 2019;19:348. doi: 10.1186/s12935-019-1069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan L., et al. Casticin inhibits breast cancer cell migration and invasion by down-regulation of PI3K/Akt signaling pathway. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20180738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong G., et al. The Cyr61 is a potential target for rotundifuran, a natural labdane-type diterpene from Vitex trifolia L., to trigger apoptosis of cervical cancer cells. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6677687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.You K.M., Son K.H., Chang H.W., Kang S.S., Kim H.P. Vitexicarpin, a flavonoid from the fruits of Vitex rotundifolia, inhibits mouse lymphocyte proliferation and growth of cell lines in vitro. Planta Med. 1998;64(6):546–550. doi: 10.1055/s-2006-957511. [DOI] [PubMed] [Google Scholar]
- 65.Li X., et al. Casticin inhibits stemness of hepatocellular carcinoma cells via disrupting the reciprocal negative regulation between DNMT1 and miR-148a-3p. Toxicol. Appl. Pharmacol. 2020;396 doi: 10.1016/j.taap.2020.114998. [DOI] [PubMed] [Google Scholar]
- 66.Hosozawa S., Kato N., Munakata K., Chen Y.-L. Antifeeding active substances for insect in plant. Agric. Biol. Chem. 2014;38(5):1045–1048. [Google Scholar]
- 67.Hu Y., et al. Estrogen-like activity of volatile components from Vitex rotundifolia L. Indian J. Med. Res. 2007;126(1):68–72. [PubMed] [Google Scholar]
- 68.Ye Q., Zhang Q.Y., Zheng C.J., Wang Y., Qin L.P. Casticin, a flavonoid isolated from Vitex rotundifolia, inhibits prolactin release in vivo and in vitro. Acta Pharmacol. Sin. 2010;31(12):1564–1568. doi: 10.1038/aps.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsui M., et al. Aqueous extract of Vitex trifolia L. (Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of Nuclear Factor kappa B translocation and expression. J. Ethnopharmacol. 2012;143(1):24–32. doi: 10.1016/j.jep.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 70.Lin S., et al. In vivo effect of casticin on acute inflammation. Zhong Xi Yi Jie He Xue Bao. 2007;5(5):573–576. doi: 10.3736/jcim20070520. [DOI] [PubMed] [Google Scholar]
- 71.Annamalai P., Thangam E.B. Vitex trifolia L. modulates inflammatory mediators via down-regulation of the NF-κB signaling pathway in carrageenan-induced acute inflammation in experimental rats. J. Ethnopharmacol. 2022;298 doi: 10.1016/j.jep.2022.115583. [DOI] [PubMed] [Google Scholar]
- 72.Ma J., et al. Casticin prevents DSS induced ulcerative colitis in mice through inhibitions of NF-κB pathway and ROS signaling. Phytother Res. 2018;32(9):1770–1783. doi: 10.1002/ptr.6108. [DOI] [PubMed] [Google Scholar]
- 73.Lee H., et al. Casticin, an active compound isolated from Vitex Fructus, ameliorates the cigarette smoke-induced acute lung inflammatory response in a murine model. Int. Immunopharm. 2015;28(2):1097–1101. doi: 10.1016/j.intimp.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 74.Li X., et al. Casticin suppresses monoiodoacetic acid-induced knee osteoarthritis through inhibiting HIF-1α/NLRP3 inflammasome signaling. Int. Immunopharm. 2020;86 doi: 10.1016/j.intimp.2020.106745. [DOI] [PubMed] [Google Scholar]
- 75.Liou C.J., et al. Casticin inhibits COX-2 and iNOS expression via suppression of NF-κB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J. Ethnopharmacol. 2014;158 Pt A:310–316. doi: 10.1016/j.jep.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 76.Kannathasan K., Senthilkumar A., Venkatesalu V., Chandrasekaran M. Larvicidal activity of fatty acid methyl esters of Vitex species against Culex quinquefasciatus. Parasitol. Res. 2008;103(4):999–1001. doi: 10.1007/s00436-008-1078-1. [DOI] [PubMed] [Google Scholar]
- 77.Kannathasan K., Senthilkumar A., Venkatesalu V. Mosquito larvicidal activity of methyl-p-hydroxybenzoate isolated from the leaves of Vitex trifolia Linn. Acta Trop. 2011;120(1–2):115–118. doi: 10.1016/j.actatropica.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Chandrasekaran T., Thyagarajan A., Santhakumari P.G., Pillai A.K.B., Krishnan U.M. Larvicidal activity of essential oil from Vitex negundo and Vitex trifolia on dengue vector mosquito Aedes aegypti. Rev. Soc. Bras. Med. Trop. 2019;52 doi: 10.1590/0037-8682-0459-2018. [DOI] [PubMed] [Google Scholar]
- 79.Manjunatha B.K., et al. Comparative evaluation of wound healing potency of Vitex trifolia L. and Vitex altissima L. Phytother Res. 2007;21(5):457–461. doi: 10.1002/ptr.2094. [DOI] [PubMed] [Google Scholar]
- 80.Zhou L., et al. Casticin attenuates liver fibrosis and hepatic stellate cell activation by blocking TGF-β/Smad signaling pathway. Oncotarget. 2017;8(34):56267–56280. doi: 10.18632/oncotarget.17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin K.H., Kang S.S., Kim H.J., Shin S.W. Isolation of an aldose reductase inhibitor from the fruits of Vitex rotundifolia Part 2 in the series "Studies on the inhibitory effects of medicinal plant constituents on cataract formation". Phytomedicine. 1994;1(2):145–147. doi: 10.1016/S0944-7113(11)80033-4. [DOI] [PubMed] [Google Scholar]
- 82.Mohanbabu A.V., et al. Evaluation of potential antiamnesic activities of aqueous extract of Vitex trifolia leaves against scopolamine induced amnesia and in normal rats. J. Basic Clin. Physiol. Pharmacol. 2015;26(2):201–209. doi: 10.1515/jbcpp-2014-0002. [DOI] [PubMed] [Google Scholar]
- 83.Lee H., et al. A new compound, 1H,8H-pyrano[3,4-c]pyran-1,8-dione, suppresses airway epithelial cell inflammatory responses in a murine model of asthma. Int. J. Immunopathol. Pharmacol. 2009;22(3):591–603. doi: 10.1177/039463200902200305. [DOI] [PubMed] [Google Scholar]
- 84.Kobayakawa J., Sato-Nishimori F., Moriyasu M., Matsukawa Y. G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifolia Linne fil.) Cancer Lett. 2004;208(1):59–64. doi: 10.1016/j.canlet.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 85.Woradulayapinij W., Soonthornchareonnon N., Wiwat C. In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J. Ethnopharmacol. 2005;101(1–3):84–89. doi: 10.1016/j.jep.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 86.Tandon S., Mittal A.K., Pant A.K. Insect growth regulatory activity of Vitex trifolia and Vitex agnus-castus essential oils against Spilosoma obliqua. Fitoterapia. 2008;79(4):283–286. doi: 10.1016/j.fitote.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 87.Alam G., et al. Tracheospasmolytic activity of viteosin-A and vitexicarpin isolated from vitex trifolia. Planta Med. 2002;68(11):1047–1049. doi: 10.1055/s-2002-35650. [DOI] [PubMed] [Google Scholar]
- 88.Lee J.H., et al. Casticin inhibits growth and enhances ionizing radiation-induced apoptosis through the suppression of STAT3 signaling cascade. J. Cell. Biochem. 2019;120(6):9787–9798. doi: 10.1002/jcb.28259. [DOI] [PubMed] [Google Scholar]
- 89.Li H.B., Chen F. Simultaneous separation and purification of five bioactive coumarins from the Chinese medicinal plant Cnidium monnieri by high-speed counter-current chromatography. J. Separ. Sci. 2005;28(3):268–272. doi: 10.1002/jssc.200400002. [DOI] [PubMed] [Google Scholar]
- 90.Sohn S.H., et al. Inhibition effects of Vitex rotundifolia on inflammatory gene expression in A549 human epithelial cells. Ann. Allergy Asthma Immunol. 2009;103(2):152–159. doi: 10.1016/S1081-1206(10)60169-X. [DOI] [PubMed] [Google Scholar]
- 91.Lee S.M., et al. Vascular protective role of vitexicarpin isolated from Vitex rotundifolia in human umbilical vein endothelial cells. Inflammation. 2012;35(2):584–593. doi: 10.1007/s10753-011-9349-x. [DOI] [PubMed] [Google Scholar]
- 92.Bae H., et al. Vitex rotundifolia L. prevented airway eosinophilic inflammation and airway remodeling in an ovalbumin-induced asthma mouse model. Int. Immunol. 2013;25(3):197–205. doi: 10.1093/intimm/dxs102. [DOI] [PubMed] [Google Scholar]
- 93.Annamalai P., Thangam E.B. Vitex trifolia L. modulates inflammatory mediators via down-regulation of the NF-kappaB signaling pathway in carrageenan-induced acute inflammation in experimental rats. J. Ethnopharmacol. 2022;298 doi: 10.1016/j.jep.2022.115583. [DOI] [PubMed] [Google Scholar]
- 94.Hong-yan L. Study on antioxidative activity of essential oil from fructus viticis in vitro. Anti-Infection Pharmacy. 2014;11(2):119–121. [Google Scholar]
- 95.WFO World Flora Online http://www.worldfloraonline.org (20 Feb).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.