Abstract
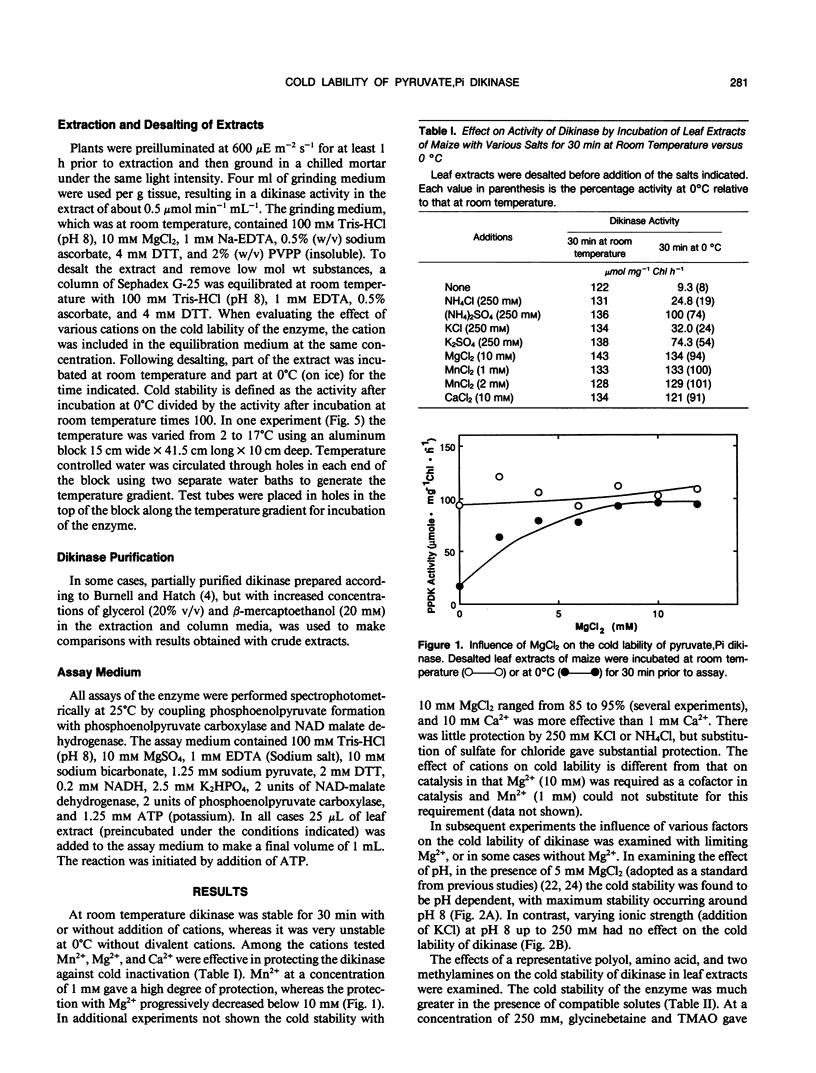
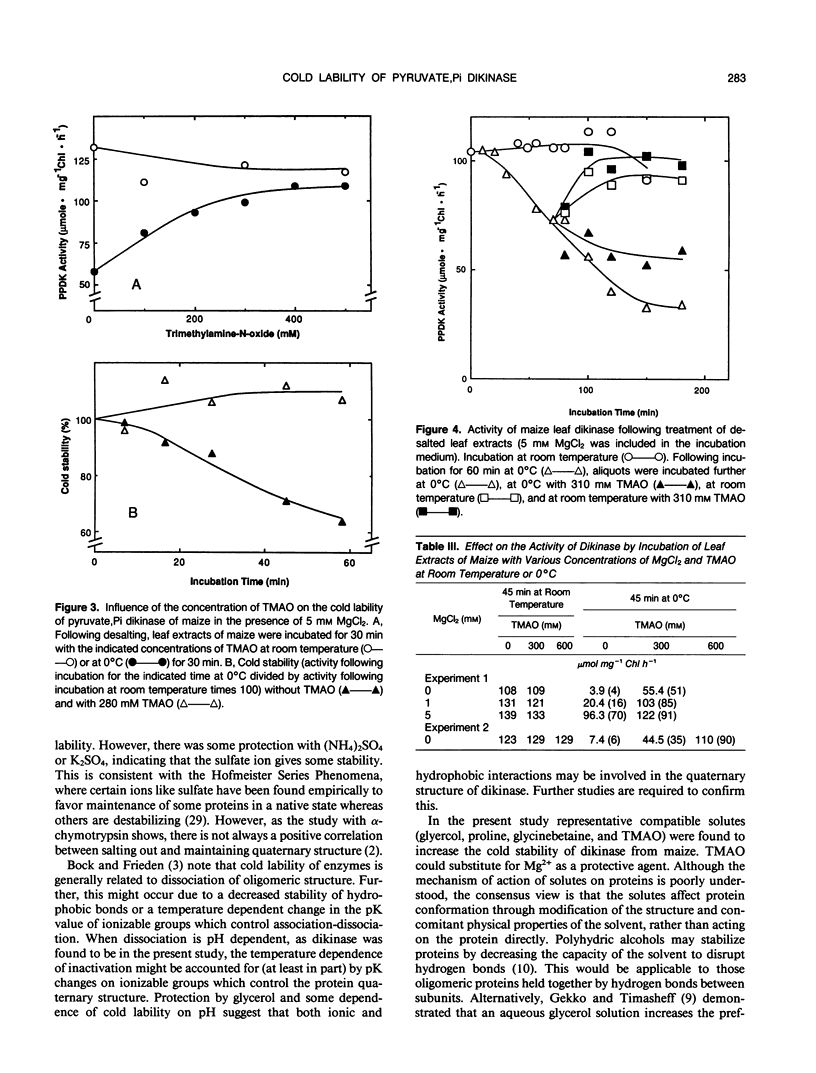
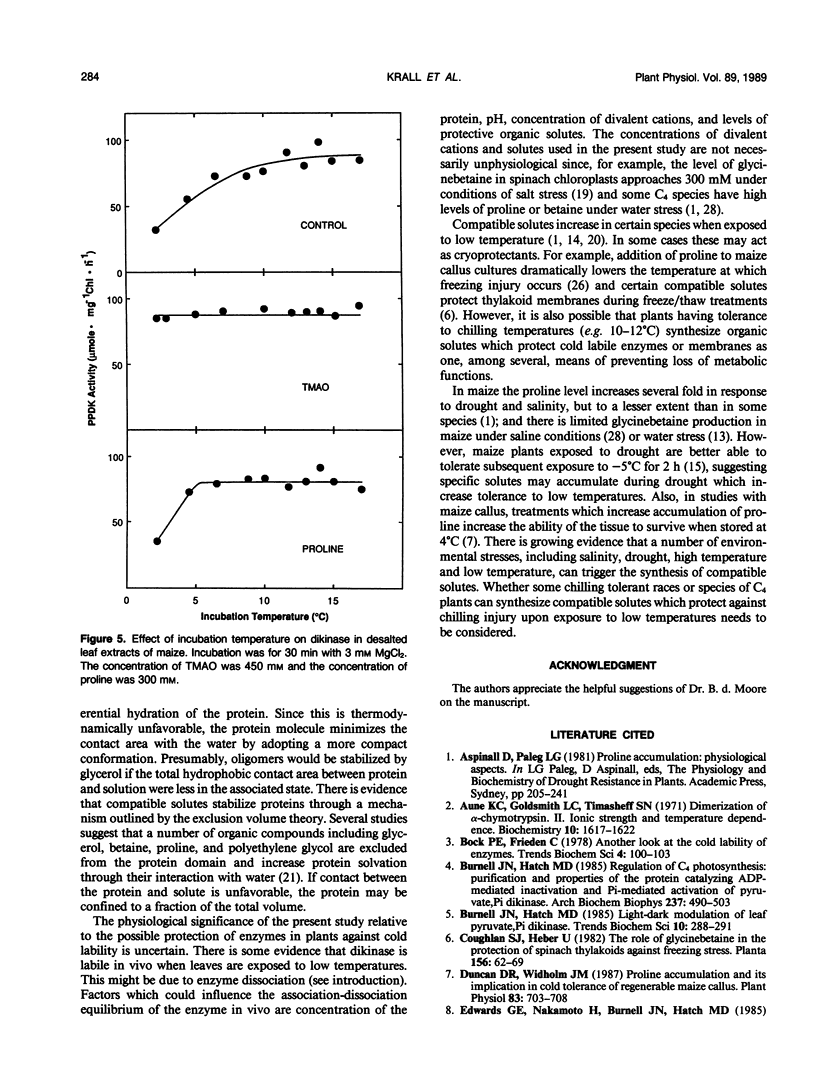
Most C4 species are chilling sensitive and certain enzymes like pyruvate,Pi dikinase of the C4 pathway are also cold labile. The ability of cations and compatible solutes to protect maize (Zea mays) dikinase against cold lability was examined. The enzyme in desalted extracts at pH 8 from preilluminated leaves could be protected against cold lability (at 0°C) by the divalent cations Mn2+, Mg2+, and Ca2+. There was substantial protection by sulfate based salts but little protection by chloride based salts of potassium or ammonium (concentration 250 millimolar). The degree of protection against cold lability under limiting MgCl2 (5 millimolar) was pH sensitive (maximum protection at pH 8), but independent of ionic strength (up to 250 millimolar by addition of KCl). In catalysis Mg2+ is required and Mn2+ could not substitute as a cofactor. Several compatible solutes reduced or prevented the cold inactivation of dikinase (in desalted extracts and the partially purified enzyme), including glycerol, proline, glycinebetaine and trimethylamine-N-oxide (TMAO). TMAO and Mg2+ had an additive effect in protecting dikinase against cold inactivation. TMAO could largely substitute for the divalent cation and addition of TMAO during cold treatment prevented further inactivation. Cold inactivation was partially reversed by incubation at room temperature; with addition of TMAO reversal was complete. The temperature dependence of inactivation at pH 8 and 3 millimolar MgCl2 was evaluated by incubation at 2 to 17°C for 45 minutes, followed by assay at room temperature. At preincubation temperatures below 11°C there was a progressive inactivation which could be prevented by TMAO (450 millimolar). The results are discussed relative to possible effects of the solutes on the quaternary structure of this enzyme, which is known to dissociate at low temperatures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune K. C., Goldsmith L. C., Timasheff S. N. Dimerization of alpha-chymotrypsin. II. Ionic strength and temperature dependence. Biochemistry. 1971 Apr 27;10(9):1617–1622. doi: 10.1021/bi00785a018. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: purification and properties of the protein catalyzing ADP-mediated inactivation and Pi-mediated activation of pyruvate,Pi dikinase. Arch Biochem Biophys. 1985 Mar;237(2):490–503. doi: 10.1016/0003-9861(85)90302-9. [DOI] [PubMed] [Google Scholar]
- Duncan D. R., Widholm J. M. Proline accumulation and its implication in cold tolerance of regenerable maize callus. Plant Physiol. 1987 Mar;83(3):703–708. doi: 10.1104/pp.83.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry. 1981 Aug 4;20(16):4667–4676. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- Gerlsma S. Y. Reversible denaturation of ribonuclease in aqueous solutions as influenced by polyhydric alcohols and some other additives. J Biol Chem. 1968 Mar 10;243(5):957–961. [PubMed] [Google Scholar]
- Hand S. C., Somero G. N. Urea and methylamine effects on rabbit muscle phosphofructokinase. Catalytic stability and aggregation state as a function of pH and temperature. J Biol Chem. 1982 Jan 25;257(2):734–741. [PubMed] [Google Scholar]
- Shirahashi K., Hayakawa S., Sugiyama T. Cold lability of pyruvate, orthophosphate dikinase in the maize leaf. Plant Physiol. 1978 Nov;62(5):826–830. doi: 10.1104/pp.62.5.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]
- Taylor A. O., Slack C. R., McPherson H. G. Plants under Climatic Stress: VI. Chilling and Light Effects on Photosynthetic Enzymes of Sorghum and Maize. Plant Physiol. 1974 Nov;54(5):696–701. doi: 10.1104/pp.54.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers L. A., King P. J. Proline: A Novel Cryoprotectant for the Freeze Preservation of Cultured Cells of Zea mays L. Plant Physiol. 1979 Nov;64(5):675–678. doi: 10.1104/pp.64.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


