Abstract
Myelodysplastic syndrome (MDS) is a hematological malignancy of undetermined etiology, possibly linked to chromosomal structural alterations, genetic mutations, presentation and carcinogenicity of variant antigens on cell surface, and the generation of pro-inflammatory microenvironment in the bone marrow. Current drugs are unable to cure this disease, and therefore, decreasing the survival and proliferation of malignant cells to delay disease progression and extend the survival time of patients becomes the primary approach to management. In recent years, the immune system has received increasing attention for its potential role in the occurrence and development of MDS, leading to the emergence of immunoregulation as a viable treatment option. The current review provides a brief overview of pathogenesis of MDS and current treatment principles. In the meantime, the significance of immune proteins in treatment and prognosis of MDS is also discussed.
Keywords: Myelodysplastic syndrome (MDS), Pathogenesis, Immune checkpoint
1. Introduction
Myelodysplastic syndrome (MDS) is a group of heterogeneous hematological malignancies identified by ineffective hematopoiesis. It is accompanied by varying degrees of myelodysplasia, decrease in hematopoietic cells, and a risk of progression to acute myelogenous leukemia (AML) [1]. By now, the pathogenesis of MDS remains an open issue. Most immune cells and downstream signaling pathways play roles in the pathogenesis and progression of MDS. The dysregulation of the immune system in AML/MDS can either facilitate or hinder the dysregulation at the balance point of malignant cloning via inducing inflammatory responses, altering adaptive immunity, and causing somatic mutation, etc. For instance, the up-regulation of some immune checkpoints helps tumor cells escape from immune surveillance, reduces the count of some immune cells (e.g., ILC1) and increases the number of some malignant cells (e.g., myeloid-derived suppressor cells, MDSC), which then promote the occurrence and development of disease. It has been reported that immune-related treatment is available for treatment of various types of MDS [2]. In this context, it may be feasible to address MDS by targeting immune-related signaling pathways and protein molecules that are involved in the onset of MDS.
Therapeutic regimens, including monoclonal antibody (mAb) therapy, CAR-T cell therapy and adoptive cellular therapy, have become hot topics in immunotherapy for MDS. mAbs for MDS mainly fall into the following three categories: antibody-drug conjugates (ADC), bispecific T cell engagers (BiTEs), and immune checkpoint inhibitors (ICIs). In recent years, the emergence of ICIs has made a significant breakthrough in cancer treatment, and their successful application in various solid tumors has changed the research direction and treatment pattern for hematological malignancies. ICI-targeted therapy for MDS works with multiple pathways, with ICIs specifically targeting tissues with distinct immune functions. Currently, several novel drugs directed towards small-molecule immune targets are undergoing preclinical evaluation. DNA-demethylating drugs remain the mainstay of first-line treatment for AML/MDS at present. Guidelines recommend that patients with relapsed/refractory AML or MDS and not suitable for allogenic transplantation can be included into clinical trials of DNA-demethylating drugs. The combination of DNA-demethylating drugs and small-molecule targeted drugs/immunosuppressive agents is the primary treatment option for MDS in current clinical trials.
This review provides a brief overview of the possible pathogenesis of MDS by describing the potentially involved signaling pathways and immune proteins (Fig. 3 and Fig. 4.). Additionally, the prognosis and progression of MDS are discussed from the aspect of ICIs (Table 1.). The main purpose of the current review is to introduce the protein molecules which have been found in studies on MDS/AML and are capable of predicting disease severity and risk of progression. Furthermore, the prospect of ICIs in treatment for MDS is also described.
Fig. 3.
Small molecules of clinically significant immune proteins expressed in tumor cells and corresponding immunosuppressants developed against these small molecules. Figures were created in BioRender.com.
Fig. 4.
Small molecules of immune proteins expressed on immune cells and affecting the development and progression of tumors and their immunosuppressants. Figures were created in BioRender.com.
Table 1.
Current progress in the development of immunosuppressants corresponding to the small molecules of immune proteins mentioned in this paper. HR-MDS high risk myelodysplastic syndromes, MDS myelodysplastic syndromes, AML acute myeloid leukemia, CLL chronic lymphocytic leukemia, MM multiple myeloma, FDA food and drug administration, PBMC peripheral blood mononuclear cell.
| MDS immune checkpoint related drugs | ||||||||
|---|---|---|---|---|---|---|---|---|
| type | drug | NCT number | experimental site | particular year | subjects | experimental progress | effect | security |
| Anti-cd47 antibody | magrolimab | NCT03248479 | America | 2017-9 to 2025 | Patients with HR-MDS (including TP53 mutation)/AML | Phase Ib | good | good |
| Anti-cd47 antibody | magrolimab | NCT04313881 | America | 2020-9 to 2026 | MDS patients | Phase III | / | / |
| Anti-cd47 antibody | magrolimab | NCT05079230 | America | 2022-7 to 2025 | AML patients | Phase III | / | / |
| Anti-cd47 antibody | magrolimab | NCT04435691 | America | 2020-7 to 2024 | AML patients | Phase Ib/II | / | / |
| Anti-cd47 antibody | magrolimab | NCT02678338 | America | 2016-2 to 2019-2 | Patients with relapsed or refractory AML/MDS | Phase I | good | good |
| Anti-cd47 antibody | magrolimab | NCT03922477 | America | 2019-10 to 2020-3 | Patients with relapsed or refractory AML | Phase Ib | / | / |
| Anti-cd47 antibody | CC-90002 | NCT02641002 | America | 2016-3 to 2018-7 | Patients with relapsed or refractory AML/HR-MDS | Phase I | Poor (forced to stop halfway, lack of objectivity) | good |
| Anti-cd27 agonist antibody | Varlilumab | NCT01460134 | America | 2011-10 to 2017-10 | Patients with B and T cell malignancies and patients with renal cell carcinoma | Phase I | good | good |
| Anti-cd70 antibody | Vorsetuzumab mafodotin (SGN-75) | NCT01015911 | America | 2009 to 2012 | Patients with relapsed or refractory NHL with CD70 positive, and patients with metastatic renal cell carcinoma | Phase I | good | good |
| Anti-cd70 antibody | cusatuzumab | NCT03030612 | France and Switzerland | 2017-1 to 2022 | Elderly patients with newly diagnosed AML and HR-MDS | Phase II | good | good |
| Anti-cd33 antibody | gemtuzumab ozogamicin | NCT04337138 | America | 2020-4 to 2020-7 | AML patients | It has been approved by the FDA for use in AML | good | good |
| Anti-cd33 antibody | gemtuzumab ozogamicin | NCT00882102 | America | 2009-4 to 2013-9 | AML/HR-MDS patients | Phase II | / | / |
| CD33/CD3 T cell binding agent | AMV564 | NCT03516591 | America | 2018-6 to 2020-7 | LR/HR-MDS patients | Phase I | good | good |
| CD33/CD3 T cell binding agent | AMV564 | NCT03144245 | America | 2017-3 to 2020-5 | Patients with relapsed or refractory AML | Phase I | / | / |
| CD16xCD33 bispecific killer cell binder | BIKE | / | America | 2014 | Blasts from patients with MDS | Preclinical in vitro cell experiments | It could enhance NK cell activity in all MDS groups | / |
| Anti-cd33 antibody | BI 836858 | NCT01690624 | America | 2012-9 to 2018 | Patients with relapsed or refractory AML | Phase I | The experiment was terminated prematurely and could not be evaluated | bad |
| Anti-cd33 antibody | BI 836858 | NCT02240706 | America | 2015-1 to 2019-11 | Patients with low-intermediate risk MDS | It has been approved by the FDA for the treatment of MDS | good | good |
| Anti-cd33 antibody | BI 836858 | NCT02632721 | America | 2016-6 to 2023-1 | AML patients | Phase I/II | / | / |
| CD33 Antibody-conjugate Drug (ADC) | SGN-CD33A | NCT02785900 | America | 2016-5 to 2017-10 | Patients with newly diagnosed AML | Phase III | / | / |
| CD33 Antibody-conjugate Drug (ADC) | SGN-CD33A | NCT01902329 | America | 2016-5 to 2017 | AML (include M3) patients | Phase III | good | bad |
| CD33 Antibody-conjugate Drug (ADC) | SGN-CD33A | NCT02706899 | America | 2016-3 to 2017-11 | Patients with intermediate and high-risk MDS | Phase I/II | / | / |
| CD33 Antibody-conjugate Drug (ADC) | SGN-CD33A | NCT02326584 | America | 2014-12 to 2018-4 | Patients with AML other than M3 | Phase I | / | / |
| Antiepileptic drugs (inhibiting NFE2 activity) | VAL | / | Britain | 2021 | Blasts from patients with AML | Preclinical in vitro cell experiments | AML cell survival time was shortened | / |
| Iron chelator (inhibits NFE2 activity) | Deferasirox | NCT01868477 | Spain | 2013-6 to 2017-4 | Patients with low-intermediate risk MDS | Phase II | good | good |
| Iron chelator (inhibits NFE2 activity) | Deferasirox | NCT01326845 | France | 2011-3 to 2012-9 | Patients with low-intermediate risk MDS | Phase IV | / | / |
| Anti-cd200 antibody | Fully humanized IgG1 antibody | / | America | 2021 | Different degrees of PBMC humanized NSG mice | Preclinical animal studies | good | good |
| Anti-cd200 antibody | Samalizumab | NCT00648739 | America | 2008-6 to 2010-12 | CLL, MM patients | Phase I/II | good | good |
| Anti-cd200 antibody | Samalizumab | NCT03013998 | America | 2017-1 to 2023 | AML patients | Phase Ib/II | good | good |
| Anti-cd200 antibody | TTI-CD200 | / | Britain | 2020 | NSG mice injected with AML cells | Preclinical animal studies | good | good |
| Anti-TIGIT antibody | Tiragolumab | NCT05259319 | America | 2022 to 2030 | Metastatic non-small cell lung cancer, metastatic bladder cancer, metastatic renal cell cancer, metastatic head and neck cancer | Phase I | / | / |
| Anti-TIGIT antibody | Tiragolumab | NCT04308785 | China | 2020-3 to 2023 | Small cell lung cancer | Phase II | / | / |
| Anti-TIGIT antibody | Etigilimab | NCT04761198 | America, Britain | 2021 to 2023 | Locally advanced or metastatic solid tumors | Phase Ib/II | / | / |
| Anti-TIGIT antibody | AB154 | NCT03628677 | America | 2018-3 to 2023 | Advanced solid tumors | Phase III | / | / |
| Anti-CD155 antibody | COM701 | NCT04354246 | America | 2020 to 2024 | Advanced solid tumors and multiple myeloma | Phase I | / | / |
| FLT3 Inhibitors | quizartinib | NCT02984995 | Japan | 2016-12 to 2018-9 | Refractory/relapsed AML | Phase III | / | / |
| FLT3 Inhibitors | quizartinib | NCT03723681 | China | 2018-11 to 2022-3 | Patients with newly diagnosed AML | Phase I | / | / |
| FLT3 Inhibitors | gilteritinib | NCT03625505 | America | 2018-10 to 2021-8 | Refractory/relapsed AML | Phase I | / | / |
| FLT3 Inhibitors | gilteritinib | NCT05024552 | America | 2021-8 to 2025-8 | Relapsed or refractory FLT3-mutated AML | Phase I | / | / |
| FLT3 Inhibitors | gilteritinib | NCT03730012 | America | 2019-6 to 2021-6 | AML and AML with FLT3 mutation | Phase II | / | / |
| FLT3 Inhibitors | gilteritinib | NCT04027309 | Australia | 2019-7 to 2033 | Patients with newly diagnosed AML and MDS-EB2 with FLT3 mutations | Phase III, which is currently suspended | good | |
| Anti-CD276 antibody | Enoblituzumab | NCT02475213 | America | 2015-6 to 2021 | Head and neck squamous cell carcinoma, non-small cell lung cancer, urothelial carcinoma | Phase I | good | good |
| Anti-CD73 antibody | IPH5301 | NCT05143970 | America | 2021-3 to 2024 | Advanced solid tumors | Phase I | / | / |
| Anti-CTLA-4 antibody | ipilimumab | NCT01757639 | America | 2012-12 to 2016-12 | Relapsed/refractory MDS and AML with MRD | Phase I | / | / |
| Anti-CTLA-4 antibody | ipilimumab | NCT00039091 | America | 2003 to 2018 | MDS patients in whom hypomethylating agents failed | Phase I | good | good |
| Anti-CTLA-4 antibody | ipilimumab | NCT03912064 | America | 2019-4 to 2024 | Patients with AML/MDS/MPN/CML/myelofibrosis | Phase I | / | / |
| Anti-CTLA-4 antibody | ipilimumab | NCT00060372 | America | 2003-5 to 2013-3 | Recurrent hematologic malignancy after hematopoietic stem cell transplantation | Phase Ib | good | good |
| VISTA monoclonal antibody | JNJ-61610588 | NCT02671955 | America | 2016-2 to 2017-7 | Patients with advanced lung, pancreatic, cervical, colorectal, head and neck cancer | Phase I, which is currently suspended | / | / |
| VISTA Inhibitors | CA-170 | NCT02812875 | America | 2016-6 to 2020-7 | Patients with advanced cancer and lymphoma | Phase I | / | / |
| VISTA monoclonal antibody | HMBD-002 | NCT05082610 | America | 2022-1 to 2025-1 | Patients with advanced solid tumors | Phase I/II | / | / |
ICIs that are already approved for clinical use in MDS treatment include.
2. CD47
2.1. Introduction of CD47 and its mechanism of action
CD47 is a cell surface glycoprotein [3] that can bind various proteins including integrin, thrombospondin-1 and signal regulatory protein α (SIRPα) [4,3]. It is ubiquitously expressed on normal cells and show excessive expression in a large number of tumor cells. Usually, CD47 functions as a receptor that facilitates immune evasion and prevents micropinocytosis, protecting normal cells against injury from the immune system (Fig. 1) [5]. It was first regarded as the regulator of self-renewal of erythrocytes and later found to exhibit wide expression in other cell types with a significant role in different homeostatic systems. It was reported that the down-regulation of CD47 could potentiate the elimination of aging and redundant cells via phagocytosis [6].
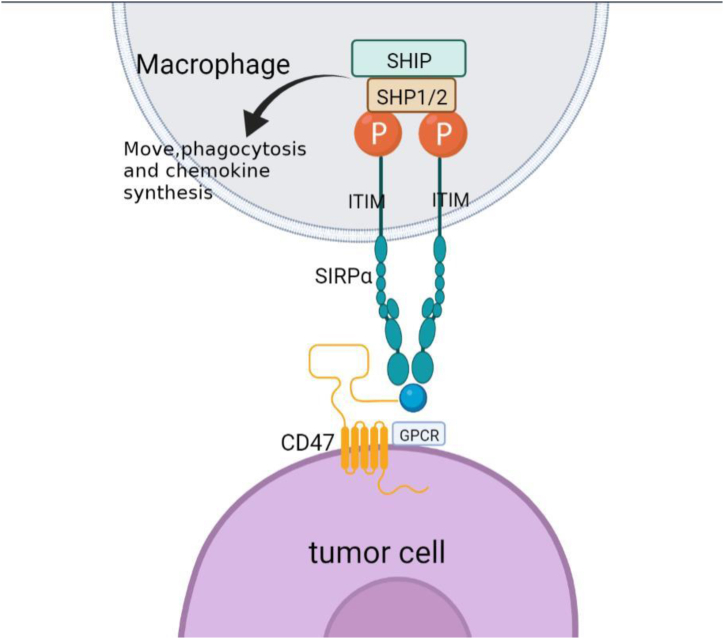
Fig. 1.
Schematic diagram of signal transmission of CD47. CD47 is a transmembrane protein containing N-terminal extra-cellular lgV-like domains,5 transmembrane domains and a short C-terminal cytoplasmic tail. CD47 induces and interacts with SIRPαto tyrosine phosphorylation of ITM motifs in SIRPαcells, resulting in the recruitment of SHP-1 and SHP-2 phosphatases, the polymerization of F-actin and the contraction of myosin. These processes control the movement, phagocytosis and chemokine synthesis of macrophages, as well as the cross antigen presentation and stimulation of the STING (IFN gene stimulator)/GAS (cyclic GMP-AMP synthase)pathway of dendritic cells. Figures were created in BioRender.com.
2.2. Role of CD47 in MDS
A previous study found that leukemic stem cells (LSC) -transplanted mice survived shorter when CD47 was highly expressed [7]. Another study found that low-risk MDS progenitors had increased expression of CRT on their surface but did not express CD47, and they were readily to be phagocytosed by macrophages. To the contrary, high-risk MDS progenitors expressed more CD47 and could evade from being phagocytosed [8]. This is likely due to that CD47 overexpression confers anti-phagocytosis activity on MDS progenitors, which allows them to escape from immune recognition by counteracting the CRT signaling on phagocytic cells. In other words, the CD47 overexpression in MDS progenitors can induce pro-tumor responses and then gradually affect the immune microenvironment in the bone marrow. Collectively, the expression of CD47 may be highly involved in the disease progression and prognosis of MDS.
2.3. Research prospect of CD47
For the past few years, CD47-targeted therapy has become a hot topic in research of MDS treatment, and multiple drugs, either alone or in combination, have advanced to clinical trials. Previous clinical trials combined Magrolimab (anti-CD47 monoclonal antibody) and Azacitidine (hypomethylating agent, HMA) in treatment of patients with myeloid malignancy (including patients with mutations in TP53). They reported that such strategy had a lasting effect with the synergistic activity of the two agents and could be well tolerated by the patients with a safe profile [9,10]. CC-90002 is another anti-CD47 antibody that can inhibit the interaction between CD47 and SIRPα. It was proved that whether CC-90002 was used alone or in combination with other anti-tumor drugs, patients with hematological malignancies still benefited from it and had a high remission rate, whereas the treatment was discontinued due to the presence of anti-drug antibodies [11]. CD47 is a key molecule with activity against phagocytosis, and the combination of anti-CD47 agent and some anti-tumor agents (e.g., AZA and Ruxolitinib) has become a new direction for research of AML and relapsed/refractory HR-MDS.
The immune regulators that are currently under exploration include.
3. CD33
3.1. Introduction of CD33 and its mechanism of action
CD33 is a transmembrane protein, myeloid-specific sialic acid-binding receptor and hematopoietic differentiation antigen. It is excessively expressed in most primitive leukemic cells and undergoes receptor-mediated endocytosis in-vivo [12]. CD33 plays an essential role in the regulation of immune functions. CD33 binding with the ligand can lead to dephosphorylation of cellular proteins and then down-regulate relevant signaling pathways [13]. Additionally, activated CD33 plays an immunosuppressive role by recruiting inhibitory proteins, activating myeloid-derived suppressor cells (MDSC) and inducing the secretion of inhibitory cytokines IL-10 and TGF-β through its ITIM-like domain [14]. Moreover, CD33 is also involved in cell adhesion and apoptosis [15,16].
3.2. Research significance of CD33 for MDS
Chen et al. [12] reported that CD33 was excessively expressed on MDS-MDSC. Upon binding with its ligand, CD33 potentiated the activation, proliferation and inhibitory function of MDSCs, contributing to the progression of inflammation in the marrow microenvironment and then leading to a hematopoietic failure [12]. Other studies found that the expression of CD33 was the highest in AML, followed by MDS [[17], [18], [19]], and its expression was positively associated with the disease risk in MDS patients [18]. Moreover, increasing evidence has suggested that cCD33 level can be used as an independent factor for prognosis of AML and MDS, which provides a novel insight for the clinical treatment of the two diseases.
3.3. Research prospect of CD33
By now, there is only one drug termed BI 836858, an anti-CD33 antibody-drug conjugate (ADC), that has been approved for use in MDS treatment, while Gemtuzumab, Ozogamicin and BI 836858 have been approved to be used in treatment of AML. Other drugs are still in testing stage. For example, the anti-CD33 ADC, SGN-CD33A, showed favorable efficacy in previous clinical trials, whereas the safety was poorly satisfied. Besides, it was abandoned in some related clinical trials in 2016, as its side effect, myelosuppression, led to an increased number of infection-related deaths [20]. The CD33/CD3+ T cell-specific binding agent AMV564 has been potentially used in treatment of myeloid solid tumors and hematological malignancies, and it can advance the activation and proliferation of T cells in MDS patients, enhance IFN-γ activity, and induce cytotoxic T cell activation [14,[13], [21], [22], [23]]. MDSCs are important participants in immune evasion and tumor progression, while the bi-specific anti-CD16/CD33 T-cell engaging (BiTE) antibody can further inhibit the immunosuppressive effect of MDSCs and enhance the CD33+- specific function of NK cells [24]. In spite of the different mechanisms of action, the drugs mentioned above eventually show favorable efficacy in treatment of MDS.
4. NFE2
4.1. Introduction of NFE2 and its function
Previous research found that NFE2 transcription factor mainly acted on hematopoietic cells such as erythroid cells, megakaryocytes and mast cells, etc., and NFE2 deficiency would result in severe disruption of platelets. Recent research also revealed its expression in non-hematopoietic structures, such as trophoblast cells and bone tissues [25]. NFE2 acts on megakaryocytes mainly via regulating their maturation and platelet transcription. The deficiency of NFE2 can affect not only hematopoietic function but also the inflammatory system. It has been proven that the NFE2/HO-1 axis plays a major role in inflammatory resistance, as NFE2 induces the expression of HO-1 gene to promote the degradation of heme to anti-inflammatory compounds including carbon monoxide and free iron. In addition, the NFE2/HO-1 axis has been used as a therapeutic target for treatment of inflammation-related diseases [26]. Another significant axis associated with inflammation is the NFE2/ARE axis. Upon activation, NF-κB activation is limited and the pro-inflammatory cytokines (e.g., IL-6, TNF-α, iNOS, IL-1 and COX-2) which are hyper-produced following NF-κB activation is disrupted. Notably, the elevation of HO-1 expression can also suppress the activity of NF-κB [27]. Activated NFE2 can prevent the lipopolysaccharide (LPS) -induced transcriptional up-regulation of pro-inflammatory cytokines, such as IL-6 and IL-1β, and also inhibit the production of downstream IL-17 and other inflammatory factors including TH1 and TH17 [28,29]. A bold guess is made that NFE2 and NF-κB signaling cooperate to control the transcription or activity of downstream target proteins and thereby inhibit inflammatory process (Fig. 2).
Fig. 2.
Schematic diagram of NFE2 functioning. NFE2 induces expression of HO-1 gene to promote the degradation of heme to anti-inflammatory compounds including carbon monoxide and free iron. Upon activation of NFE2/ARE axis, NF-κB activation will be limited and the pro-inflammatory cytokines (e.g., IL-6, TNF-α, iNOS, IL-1 and COX-2) which are hyper-produced following NF-κB activation will be disrupted. Figures were created in BioRender.com.
4.2. Significance of NFE2 in hematologic diseases
NFE2 exhibits increased expression in a variety of malignancies, such as AML [30], gastric cancer [31], ovarian cancer [32] and gallbladder cancer [27]. In addition, NFE2 is also overexpressed in patients with polycythemia vera (PV) and myeloproliterative neoplasms (MPNs), showing a relationship with the disease severity [33,34]. Other research demonstrated that NFE2 could induce AML chemoresistance via activating the JNK/c-Jun signaling pathway and decreasing the expression of MSH2 in AML cells [30]. A recent study also identified the role of NFE2 in chronic lymphocytic leukemia (CLL) chemoresistance [35]. Lin et al. [36] found that NFE2 was excessively expressed in MDS patients, especially in those at a high risk, and higher levels of NFE2 were prognostic for poorer outcomes in this population. Another study indicated that NFE2 could mediate the Ara-C (cytarabine) resistance in high-risk MDS patients by directly targeting DUSP1, and NFE2 knock-out could lead to re-sensitization [36]. These findings suggest that NFE2 is also the basis of development of drug resistance in MDS cells.
4.3. Research prospect of NFE2
Previous research found that VAL, an inhibitor of NFE2 activity, in combination with low-dose medroxyprogesterone acetate (MPA), could correct the counts of platelet, erythrocyte and neutrophil in AML patients, reduce transfusion dependency and delay or decrease the conversion from MDS to AML, offering positive implications for the improvement of patients's quality of life and survival [37]. The iron chelator Deferasirox could act as an inhibitor of NFE2 by inducing down-regulation of genes related to the NF-κB signaling pathway. Previous study also reported that Deferasirox could reduce inflammatory responses and increase hematopoiesis in MDS patients [38]. Collectively, agents targeting NFE2 may have effects in the management of inflammatory symptoms, as well as in the prevention and treatment of diseases such as myeloproliferative disease and cancer. However, the role of NFE2 signaling in MDS has not been adequately studied yet.
5. CD200
5.1. Introduction of CD200 and its mechanism of action
CD200 (also known as a single-channel protein), a type 1a transmembrane glycoprotein belonging to the immunoglobulin superfamily, exhibits extensive expression in neurons, endothelial cells, thymocytes, B and T lymphocytes [39]. CD200 receptor has a relatively limited expression range as confined to immune cells only (mainly bone marrow cells, NK cells, B and T lymphocytes) [40]. It remains unclear about the role of CD200 in hematopoiesis, but the immunosuppressive implications instead upon binding with its receptor. For the past few years, growing evidence has supported the role of CD200 in human bone marrow cells as an immune suppressor, except the conventional immunoregulatory role in cases of infection, autoimmune disease (AID) and allergic diseases [41]. CD200 binding with its receptor induces T-lymphocyte in-vitro differentiation to CD4CD25Foxp3 Tregs [42] and suppresses corresponding inflammatory responses in various inflammatory diseases. In the meantime, their interaction can also regulate tumor microenvironment via affecting tumor-related bone marrow cells, thereby playing a key role in tumor-related inflammation and angiogenesis [42,43].
5.2. Significance of CD200 in MDS/AML
CD200 has excessive expression in MM, AML and CLL [42,[44], [45], [46]]. In addition, CD200 also has implications for progression of head and neck carcinoma, renal carcinoma, colon carcinoma and neurodegenerative disease [47,48]. Jenny M Ho et al. [49] found that CD200 exhibited extremely high expression in LSCs of AML patients but were absent in normal hematopoietic stem cells (HSCs) and LSCs. Shelley Herbrich et al. [50] found high levels of CD200 in some diseases with complex karyotype or a tendency of recurrence and reported a significantly decreased survival rate in this population. Another study also demonstrated that high expression levels of CD200 were associated with a poorer outcome, a shorter overall survival time and a higher risk of progression to AML in MDS patients [51]. Moreover, Chen et al. [52] proved that the CD200-positive rate increased with increasing malignancy degree of MDS, i.e., CD200 expression was tightly associated with the subclassification and clinical staging of MDS. The studies above indicate that CD200 may be a marker of primitive leukemic hematopoietic cells and is significantly associated with the malignancy degree and prognosis of MDS/AML.
5.3. Research prospect of CD200
Shelley Herbrich et al. [50] developed a CD200-targeted, fully humanized IgG1 antibody and applied it in humanized peripheral blood mononuclear cell-engrafted NSG mice with various degrees of graft-versus-host disease (GVHD). They found that mice administrated with such antibody improved significantly and eventually were free of leukemia. Currently, multiple CD200-targeted drugs have been developed. For instance, the humanized monoclonal antibody Samalizumab has advanced to phase I clinical trials for CLL and MM, showing good efficacy (partial remission) and tolerance [53]. TTI-CD200 is a fully humanized antibody capable of enhancing autoimmune cell functions and evidently increasing the effects of immune cells on AML cells. Paraskevi Diamanti et al. [54,55] found that NGS mice treated with TTI-CD200 had an increased survival rate. CD200 as an immune suppressor with high expression in hematological malignancies should be highly valued for its potential role as a therapeutic target.
6. CD155
6.1. Introduction of CD155
CD155 (a type I transmembrane glycoprotein of the immunoglobulin superfamily) is one of adhesion molecules that was first discovered as a poliovirus receptor [56]. It is distributed in epithelial and endothelial cells of multiple tissues with low expression levels. In malignant cells, it reversely has excessive expression and participates in cell adhesion, migration and proliferation. In addition, CD155 is also tightly associated with the outcome of some cancers, including breast cancer [57], melanoma [58], glioblastoma [59], and soft tissue sarcoma [60], etc. Recently, CD155 has been proved as an immunomodulatory receptor that can either activate NK cells via interacting with CD226 and CD96 or suppressing their immune activity through acting on TIGIT. Notably, TIGIT is increasingly expressed in NK cells during the process of cancerization [59,[61], [62], [63]].
6.2. Research significance of CD155 in hematological malignancies
Meng et al. [64] reported that the decline in the proportion and function of NK and T cells in MDS patients was associated with the abnormal expression of TIGIT and CD155. Another study found that NK cells in MDS patients were largely suppressed by MDSCs, which could be reversed by knock-out of TIGIT [65]. The study of Anna Chashchina et al. [66] revealed that CD226 and CD155 cooperated to induce the release of immunoregulatory cytokines (IL-6, IL-8, IL-10 and TNF-α) from AML cells, and that the elevation of CD226 expression in AML cells was prognostic for the prolonged survival in these patients. In all, C155-TIGIT blockade or CD155-CD226 augmentation may be a viable treatment option for MDS/AML.
6.3. Research prospect of CD155
Since CD155 and TIGIT are usually excessively expressed in cancers, blockade of the interaction between CD155 and TIGIT may provide a new insight for anti-tumor treatment. By now, anti-TIGIT antibodies are in testing stage for treatment of solid tumors. For example, the anti-TIGIT monoclonal antibody OMP-313M32 has advanced to phase I clinical trials as a monotherapy or combination therapy with anti-PD-1 antibodies for solid tumors. Tiragolumab, another anti-TIGIT monoclonal antibody developed by Genentech/Roche (also known as MTIG7192A or RG6058), is in phase II clinical trial [67]. Although the intervention of CD155-TIGIT blockade has not been applied in treatment of hematological diseases yet, there is still some experience that can be obtained from its successful application in treatment of solid tumors. It was also reported that FLT3 inhibitors (Quizartinib and Gilteritinib) could down-regulate the expression of CD155 in AML cells [68].
7. VSIR
7.1. Introduction of VSIR
VSIR is a type of immunoregulatory receptor that can suppress the effector function of T cells. It was reported that the increase in VSIR in tumor is closely related to tumor immune infiltration, expression of related antigens and canonical immune checkpoints. Additionally, VSIR also exhibits high expression in many tumor cells and hematopoietic tissues, and it contributes to an immunosuppressive tumor microenvironment in some cancers and then results in tumor progression and a lower survival rate [69].
7.2. Clinical significance of VSIR in AML/MDS
VSIR can inhibit T-cell activation, and immunodeficiency represents one of the important mechanisms that leads to AML/MDS. Under this background, the clinical significance of VSIR in AML/MDS has been increasingly studied. The analytical results reveal that VSIR has the highest expression in AML among all cancers, and meanwhile, it is also the protein with the highest expression in AML among all immune checkpoints. Moreover, the increased expression of VSIR was associated with a shorter survival time of AML patients and an increased risk of progression from MDS to AML [70]. In this context, VSIR has the potential to be employed as a target in AML/MDS immunotherapy, and it may play an important role in preventing the progression from proliferating MDS primitive cells to AML.
7.3. Current progress in VSIR
Currently, VSIR has been studied in multiple diseases such as renal carcinoma, AML, MDS, and psoriasis. However, whether VSIR can be applied as an emerging immune target in clinical AML/MDS treatment requires validation with huge number of studies and experimental data.
Small-molecule targets that have the potential to be used in MDS immunotherapy but have not been studied include.
8. CD27/CD70
8.1. Introduction of CD27/CD70 and their mechanism of action
CD27 is a member of the tumor necrosis factor (TNF) receptor superfamily and widely expressed in human lymphocytes [71]. CD70 is the only ligand of CD27 that belongs to the TNF superfamily and only exhibits expression in activated lymphocytes and myeloid cells [72]. CD27−CD70 binding is key to the activation of T cells and B cells. Upon binding, CD27 activates the NF-κB signaling pathway via binding TRAF2 and TRAF5, resulting in activation of T, B and natural killer (NK) cells followed by cytokine production and cytotoxic T-cell responses [73,74]. Usually, the CD27−CD70 binding is tightly regulated to prevent excessive expression and lymphocyte over-activation. The co-stimulation subsequent to their binding has self-regulation skills. CD70 down-regulates CD27 expression after binding and reverses the signal transduction. In the meantime, CD27 also decreases the level of CD70, and the deficiency of CD27 can further stimulate the high expression of CD70 [[75], [76], [77]]. It is significantly different from our previous conclusion that CD27 and CD70 exhibited high expression in multiple hematological malignancies and solid tumors, such as lymphoma [78], renal cell carcinoma (RCC) [79], nasopharyngeal carcinoma (NPC) [80] and breast cancer [81], and predicted poor prognosis. Thus, we reasoned that CD27−CD70 binding may play a role in tumor cell survival and proliferation.
8.2. Role of CD27/CD70 in hematological malignancies
The dysregulation of CD70−CD27 axis is associated with tumor progression and immunosuppression. In hematological malignancies, CD70 and CD27 are co-expressed in tumor cells and interact with each other to increase the number of inhibitory regulatory T cells (Tregs), inducing T cell exhaustion and apoptosis, which potentiates the survival and proliferation of tumor cells [82]. Research found that both CD70 and CD27 were expressed by LSCs in patients with AML, chronic myelogenous leukemia (CML) and non-Hodgkin lymphoma (NHL) subtypes. It was also reported that sCD27 presented a high level in serum samples of AML patients and predicted a poor disease prognosis [[71], [83], [84]]. Other studies also noted that the CD27 signal transduction in patients with AML and CML could induce Wnt pathway activation, while impaired Wnt signaling activation could result in proliferation and drug resistance in LSCs [71,83]. To the contrary, CD27 deficiency could increase the susceptibility to EBV infection and relevant lymphoproliferative disorders (PTLD) [85]. Moreover, expression of CD70 and CD27 could be also observed in the malignant plasma cells of patients with multiple myeloma (MM), and the expression of CD27 would decrease with disease progression [86]. Collectively, we believed that CD27 deficiency may be a high risk factor of EBV infection, PTLD and plasma cell proliferative disorders. An interesting finding reported by Suwen Zhao et al. [87] is that patients suffering from aplastic anemia had increased CD27 but decreased CD70. This result suggests that CD27 may be important for abnormal T-cell activation in aplastic anemia patients, and CD70 may play its role dependent on their pathological environment.
8.3. Clinical prospect of CD27/CD70
Currently, increasing drugs targeting CD27/CD70 have advanced to clinical trials. For example, Varlilumab, the agonist antibody targeting CD27 developed by Celldex Therapeutics for treatment of solid tumors and lymphoid malignancies, is still in testing stage and has shown good efficacy [88]. Experimental study found that Varlilumab and ICIs (Nivolumab or Atezolizumab) could exhibit synergistic effect against the tumor microenvironment in patients with T-cell, B-cell malignancies and renal carcinoma [89]. Antibodies targeting CD70 have shown significance in chemotherapy for tumors, as they can suppress proliferation of tumor cells and decrease chemo-resistance [90,91]. However, such strategy may increase the effect of anti-CD70 antibodies on malignant cells, which prompts us to obtain more experimental data to help formulate more suitable treatment regimens. The CD70−CD27 axis is currently considered as a promising target in immunotherapy for cancers. Nan Guo Ring et al. [82] used anti-CD70 antibody Vorsetuzumab and KWAR23 to treat SRG mice with Burkitt lymphoma and obtained similar outcome with Rituximab treatment. Since most existing clinical trials were performed in lymphomas, the efficacy of antibodies targeting CD70 and CD27 in treatment for MDS remains an open issue, which may be a direction in future research.
9. CD276
9.1. Introduction of CD276 and its function
CD276 is a recently identified immunoregulatory protein belonging to the B7 family, and it has activities against T-cell activation and is involved in cell proliferation, apoptosis, invasion, differentiation, autophagy, epithelial-mesenchymal transition (EMT), and formation of tumor microenvironment [92,93]. Interestingly, CD276 plays a dual role in the immune system. On the one hand, CD276 advances proliferation of CD4+ and CD8+ T cells and augments cytotoxic T lymphocyte (CTL) activity [94]. On the other hand, CD276 decreases the production of IL-2 and IFN-γ via suppressing a series of signaling pathways (e.g., NF-κB, nuclear factor of activated T cells and activator protein-1, etc.) and concurrently inhibits proliferation of CD4+ and CD8+ T cells as well as NK cell activity [[95], [96], [97], [98]]. Usually, CD276 is poorly expressed in the majority of normal tissues but increasingly expressed in malignant tumor tissues. Studies also reported that high levels of CD276 are prognostic for poor outcomes of patients with cancers, such as bladder cancer [99], breast cancer [100] and colorectal cancer [101].
9.2. Clinical significance of CD276 in hematologic diseases
Multiple studies revealed that CD276 exhibited significantly increased expression in AML patients and was remarkably associated with poor outcomes [102,103]. Despite that there is no research on the role of CD276 in MDS patients, the current evidence also suggests its potential prognostic significance.
9.3. Current research progress on CD276
Anti-CD276 strategies have advanced to pre-clinical and clinical trials. For instance, the humanized anti-CD276 monoclonal antibody Enoblituzumab (MGA271) can mediate the antibody-dependent cell-mediated cytotoxicity (ADCC) in diverse tumors and has been used in treatment for melanoma, osteosarcoma, Ewing's Sarcoma, renal carcinoma and bladder cancer [104]. Clinical trials concentrating on CD276 activity suppression have been performed in both solid tumors and hematological malignancies. A previous animal experiment assessed the efficacy of CD276-specific chimeric antigen receptor T cells in AML mice and reported CD276 as a promising therapeutic target for treatment of AML with a safety profile [105]. Thus, anti-CD276 mAb is a promising therapeutic target for AML treatment, but further study is warranted to explore its role in MDS progression.
10. CD73
10.1. Introduction of CD73 and its mechanism of action
CD73 is an enzyme that catalyzes AMP to immunosuppressive adenosine. It interacts with CD39 in normal tissues to prevent excessive immune activation. However, it can also help tumor cells achieve self-protection and evade immune cell attacks, conducive to tumor growth, metastasis and formation of tumor microenvironment [[106], [107], [108]]. CD73 exhibits high expression in most solid tumors, such as malignant glioma, melanoma, ovarian cancer, colon carcinoma and hematological malignancies, and it is highly associated with the invasiveness and metastasis of these tumors [109,110].
10.2. Clinical significance of CD73 overexpression in hematological malignancies
CD73 with activities against anti-tumor immunity is excessively expressed by tumor cells. Either in solid tumors or hematological malignancies, CD73 up-regulation usually results in production of extracellular adenosine, which activates adenosine signaling and eventually advances tumor progression [111]. To the contrary, Kong et al. [112] found that increased CD73 expression in CD8+ T cells was prognostic for increased immune responses in and good prognosis of AML, and that CD73− CD8+ T cells showed progressive depletion. Similarly, a German study reported the same research result [113]. These studies suggest that CD37 may represent a double-edged sword. More experiments are required to validate its immunoregulatory role in hematological diseases, and meanwhile, a good understanding on the specific role of CD73 in various cancers and under different disease statuses is essential for further clinical cancer treatment.
10.3. Research prospect of CD73
A French trial [114] revealed that anti-CD73 monoclonal antibody IPH5301 could increase ATP and induce anti-tumor activity of Oxaliplatin in CD73 knock-out mice. By now, few studies are concentrating on the role of CD73 in hematologic diseases. Nevertheless, all results of the studies above indicate that the combination of CD73-targeted agents and other ICIs has the potential as a promising treatment scheme for hematologic diseases.
11. CD161
11.1. Introduction of CD161 and its mechanism of action
CD161 is a C-type lectin receptor (CLR) highly expressed on immune cells such as NK, NKT, CD8+ T and CD4+ T cells, and binding with its ligand LLT1 can inhibit the function of NK cells [115]. RNAseq data revealed that CD161 expression is closely associated with T cell infiltration and expression of related immune checkpoints, immune activation genes, immunosuppressive genes, chemokines and their receptors. CD161, therefore, has the potential to be a tumor marker and may play a role in regulating immune environment by cooperating with other immune checkpoints [116].
11.2. Clinical significance of CD161 in AML
Previous research found that CD161 is a marker of long-lived antigen-specific memory T cells. T cells expressing CD161 can selectively express multidrug resistance membrane transporter (MDR1), conducive to prolonging the survival time of virus-specific memory T cells. Thus, CD161-positive T cells may play a key role in immunotherapy for viral disorders and tumor [117]. It was reported that Valpha24 TC R + CD161+ NKT (Valpha24+ NKT) cells can be activated by α-galactosylceramide and then exhibit anti-tumor effect on multiple tumor cells. The number of Valpha24+ NKT cells decreases significantly in hematopoietic malignancies, such as AML and MDS [118]. In contrast, a recent study of Chevalier MF et al. found that the CD4+ T cells expressing TIGIT and CD161 were associated with AML relapse in patients undergoing haemopoietic stem-cell transplant (HSCT) [119].
11.3. Current progress on CD161
There have been studies that identified expression of inhibitory CD161 receptor on infiltrating T cells in liver cancer and glioma using single-cell sequencing and related experiments [120,121]. Thus, CD161 may provide a deeper insight into the immune evasion mechanisms related to tumor progression and relapse. Relevant studies proved that ILC1 cells help control the growth of AML tumor cells, and human ILC1 cells can eliminate leukemia stem cells (LSCs) by inducing the production of IFN-γ. A recent study found that ILC1 cells expressing CD161 targeted LSCs and affected theprogression of AML [122]. Although no validation has yet been made, this suggests the necessity of a further study on the role of CD161 in hematologic tumors, especially in AML/MDS.
12. GPX1
12.1. Introduction of GPX1 and its mechanism of action
GPX1 is the first reported selenoprotein and antioxidant enzyme. It is widely and abundantly expressed and crucial to maintain organismal homeostasis and cellular physiological functions. Previous research reported that the expression of GPX1 is abnormally elevated in most cancers, such as prostate cancer, lung cancer, bladder cancer, and breast cancer, etc [123].
12.2. Clinical significance of GPX1 in AML
Zhang et al. applied bioinformatics methods to analyze the transcript expression profiles in human AML samples and then performed validation with GEO sample data and basic experiments. They found that GPX1 played a significant role in the phase of immunosuppression in AML, and meanwhile, it had positive associations with the depletion of myeloid-derived suppressor cells (MDSCs), monocytes and T cells and the expression of MDSC markers but negative associations with the levels of CD4+ and CD8+ T cells. Moreover, this study noted that AML patients highly expressing GPX1 had poor outcomes and increased levels of NOTCH, WNT and TLR signal transduction that can promote drug resistance [124]. Recent studies have shown that GPX1 is a downstream target of miR-185–5p, and overexpression of miR-185–5p can suppress the proliferation of AML cells and decrease the expression of GPX1 both in vivo and in vitro, impeding the progression of AML [125]. Thus, the miR-185–5p/GPX1 axis may play a key role in AML treatment.
12.3. Current progress on GPX1
Current studies of GPX1 are still in basic experiments, and no related clinical trials have yet been performed. In spite of the many studies of GPX1 in AML, the role of GPX1 in MDS has not yet been investigated. We believe that GPX1 may be a new target that deserves analysis in MDS treatment.
13. VISTA
13.1. Introduction of VISTA
VISTA is a recognized anti-tumor regulator of immunity, and it is also one of the targets that have been extensively studied in tumor immunotherapy. Additionally, VISTA is an immunoglobulin highly expressed on naive T cells, hematopoietic stem cells (HSCs), myeloid progenitors, MDSCs and APCs [126,127]. Relevant research revealed that VISTA has important clinical implications for hematologic diseases.
13.2. Clinical significance of VISTA in AML
A recent study proved that VISTA is highly expressed in AML and associated with the poor prognosis of AML patients. Zheng et al. found increased expression of VISTA on peripheral MDSCs of AML patients, and that specific VISTA knock-down restored the immune activity of CD8+ T cells suppressed by MDSCs. Moreover, the expression of VISTA on MDSCs was found to be positively associated with the expression of PD-1 on T cells [127]. Another study reported that high expression of VISTA was positively associated with the expression of STAT3 in AML patients, and suppression of STAT3 down-regulated the expression of VISTA and then activated T cell immune activity [128]. In addition, it was also found that Gal-9 was a ligand of VISTA, and interaction between VISTA and Gal-9 facilitated the Gal-9-mediated T-cell apoptosis and then suppressed immunity [129]. These findings indicate that targeting VISTA alone or suppressing both VISTA and PD-1 or STAT3 may play a significant role in the effective control of leukemia. VISTA may serve as a regulator of early immune response to suppress the immune activation in cancer, providing a basis for diagnosing patients at a high risk of recurrence.
13.3. Current progress on VISTA
Zhang et al. developed an effective inhibitor for STAT3, named W1046. W1046 can downregulate the expression of VISTA by inhibiting STAT3 signal transduction, thereby activating T cells and strengthening the function of VISTA-targeting mAb, eventually significantly inhibiting the proliferation and activity of malignant AML hematopoietic cells [130]. JNJ-61610588 is a VISTA-targeted mAb produced by Johnson & Johnson and used for assessing the therapeutic efficacy and safety in patients with advanced solid tumor [131]. Other inhibitors for VISTA include CA-170 and HMBD-002. The former can target both PD-1 and VISTA, while the latter has been proved to be effective in treatment for colorectal cancer, breast cancer and lung cancer. By now, the role of VISTA in AML has been explored in several studies. However, no relevant clinical trials have yet been carried out, and its role in MDS model is still a blank. We hold the view that VISTA is a promising target in MDS immunotherapy.
14. CTLA-4
14.1. Introduction of CTLA-4 and its mechanism of action
CTLA-4 is also known as CD152. It is highly homologous to CD28, and both of them are receptors predominantly distributed on T lymphocytes with reverse mediating effects on T cells. CD28 mediates T-cell co-stimulatory signaling by interacting with CD80 and CD86 receptors, while CTLA-4 inhibits T-cell immune responses via binding the two receptors [[132], [133], [134]]. There are two possible speculations: 1) CTLA-4 competes for common ligands with CD28 and then mediates the endocytosis of CD80 and CD86 [135]; 2) CTLA-4 enhances the function of Treg by inducing their growth, thereby suppressing immune responses [136].
14.2. Clinical significance of CTLA-4 in MDS/AML
Studies demonstrated that proportions of CTLA-4+ CD3+, CTLA-4+ CD4+ and CTLA-4+ CD8+ T cells increased in patients with CLL [137] and AML [138]. Similar changing trend of CTLA-4+ cells could also be obtained in MDS patients. In a study involving 57 MDS patients, the serum level of CTLA-4 was increased and positively associated with the risk stratification in these patients [139].
14.3. Current research progress on CTLA-4
There have been multiple AML-related antigens approved or in the testing stage as potential therapeutic targets, including the anti-CTLA-4 antibody Ipilimumab. In a phase I trial that applied Ipilimumab to treat the recurrent hematological malignancies following hematopoietic stem cell transplantation, CTLA-4 expression was suppressed and the risk of recurrence was effectively reduced [140]. Another multi-center phase Ib study showed that Ipilimumab could enhance T-cell activation and immune responses in patients with high-risk MDS, with a safety profile [141]. Collectively, CTLA-4-targeted agents are expected to be applied in clinical treatment for MDS.
15. Summary and future prospect
In conclusion, primary MDS cells alter immune microenvironment under multiple mechanisms. For instance, the up-regulation of some immune checkpoints help malignant hematopoietic cells escape from immunosurveillance, and myeloid malignancies exhibit varying degrees of immune dysregulation at different clinical stages. HMA remains the preferred therapeutic agent for managing high-risk MDS at present, but its efficacy is limited to only less than 20% of patients and the effect is not sustained [142]. Therefore, it is urgent to find alternatives. The latest guideline recommends that patients with relapsed/refractory AML or MDS and not suitable for allogenic transplantation can be included into clinical trials of developing drugs. Similar to some solid tumors, MDS/AML can be well managed by treatment strategies that can enhance the anti-tumor immunity, such as immunosuppression of tumor microenvironment, and therapies targeting relevant vaccines and cytokines. Statistically, approximately 30–40% of MDS cases will progress to AML, which makes early prevention of tumor progression a focus that deserves attention in hematologists.
In future immune-targeted therapy for MDS, how immune regulator proteins and small-molecule targets act during the initiation and progression of MDS should be clarified at first. In addition, the conditions in clinical application of ICIs and the effect of their combinations should be more distinct. Before drug application, large-scale clinical trials are warranted to comprehensively analyze the safety, effectiveness, universal applicability and the optimal timing of the use of ICIs. Moreover, we are encouraged to look for more immune targets and then perform a large number of basic experiments to validate their clinical implications. It is necessary for the development of treatment for AML/MDS. In the context of the current pandemic, whether SARS-CoV has an impact on the efficacy of immunosuppressive drugs also deserves more attention in the future.
Immune phenotypes with clinical significance are tightly associated with the malignancy degree, treatment and prognosis of MDS/AML, and immunosuppressive agents targeting these phenotypes are potentially effective and have received attention in current research. Notably, due to the risk of MDS progression to AML, immune proteins that have implications for AML may also have important clinical significance for MDS, and AML-targeting molecular drugs can be also available for treatment of MDS. As the pathogenesis and clinical features of MDS are increasingly well understood, it is possible to formulate preventive strategies for the healthy people at a high risk of having MDS in the near future.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Contributor Information
Xinyu Guo, Email: xinyuguo6@outlook.com.
Shunjie Yu, Email: shunjieyu@outlook.com.
Xiaotong Ren, Email: renxiaotong0580@163.com.
Lijuan Li, Email: lilijuan20@qq.com.
References
- 1.Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Yang Y., Gao S., Chen J., Yu J., Zhang H., Li M., Zhan X., Li W. Crit rev oncol hematol. Immune dysregulation in myelodysplastic syndrome. Clinical features, pathogenesis and therapeutic strategies. 2018 Feb;122:123–132. doi: 10.1016/j.critrevonc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Z., Sun H., Yu J., Tian W., Song Y., Oncol J Hematol. Targeting CD47 for cancer immunotherapy. 2021 Oct 30;14(1):180. doi: 10.1186/s13045-021-01197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mushegian A. Proteins. Refining structural and functional predictions for secretasome components by comparative sequence analysis. 2002 Apr 1;47(1):69–74. PMID: 11870866. [PubMed] [Google Scholar]
- 5.McCracken M.N., Cha A.C., Weissman I.L. Clin cancer res. Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 "Don't Eat Me" Signals. 2015 Aug 15;21(16):3597–3601. doi: 10.1158/1078-0432.CCR-14-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., Lovelace P., Scheeren F.A., Chao M.P., Weiskopf K., Tang C., Volkmer A.K., Naik T.J., Storm T.A., Mosley A.R., Edris B., Schmid S.M., Sun C.K., Chua M.S., Murillo O., Rajendran P., Cha A.C., Chin R.K., Kim D., Adorno M., Raveh T., Tseng D., Jaiswal S., Enger P.Ø., Steinberg G.K., Li G., So S.K., Majeti R., Harsh G.R., van de Rijn M., Teng N.N., Sunwoo J.B., Alizadeh A.A., Clarke M.F., Weissman I.L. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U. S. A. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong X., Han Y., Liu Y., Yang L., Niu H., Yan L., Liu C., Shao Z., Xing L., Wang H. Phagocytosis checkpoints on hematopoietic stem cells in patients with myelodysplastic syndromes. Asia pac J clin oncol. 2022 Apr;18(2):e119–e128. doi: 10.1111/ajco.13566. [DOI] [PubMed] [Google Scholar]
- 8.Pang W.W., Pluvinage J.V., Price E.A., Sridhar K., Arber D.A., Greenberg P.L., Schrier S.L., Park C.Y., Weissman I.L. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3011–3016. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swoboda D.M., Sallman D.A. Best pract res clin haematol. The promise of macrophage directed checkpoint inhibitors in myeloid malignancies. 2020;33(4) doi: 10.1016/j.beha.2020.101221. [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Sallman D.A. The cluster of differentiation 47/signal-regulatory protein alpha axis in myeloid malignancies. Curr Opin Hematol. Targeting. 2022;29(1):44–52. doi: 10.1097/MOH.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 11.Zeidan A.M., DeAngelo D.J., Palmer J., Seet C.S., Tallman M.S., Wei X., Raymon H., Sriraman P., Kopytek S., Bewersdorf J.P., Burgess M.R., Hege K., Stock W. Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in patients with relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndromes. Ann hematol. 2022 Mar;101(3):557–569. doi: 10.1007/s00277-021-04734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laszlo G.S., Estey E.H., Walter R.B. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood rev. 2014;28(4):143–153. doi: 10.1016/j.blre.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013 Oct;13(10):739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X., Eksioglu E.A., Zhou J., Zhang L., Djeu J., Fortenbery N., Epling-Burnette P., Van Bijnen S., Dolstra H., Cannon J., Youn J.I., Donatelli S.S., Qin D., De Witte T., Tao J., Wang H., Cheng P., Gabrilovich D.I., List A., Wei S. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013;123(11):4595–4611. doi: 10.1172/JCI67580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crocker P.R., McMillan S.J., Richards H.E. CD33-related siglecs as potential modulators of inflammatory responses. Ann N Y acad sci. 2012;1253:102–111. doi: 10.1111/j.1749-6632.2011.06449.x. [DOI] [PubMed] [Google Scholar]
- 16.Tateno H., Li H., Schur M.J., Bovin N., Crocker P.R., Wakarchuk W.W., Paulson J.C. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol cell biol. 2007;27(16):5699–5710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilani I., Estey E., Huh Y., Joe Y., Manshouri T., Yared M., Giles F., Kantarjian H., Cortes J., Thomas D., Keating M., Freireich E., Albitar M. Differences in CD33 intensity between various myeloid neoplasms. Am. J. Clin. Pathol. 2002;118(4):560–566. doi: 10.1309/1WMW-CMXX-4WN4-T55U. [DOI] [PubMed] [Google Scholar]
- 18.Ogata K., Sei K., Saft L., Kawahara N., Porta M.G.D., Chapuis N., Yamamoto Y. Revising flow cytometric mini-panel for diagnosing low-grade myelodysplastic syndromes: Introducing a parameter quantifying CD33 expression on CD34+ cells. Leuk Res. 2018;71:75–81. doi: 10.1016/j.leukres.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Abdool A., Yeh C.H., Kantarjian H., O'Brien S., Bruey J., Giles F., Albitar M. Circulating CD33 and its clinical value in acute leukemia. Exp hematol. 2010;38(6):462–471. doi: 10.1016/j.exphem.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Stein E.M., Walter R.B., Erba H.P., Fathi A.T., Advani A.S., Lancet J.E., Ravandi F., Kovacsovics T., DeAngelo D.J., Bixby D., Faderl S., Jillella A.P., Ho P.A., O'Meara M.M., Zhao B., Biddle-Snead C., Stein A.S. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood. 2018;131(4):387–396. doi: 10.1182/blood-2017-06-789800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat rev immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P., Chen X., Dalton R., Calescibetta A., So T., Gilvary D., Ward G., Smith V., Eckard S., Fox J.A., Guenot J., Markowitz J., Cleveland J.L., Wright K.L., List A.F., Wei S., Eksioglu E.A. Immunodepletion of MDSC by AMV564, a novel bivalent, bispecific CD33/CD3 T cell engager, ex vivo in MDS and melanoma. Mol Ther. 2022;30(6):2315–2326. doi: 10.1016/j.ymthe.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleason M.K., Ross J.A., Warlick E.D., Lund T.C., Verneris M.R., Wiernik A., Spellman S., Haagenson M.D., Lenvik A.J., Litzow M.R., Epling-Burnette P.K., Blazar B.R., Weiner L.M., Weisdorf D.J., Vallera D.A., Miller J.S. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasiorek J.J., Blank V. Regulation and function of the NFE2 transcription factor in hematopoietic and non-hematopoietic cells. Cell mol life sci. 2015;72(12):2323–2335. doi: 10.1007/s00018-015-1866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed S.M., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim biophys acta mol basis dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Zhang M., Zhang L., Cai H., Zhou S., Zhang J., Wang Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. Y. J Surg Res. 2010;164(1):e99. doi: 10.1016/j.jss.2010.05.058. Nov. 2899. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat commun. 2016;7 doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pareek T.K., Belkadi A., Kesavapany S., Zaremba A., Loh S.L., Bai L., Cohen M.L., Meyer C., Liby K.T., Miller R.H., Sporn M.B., Letterio J.J. Triterpenoid modulation of IL-17 and Nrf-2 expression ameliorates neuroinflammation and promotes remyelination in autoimmune encephalomyelitis. Sci rep. 2011;1:201. doi: 10.1038/srep00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P., Ma D., Wang P., Pan C., Fang Q., Wang J. Nrf2 overexpression increases risk of high tumor mutation burden in acute myeloid leukemia by inhibiting Inside MS. Cell Death Dis. 2021;12(1):20. doi: 10.1038/s41419-020-03331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng H., Nong Z., Lu G. Correlation between nuclear factor E2-related factor 2 expression and gastric cancer progression. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2015 Sep 27;21:2893–2899. doi: 10.12659/MSM.894467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., Chen Y., Shi Y., Wu L., Tan Y., Li T., Chen Y., Xia J., Hu R. FAM117B promotes gastric cancer growth and drug resistance by targeting the KEAP1/NRF2 signaling pathway. J. Clin. Invest. 2023;133(3) doi: 10.1172/JCI158705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goerttler P.S., Kreutz C., Donauer J., Faller D., Maiwald T., März E., Rumberger B., Sparna T., Schmitt-Gräff A., Wilpert J., Timmer J., Walz G., Pahl H.L. Gene expression profiling in polycythaemia vera: overexpression of transcription factor NF-E2. Br J haematol. 2005;129(1):138–150. doi: 10.1111/j.1365-2141.2005.05416.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Schwemmers S., Hexner E.O., Pahl H.L. AML1 is overexpressed in patients with myeloproliferative neoplasms and mediates JAK2V617F-independent overexpression of NF-E2. Blood. 2010;116(2):254–266. doi: 10.1182/blood-2009-11-254664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu R.P., Hayashi T., Cottam H.B., Jin G., Yao S., Wu C.C., Rosenbach M.D., Corr M., Schwab R.B., Carson D.A. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2010;107(16):7479–7484. doi: 10.1073/pnas.1002890107. Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin P., Ren Y., Yan X., Luo Y., Zhang H., Kesarwani M., Bu J., Zhan D., Zhou Y., Tang Y., Zhu S., Xu W., Zhou X., Mei C., Ma L., Ye L., Hu C., Azam M., Ding W., Jin J., Huang G., Tong H. The high NRF2 expression confers chemotherapy resistance partly through up-regulated DUSP1 in myelodysplastic syndromes. Haematologica. 2019;104(3):485–496. doi: 10.3324/haematol.2018.197749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y., Southam A.D., Trova S., Beke F., Alhazmi B., Francis T., Radotra A., di Maio A., Drayson M.T., Bunce C.M., Khanim F.L. Valproic acid disables the Nrf2 anti-oxidant response in acute myeloid leukaemia cells enhancing reactive oxygen species-mediated killing. Br J Cancer. 2022;126(2):275–286. doi: 10.1038/s41416-021-01570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez J.M.H., Lumbreras E., Díez-Campelo M., González T., López D.A., Abáigar M., Del Rey M., Martín A.Á., de Paz R., Erquiaga S., Arrizabalaga B., Hernández-Rivas J.M., Vicente A.E.R. Genome-wide transcriptomics leads to the identification of deregulated genes after deferasirox therapy in low-risk MDS patients. Pharmacogenomics J. 2020;20(5):664–671. doi: 10.1038/s41397-020-0154-5. [DOI] [PubMed] [Google Scholar]
- 39.Barclay A.N., Wright G.J., Brooke G., Brown M.H. CD200 and membrane protein interactions in the control of myeloid cells. Trends immunol. 2002;23(6):285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 40.Manich G., Recasens M., Valente T., Almolda B., González B., Castellano B. Role of the CD200-CD200R Axis During Homeostasis and Neuroinflammation. Neuroscience. 2019;405:118–136. doi: 10.1016/j.neuroscience.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Kotwica-Mojzych K., Jodłowska-Jędrych B., Mojzych M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int J Mol Sci. 2021;22(4):1602. doi: 10.3390/ijms22041602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Arena Giovanni, et al. CD200 and chronic lymphocytic leukemia. Biological and Clinical Relevance. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.584427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Arena G., De Feo V., Pietrantuono G., Seneca E., Mansueto G., Villani O., La Rocca F., D'Auria F., Statuto T., Valvano L., Arruga F., Deaglio S., Efremov D.G., Sgambato A., Laurenti L. CD200 and chronic lymphocytic leukemia: Biological and Clinical Relevance. Front oncol. 2020;10 doi: 10.3389/fonc.2020.584427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorczynski L., Chen Z., Hu J., Kai Y., Lei J., Ramakrishna V., Gorczynski R.M. Evidence that an OX-2-positive cell can inhibit the stimulation of type 1 cytokine production by bone marrow-derived B7-1 (and B7-2)-positive dendritic cells. J Immunol. 1999;162(2):774–781. PMID: 9916698. [PubMed] [Google Scholar]
- 45.Moreaux J., Hose D., Reme T., Jourdan E., Hundemer M., Legouffe E., Moine P., Bourin P., Moos M., Corre J., Möhler T., De Vos J., Rossi J.F., Goldschmidt H., Klein B. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108(13):4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 46.Tonks A., Hills R., White P., Rosie B., Mills K.I., Burnett A.K., Darley R.L. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21(3):566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 47.Moreaux J., Veyrune J.L., Reme T., De Vos J., Klein B. CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun. 2008;366(1):117–122. doi: 10.1016/j.bbrc.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 48.Li D., Liu S., Xu J., Chen L., Xu C., Chen F., Xu Z., Zhang Y., Xia S., Shao Y., Wang Y. Ferroptosis-related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. J. Cell Mol. Med. 2021 Apr;25(7):3610–3621. doi: 10.1111/jcmm.16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho J.M., Dobson S.M., Voisin V., McLeod J., Kennedy J.A., Mitchell A., Jin L., Eppert K., Bader G., Minden M.D., Dick J.E., Wang J.C.Y. CD200 expression marks leukemia stem cells in human AML. Blood adv. 2020;4(21):5402–5413. doi: 10.1182/bloodadvances.2020001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbrich S., Baran N., Cai T., Weng C., Aitken M.J.L., Post S.M., Henderson J., Shi C., Richard-Carpentier G., Sauvageau G., Baggerly K., Al-Atrash G., Davis R.E., Daver N., Zha D., Konopleva M. Overexpression of CD200 is a Stem Cell-Specific Mechanism of Immune Evasion in AML. J Immunother Cancer. 2021;9(7) doi: 10.1136/jitc-2021-002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aref S., Abousamra N., El-Helaly E., Mabed M. Clinical Significance of CD200 and CD56 expression in patients with acute myeloid leukemia. Asian Pac J Cancer Prev. 2020;21(3):743–748. doi: 10.31557/APJCP.2020.21.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J.X., Mei L.P., Chen B.G., Wang D.L., Luo W.D., Luo L.F., Lu R., Zheng R., Zhang L. Over-expression of CD200 predicts poor prognosis in MDS. Leuk res. 2017;56:1–6. doi: 10.1016/j.leukres.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Mahadevan D., Lanasa M.C., Farber C., Pandey M., Whelden M., Faas S.J., Ulery T., Kukreja A., Li L., Bedrosian C.L., Zhang X., Heffner L.T. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J Immunother Cancer. 2019;7(1):227. doi: 10.1186/s40425-019-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rastogi N., Baker S., Man S., Uger R.A., Wong M., Coles S.J., Hodges M., Gilkes A.F., Knapper S., Darley R.L., Tonks A. Use of an anti-CD200-blocking antibody improves immune responses to AML in vitro and in vivo. Br J Haematol. 2021;193(1):155–159. doi: 10.1111/bjh.17125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diamanti P., Cox C.V., Ede B.C., Uger R.A., Moppett J.P., Blair A. Targeting pediatric leukemia-propagating cells with anti-CD200 antibody therapy. Blood adv. 2021;5(18):3694–3708. doi: 10.1182/bloodadvances.2020003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castriconi R., Dondero A., Corrias M.V., Lanino E., Pende D., Moretta L., Bottino C., Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64(24):9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 57.Li Y.C., Zhou Q., Song Q.K., Wang R.B., Lyu S., Guan X., Zhao Y.J., Wu J.P. Overexpression of an immune checkpoint (CD155) in breast cancer associated with prognostic significance and exhausted tumor-infiltrating lymphocytes: A Cohort Study. J immunol res. 2020;2020 doi: 10.1155/2020/3948928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bevelacqua V., Bevelacqua Y., Candido S., Skarmoutsou E., Amoroso A., Guarneri C., Strazzanti A., Gangemi P., Mazzarino M.C., D'Amico F., McCubrey J.A., Libra M., Malaponte G. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget. 2012;3(8):882–892. doi: 10.18632/oncotarget.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lupo K.B., Matosevic S. CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J Hematol Oncol. 2020;13(1):76. doi: 10.1186/s13045-020-00913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atsumi S., Matsumine A., Toyoda H., Niimi R., Iino T., Sudo A. Prognostic significance of CD155 mRNA expression in soft tissue sarcomas. Oncol lett. 2013;5(6):1771–1776. doi: 10.3892/ol.2013.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F., Hou H., Wu S., Tang Q., Liu W., Huang M., Yin B., Huang J., Mao L., Lu Y., Sun Z. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J immunol. 2015;45(10):2886–2897. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 62.Castriconi R., Dondero A., Corrias M.V., Lanino E., Pende D., Moretta L., Bottino C., Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64(24):9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 63.Fuchs A., Cella M., Giurisato E., Shaw A.S., Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172(7):3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 64.Meng F., Li L., Lu F., Yue J., Liu Z., Zhang W., Fu R. Overexpression of TIGIT in NK and T Cells Contributes to Tumor Immune Escape in Myelodysplastic Syndromes. Front oncol. 2020;10:1595. doi: 10.3389/fonc.2020.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarhan D., Cichocki F., Zhang B., Yingst A., Spellman S.R., Cooley S., Verneris M.R., Blazar B.R., Miller J.S. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer res. 2016;76(19):5696–5706. doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chashchina A., Märklin M., Hinterleitner C., Salih H.R., Heitmann J.S., Klimovich B. DNAM-1/CD226 is functionally expressed on acute myeloid leukemia (AML) cells and is associated with favorable prognosis. Sci rep. 2021;11(1) doi: 10.1038/s41598-021-97400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanchez-Correa B., Valhondo I., Hassouneh F., Lopez-Sejas N., Pera A., Bergua J.M., Arcos M.J., Bañas H., Casas-Avilés I., Durán E., Alonso C., Solana R., Tarazona R. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers (basel) 2019;11(6):877. doi: 10.3390/cancers11060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaito Y., Hirano M., Futami M., Nojima M., Tamura H., Tojo A., Imai Y. CD155 and CD112 as possible therapeutic targets of FLT3 inhibitors for acute myeloid leukemia. Oncol lett. 2022;23(2):51. doi: 10.3892/ol.2021.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Zhang J., Wang Z., Zhang X., Dai Z., Wu W., Zhang N., Liu Z., Zhang J., Luo P., Wen Z., Yu J., Zhang H., Yang T., Cheng Q. Identify the prognostic and immune profile of VSIR in the tumor microenvironment: a pan-cancer analysis. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.821649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao K., Zhou E., Schaafsma E., Zhang B., Cheng C. Immune checkpoint gene VSIR predicts patient prognosis in acute myeloid leukemia and myelodysplastic syndromes. Cancer med. 2023;12(5):5590–5602. doi: 10.1002/cam4.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riether C., Schürch C.M., Bührer E.D., Hinterbrandner M., Huguenin A.L., Hoepner S., Zlobec I., Pabst T., Radpour R., Ochsenbein A.F. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med. 2017;214(2):359–380. doi: 10.1084/jem.20152008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nolte M.A., van Olffen R.W., van Gisbergen K.P., van Lier R.A. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol rev. 2009;229(1):216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 73.Jacobs J., Deschoolmeester V., Zwaenepoel K., Rolfo C., Silence K., Rottey S., Lardon F., Smits E., Pauwels P. CD70: An emerging target in cancer immunotherapy. Pharmacol ther. 2015;155:1–10. doi: 10.1016/j.pharmthera.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Lens S.M., Tesselaar K., van Oers M.H., van Lier R.A. Control of lymphocyte function through CD27-CD70 interactions. Semin. Immunol. 1998;10(6):491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 75.Kuka M., Munitic I., Giardino Torchia M.L., Ashwell J.D. CD70 is downregulated by interaction with CD27. J Immunol. 2013;191(5):2282–2289. doi: 10.4049/jimmunol.1300868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hintzen R.Q., Lens S.M., Beckmann M.P., Goodwin R.G., Lynch D., van Lier R.A. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J immunol. 1994;152(4):1762–1773. PMID: 8120385. [PubMed] [Google Scholar]
- 77.Libregts S., van Olffen R.W., van der Sluijs K.F., van Lier R.A., Nolte M.A. Function of CD27 in helper T cell differentiation. Immunol Lett. 2011;136(2):177–186. doi: 10.1016/j.imlet.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 78.Bassig B.A., Shu X.O., Koh W.P., Gao Y.T., Purdue M.P., Butler L.M., Adams-Haduch J., Xiang Y.B., Kemp T.J., Wang R., Pinto L.A., Zheng T., Ji B.T., Hosgood H.D., Hu W., Yang G., Zhang H., Chow W.H., Kim C., Seow W.J., Zheng W., Yuan J.M., Lan Q., Rothman N. Soluble levels of CD27 and CD30 are associated with risk of non-Hodgkin lymphoma in three Chinese prospective cohorts. Int J Cancer. 2015;137(11):2688–2695. doi: 10.1002/ijc.29637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jilaveanu L.B., Sznol J., Aziz S.A., Duchen D., Kluger H.M. Hum Pathol. CD70 expression patterns in renal cell carcinoma. Camp RL. 2012;43(9):1394–1399. doi: 10.1016/j.humpath.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agathanggelou A., Niedobitek G., Chen R., Nicholls J., Yin W., Young L.S. Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am J Pathol. 1995;147(4):1152–1160. [PMC free article] [PubMed] [Google Scholar]
- 81.Petrau C., Cornic M., Bertrand P., Maingonnat C., Marchand V., Picquenot J.M., Jardin F., Clatot F. CD70: A Potential Target in Breast Cancer? J Cancer. 2014;5(9):761–764. doi: 10.7150/jca.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ring N.G., Herndler-Brandstetter D., Weiskopf K., Shan L., Volkmer J.P., George B.M., Lietzenmayer M., McKenna K.M., Naik T.J., McCarty A., Zheng Y., Ring A.M., Flavell R.A., Weissman I.L. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114(49) doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schürch C., Riether C., Matter M.S., Tzankov A., Ochsenbein A.F. CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J. Clin. Invest. 2012;122(2):624–638. doi: 10.1172/JCI45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kok M., Bonfrer J.M., Korse C.M., de Jong D., Kersten M.J. Serum soluble CD27, but not thymidine kinase, is an independent prognostic factor for outcome in indolent non-Hodgkin's lymphoma. Tumour biol. 2003;24(1):53–60. doi: 10.1159/000070661. [DOI] [PubMed] [Google Scholar]
- 85.Alkhairy O.K., Perez-Becker R., Driessen G.J., Abolhassani H., van Montfrans J., Borte S., Choo S., Wang N., Tesselaar K., Fang M., Bienemann K., Boztug K., Daneva A., Mechinaud F., Wiesel T., Becker C., Dückers G., Siepermann K., van Zelm M.C., Rezaei N., van der Burg M., Aghamohammadi A., Seidel M.G., Niehues T., Hammarström L. Novel mutations in TNFRSF7/CD27: clinical, immunologic, and genetic characterization of human CD27 deficiency. J. Allergy Clin. Immunol. 2015;136(3):703–712.e10. doi: 10.1016/j.jaci.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 86.Guikema J.E., Hovenga S., Vellenga E., Conradie J.J., Abdulahad W.H., Bekkema R., Smit J.W., Zhan F., Shaughnessy J., Jr., Bos N.A. CD27 is heterogeneously expressed in multiple myeloma: low CD27 expression in patients with high-risk disease. Br J Haematol. 2003;121(1):36–43. doi: 10.1046/j.1365-2141.2003.04260.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhao S., Zhang Y., Huang G., Luo W., Li Y., Xiao Y., Zhou M., Li Y., Lai J., Li Y., Li B. Increased CD8+CD27+perforin+ T cells and decreased CD8+CD70+ T cells may be immune biomarkers for aplastic anemia severity. Blood cells mol dis. 2019;77:34–42. doi: 10.1016/j.bcmd.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Burris H.A., Infante J.R., Ansell S.M., Nemunaitis J.J., Weiss G.R., Villalobos V.M., Sikic B.I., Taylor M.H., Northfelt D.W., Carson W.E., 3rd, Hawthorne T.R., Davis T.A., Yellin M.J., Keler T., Bullock T. Safety and activity of Varlilumab, a novel and first-in-class agonist anti-CD27 antibody, in patients with advanced solid tumors. J. Clin. Oncol. 2017;35(18):2028–2036. doi: 10.1200/JCO.2016.70.1508. [DOI] [PubMed] [Google Scholar]
- 89.Starzer A.M., Berghoff A.S. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7) ESMO Open. 2020;4(Suppl 3) doi: 10.1136/esmoopen-2019-000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacobs J., Deschoolmeester V., Rolfo C., Zwaenepoel K., Van den Bossche J., Deben C., Silence K., de Haard H., Hermans C., Rottey S., Vangestel C., Lardon F., Smits E., Pauwels P. Preclinical data on the combination of cisplatin and anti-CD70 therapy in non-small cell lung cancer as an excellent match in the era of combination therapy. Oncotarget. 2017;8(43):74058–74067. doi: 10.18632/oncotarget.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu N., Sheng X., Liu Y., Zhang X., Yu J. Increased CD70 expression is associated with clinical resistance to cisplatin-based chemotherapy and poor survival in advanced ovarian carcinomas. Onco Targets Ther. 2013;6:615–619. doi: 10.2147/OTT.S44445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G., Quan Y., Che F., Wang L. B7-H3 in tumors: friend or foe for tumor immunity? Cancer chemother pharmacol. 2018;81(2):245–253. doi: 10.1007/s00280-017-3508-1. [DOI] [PubMed] [Google Scholar]
- 93.Zhou W.T., Jin W.L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapoval A.I., Ni J., Lau J.S., Wilcox R.A., Flies D.B., Liu D., Dong H., Sica G.L., Zhu G., Tamada K., Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 95.Suh W.K., Gajewska B.U., Okada H., Gronski M.A., Bertram E.M., Dawicki W., Duncan G.S., Bukczynski J., Plyte S., Elia A., Wakeham A., Itie A., Chung S., Da Costa J., Arya S., Horan T., Campbell P., Gaida K., Ohashi P.S., Watts T.H., Yoshinaga S.K., Bray M.R., Jordana M., Mak T.W. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 96.Prasad D.V., Nguyen T., Li Z., Yang Y., Duong J., Wang Y., Dong C. Murine B7-H3 is a negative regulator of T cells. J immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 97.Castriconi R., Dondero A., Augugliaro R., Cantoni C., Carnemolla B., Sementa A.R., Negri F., Conte R., Corrias M.V., Moretta L., Moretta A., Bottino C. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101(34):12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinberger P., Majdic O., Derdak S.V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W.F., Stöckl J., Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172(4):2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 99.Aicher W.K., Korn M., Reitnauer L., Maurer F.B., Hennenlotter J., Black P.C., Todenhofer T., Bedke J., Stenzl A. Expression patterns of the immune checkpoint ligand CD276 in urothelial carcinoma. BMC Urol. 2021;21(1):60. doi: 10.1186/s12894-021-00829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang J., Chen F., Liu D., Gu F., Chen Z., Wang Y. Prognostic value of immune checkpoint molecules in breast cancer. Biosci rep. 2020;40(7) doi: 10.1042/BSR20201054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang R., Ma Y., Zhan S., Zhang G., Cao L., Zhang X., Shi T., Chen W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 2020;11(1):55. doi: 10.1038/s41419-020-2252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang L.Y., Jin Y., Xia P.H., Lin J., Ma J.C., Li T., Liu Z.Q., Xiang H.L., Cheng C., Xu Z.J., Zhou H., Qian J. Integrated analysis reveals distinct molecular, clinical, and immunological features of B7-H3 in acute myeloid leukemia. Cancer Med. 2021;10(21):7831–7846. doi: 10.1002/cam4.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W., Zhang L., Qian J., Lin J., Chen Q., Yuan Q., Zhou J., Zhang T., Shi J., Zhou H. Expression characteristic of 4Ig B7-H3 and 2Ig B7-H3 in acute myeloid leukemia. Bioengineered. 2021;12(2):11987–12002. doi: 10.1080/21655979.2021.2001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loo D., Alderson R.F., Chen F.Z., Huang L., Zhang W., Gorlatov S., Burke S., Ciccarone V., Li H., Yang Y., Son T., Chen Y., Easton A.N., Li J.C., Rillema J.R., Licea M., Fieger C., Liang T.W., Mather J.P., Koenig S., Stewart S.J., Johnson S., Bonvini E., Moore P.A. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin cancer res. 2012;18(14):3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 105.Lichtman E.I., Du H., Shou P., Song F., Suzuki K., Ahn S., Li G., Ferrone S., Su L., Savoldo B., Dotti G. Preclinical Evaluation of B7-H3-specific Chimeric Antigen Receptor T Cells for the Treatment of Acute Myeloid Leukemia. Clin cancer res. 2021;27(11):3141–3153. doi: 10.1158/1078-0432.CCR-20-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antonioli L., Pacher P., Vizi E.S., Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu J., Wang X., Lu Q., Wang J., Li L., Liao X., Zhu W., Lv L., Zhi X., Yu J., Jin Y., Zou Q., Ou Z., Liu X., Zhou P. Extracellular 5'-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3β/β-catenin/cyclinD1 signaling pathway. Int J Cancer. 2018;142(5):959–967. doi: 10.1002/ijc.31112. [DOI] [PubMed] [Google Scholar]
- 108.Bynoe M.S., Waickman A.T., Mahamed D.A., Mueller C., Mills J.H., Czopik A. CD73 is critical for the resolution of murine colonic inflammation. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/260983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen S., Wainwright D.A., Wu J.D., Wan Y., Matei D.E., Zhang Y., Zhang B. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy. 2019;11(11):983–997. doi: 10.2217/imt-2018-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Q., Pu N., Yin H., Zhang J., Zhao G., Lou W., Wu W. CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer. J. Cell Mol. Med. 2020;24(15):8674–8686. doi: 10.1111/jcmm.15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jakobsen J.S., Laursen L.G., Schuster M.B., Pundhir S., Schoof E., Ge Y., d'Altri T., Vitting-Seerup K., Rapin N., Gentil C., Jendholm J., Theilgaard-Mönch K., Reckzeh K., Bullinger L., Döhner K., Hokland P., Fitzgibbon J., Porse B.T. Mutant CEBPA directly drives the expression of the targetable tumor-promoting factor CD73 in AML. Sci adv. 2019;5(7) doi: 10.1126/sciadv.aaw4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kong Y., Jia B., Zhao C., Claxton D.F., Sharma A., Annageldiyev C., Fotos J.S., Zeng H., Paulson R.F., Prabhu K.S., Zheng H. Downregulation of CD73 associates with T cell exhaustion in AML patients. J hematol oncol. 2019;12(1):40. doi: 10.1186/s13045-019-0728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brauneck F., Haag F., Woost R., Wildner N., Tolosa E., Rissiek A., Vohwinkel G., Wellbrock J., Bokemeyer C., Schulze Zur Wiesch J., Ackermann C., Fiedler W. Increased frequency of TIGIT+CD73-CD8+ T cells with a TOX+ TCF-1low profile in patients with newly diagnosed and relapsed AML. Oncoimmunology. 2021;10(1) doi: 10.1080/2162402X.2021.1930391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perrot I., Michaud H.A., Giraudon-Paoli M., Augier S., Docquier A., Gros L., Courtois R., Déjou C., Jecko D., Becquart O., Rispaud-Blanc H., Gauthier L., Rossi B., Chanteux S., Gourdin N., Amigues B., Roussel A., Bensussan A., Eliaou J.F., Bastid J., Romagné F., Morel Y., Narni-Mancinelli E., Vivier E., Paturel C., Bonnefoy N. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell rep. 2019;27(8):2411–2425.e9. doi: 10.1016/j.celrep.2019.04.091. [DOI] [PubMed] [Google Scholar]
- 115.Konduri V., Oyewole-Said D., Vazquez-Perez J., Weldon S.A., Halpert M.M., Levitt J.M., Decker W.K. CD8+CD161+ T-Cells: Cytotoxic Memory Cells With High Therapeutic Potential. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.613204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou X., Du J., Liu C., Zeng H., Chen Y., Liu L., Wu D. A pan-cancer analysis of CD161, a Potential New Immune Checkpoint. Front immunol. 2021;12 doi: 10.3389/fimmu.2021.688215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alsuliman A., Muftuoglu M., Khoder A., Ahn Y.O., Basar R., Verneris M.R., Muranski P., Barrett A.J., Liu E., Li L., Stringaris K., Armstrong-James D., Shaim H., Kondo K., Imahashi N., Andersson B., Marin D., Champlin R.E., Shpall E.J., Rezvani K. A subset of virus-specific CD161+ T cells selectively express the multidrug transporter MDR1 and are resistant to chemotherapy in AML. Blood. 2017;129(6):740–758. doi: 10.1182/blood-2016-05-713347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoneda K., Morii T., Nieda M., Tsukaguchi N., Amano I., Tanaka H., Yagi H., Narita N., Kimura H. The peripheral blood Valpha24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk res. 2005;29(2):147–152. doi: 10.1016/j.leukres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 119.Gournay V., Vallet N., Peux V., Vera K., Bordenave J., Lambert M., Corneau A., Michonneau D., Peffault de Latour R., Caillat-Zucman S., Socié G., Chevalier M.F. Immune landscape after allo-HSCT: TIGIT- and CD161-expressing CD4 T cells are associated with subsequent leukemia relapse. Blood. 2022;140(11):1305–1321. doi: 10.1182/blood.2022015522. [DOI] [PubMed] [Google Scholar]
- 120.Mathewson N.D., Ashenberg O., Tirosh I., Gritsch S., Perez E.M., Marx S., Jerby-Arnon L., Chanoch-Myers R., Hara T., Richman A.R., Ito Y., Pyrdol J., Friedrich M., Schumann K., Poitras M.J., Gokhale P.C., Gonzalez Castro L.N., Shore M.E., Hebert C.M., Shaw B., Cahill H.L., Drummond M., Zhang W., Olawoyin O., Wakimoto H., Rozenblatt-Rosen O., Brastianos P.K., Liu X.S., Jones P.S., Cahill D.P., Frosch M.P., Louis D.N., Freeman G.J., Ligon K.L., Marson A., Chiocca E.A., Reardon D.A., Regev A., Suvà M.L., Wucherpfennig K.W. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell. 2021;184(5):1281–1298.e26. doi: 10.1016/j.cell.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun Y., Wu L., Zhong Y., Zhou K., Hou Y., Wang Z., Zhang Z., Xie J., Wang C., Chen D., Huang Y., Wei X., Shi Y., Zhao Z., Li Y., Guo Z., Yu Q., Xu L., Volpe G., Qiu S., Zhou J., Ward C., Sun H., Yin Y., Xu X., Wang X., Esteban M.A., Yang H., Wang J., Dean M., Zhang Y., Liu S., Yang X., Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184(2):404–421.e16. doi: 10.1016/j.cell.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 122.Caligiuri M., Li Z., Ma R., Tang H., Zhang J., Marcucci G., Yu J. Human ILC1s Target Leukemia Stem Cells and Control Development of AML. Res Sq [Preprint] 2023 doi: 10.21203/rs.3.rs-2319959/v1. rs.3.rs-2319959. [DOI] [Google Scholar]
- 123.Zhao Y., Wang H., Zhou J., Shao Q. Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer. Cancers (basel) 2022;14(10):2560. doi: 10.3390/cancers14102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J., Peng Y., He Y., Xiao Y., Wang Q., Zhao Y., Zhang T., Wu C., Xie Y., Zhou J., Yu W., Lu D., Bai H., Chen T., Guo P., Zhang Q. GPX1-associated prognostic signature predicts poor survival in patients with acute myeloid leukemia and involves in immunosuppression. Biochim biophys acta mol basis dis. 2022;1868(1) doi: 10.1016/j.bbadis.2021.166268. [DOI] [PubMed] [Google Scholar]
- 125.Pang B., Mao H., Wang J., Yang W. MiR-185-5p suppresses acute myeloid leukemia by inhibiting GPX1. Microvasc res. 2022;140 doi: 10.1016/j.mvr.2021.104296. [DOI] [PubMed] [Google Scholar]
- 126.Xu W., Dong J., Zheng Y., Zhou J., Yuan Y., Ta H.M., Miller H.E., Olson M., Rajasekaran K., Ernstoff M.S., Wang D., Malarkannan S., Wang L. Immune-Checkpoint Protein VISTA Regulates Antitumor Immunity by Controlling Myeloid Cell-Mediated Inflammation and Immunosuppression. Cancer immunol res. 2019;7(9):1497–1510. doi: 10.1158/2326-6066.CIR-18-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang L., Jia B., Claxton D.F., Ehmann W.C., Rybka W.B., Mineishi S., Naik S., Khawaja M.R., Sivik J., Han J., Hohl R.J., Zheng H. Oncoimmunology. VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. 2018;7(9) doi: 10.1080/2162402X.2018.1469594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mo J., Deng L., Peng K., Ouyang S., Ding W., Lou L., Lin Z., Zhu J., Li J., Zhang Q., Wang P., Wen Y., Chen X., Yue P., Lu J.J., Zhu K., Zheng Y., Wang Y., Zhang X. STAT3-VISTA axis to suppress tumor aggression and burden in acute myeloid leukemia. J Hematol Oncol. Targeting. 2023;16(1):15. doi: 10.1186/s13045-023-01410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yasinska I.M., Meyer N.H., Schlichtner S., Hussain R., Siligardi G., Casely-Hayford M., Fiedler W., Wellbrock J., Desmet C., Calzolai L., Varani L., Berger S.M., Raap U., Gibbs B.F., Fasler-Kan E., Sumbayev V.V. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front immunol. 2020;11 doi: 10.3389/fimmu.2020.580557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mo J., Deng L., Peng K., Ouyang S., Ding W., Lou L., Lin Z., Zhu J., Li J., Zhang Q., Wang P., Wen Y., Chen X., Yue P., Lu J.J., Zhu K., Zheng Y., Wang Y., Zhang X. STAT3-VISTA axis to suppress tumor aggression and burden in acute myeloid leukemia. J Hematol Oncol. Targeting. 2023;16(1):15. doi: 10.1186/s13045-023-01410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dempke W.C.M., Fenchel K., Uciechowski P., Dale S.P. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur J cancer. 2017;74:55–72. doi: 10.1016/j.ejca.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 132.Schwartz J.C., Zhang X., Fedorov A.A., Nathenson S.G., Almo S.C. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410(6828):604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 133.Stamper C.C., Zhang Y., Tobin J.F., Erbe D.V., Ikemizu S., Davis S.J., Stahl M.L., Seehra J., Somers W.S., Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410(6828):608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 134.Collins A.V., Brodie D.W., Gilbert R.J., Iaboni A., Manso-Sancho R., Walse B., Stuart D.I., van der Merwe P.A., Davis S.J. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17(2):201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 135.Sehgal A., Whiteside T.L., Boyiadzis M. Programmed death-1 checkpoint blockade in acute myeloid leukemia. Expert Opin Biol Ther. 2015;15(8):1191–1203. doi: 10.1517/14712598.2015.1051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 137.Palma M., Gentilcore G., Heimersson K., Mozaffari F., Näsman-Glaser B., Young E., Rosenquist R., Hansson L., Österborg A., Mellstedt H. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica. 2017;102(3):562–572. doi: 10.3324/haematol.2016.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen Y., Tan J., Huang S., Huang X., Huang J., Chen J., Yu Z., Lu Y., Weng J., Du X., Li Y., Zha X., Chen S. Higher frequency of the CTLA-4+ LAG-3+ T-cell subset in patients with newly diagnosed acute myeloid leukemia. Asia Pac J Clin Oncol. 2020;16(2):e12–e18. doi: 10.1111/ajco.13236. [DOI] [PubMed] [Google Scholar]
- 139.Aref S., El Agdar M., El Sebaie A., Abouzeid T., Sabry M., Ibrahim L. Prognostic Value of CD200 Expression and Soluble CTLA-4 Concentrations in Intermediate and High-Risk Myelodysplastic Syndrome Patients. Asian pac J cancer prev. 2020;21(8):2225–2230. doi: 10.31557/APJCP.2020.21.8.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davids M.S., Kim H.T., Bachireddy P., Costello C., Liguori R., Savell A., Lukez A.P., Avigan D., Chen Y.B., McSweeney P., LeBoeuf N.R., Rooney M.S., Bowden M., Zhou C.W., Granter S.R., Hornick J.L., Rodig S.J., Hirakawa M., Severgnini M., Hodi F.S., Wu C.J., Ho V.T., Cutler C., Koreth J., Alyea E.P., Antin J.H., Armand P., Streicher H., Ball E.D., Ritz J., Bashey A., Soiffer R.J., Leukemia and lymphoma society blood cancer research partnership Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med. 2016;375(2):143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 142.Bewersdorf J.P., Zeidan A.M. Management of higher risk myelodysplastic syndromes after hypomethylating agents failure: are we about to exit the black hole? Expert Rev Hematol. 2020;13(10):1131–1142. doi: 10.1080/17474086.2020.1819233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.