Abstract
Background
Co-stimulatory molecules have been shown to enhance antitumor immune responses, but their role in Diffuse Large B-cell Lymphoma (DLBCL) remains unexplored.
Methods
This study aimed to explore the molecular typing of DLBCL with co-stimulatory molecule genes and to construct a prognostic profile to improve treatment decisions and clinical outcomes.
Results
We conducted the first comprehensive analysis of co-stimulatory molecules in DLBCL patients and identified five co-stimulatory molecule genes with prognostic and diagnostic values. Consensus cluster analysis based on these five co-stimulatory molecule genes revealed that the two identified clusters had different distribution patterns and prognostic differences. Co-stimulatory molecular correlation signatures were then constructed based on these five co-stimulatory molecular genes and validated in an external dataset, showing good performance in predicting patient prognosis. The signature is an independent risk factor for DLBCL patients and significantly correlates with clinical factors in patients and can be used as a complement to clinical factors. Furthermore, the signature was associated with the tumor immune microenvironment. Patients identified as being at high risk according to our signature exhibit high levels of immune cell infiltration microenvironment.
Conclusions
In conclusion, our signature can provide clinicians with prognostic predictions and help guide the treatment of patients with DLBCL.
Keywords: Diffuse large B-Cell lymphoma, Co-stimulatory molecule, Prognosis, Immune microenvironment, Infiltration
1. Introduction
Diffuse large B-cell lymphoma is one of the most common types of adult lymphoma and is a subset of malignancies that is highly heterogeneous in terms of clinical presentation and prognosis [1,2]. DLBCL is the most common malignancy of the lymphatic system in adults, accounting for almost 35–40% of lymphomas in Western countries, and generally higher than 40% in Asian countries [3]. Most patients have no obvious clinical symptoms, but about 1/3 of patients have symptoms such as fever, night sweats, and weight loss, and the appearance of symptoms is mostly related to the site of tumor involvement. In recent years, chemotherapy and immunotherapy have further improved the prognosis of DLBCL [4,5]. However, only a small percentage of DLBCL patients will respond well to this therapy [6]. Finding novel indicators to forecast patient survival and responsiveness to immunotherapies and targeted medicines is therefore urgently needed.
DLBCL is commonly classified as activated B-cell-like (ABC), germinal centre B-cell-like (GCB) and unclassified, based on the different responses of the cells of origin to chemotherapy and targeted drugs. Since the introduction of the anti-CD20 monoclonal antibody (rituximab), the R–CHOP (Rituximab, Cyclophosphamide, Hydroxydaunomycin, Oncovin and Prednisone) regimen of rituximab combined with chemotherapy has significantly improved the five-year survival rate in DLBCL [7,8]. However, there are still some patients who fail R–CHOP and enter the relapsed/refractory phase of the disease. The efficacy of rituximab in DLBCL depends on the level of CD20 expression in tumor cells, which is heterogeneous in DLBCL, leading to resistance during treatment [9,10]. Tumor-infiltrating immune cells are thought to be relevant in this regard. Tumour cells, which normally colonize normal tissues, can form the tumor microenvironment (TME) together with components such as immune cells and their secretory factors, stromal cells, vascular endothelial cells and extracellular stroma [11]. During the early stages of tumor progression, the main cellular components that maintain the immunosuppressive microenvironment play an anti-tumor role. By targeting TME, the inherent tumor suppressive capacity of the immune system can be stimulated or restored, and a positive immune microenvironment can be reshaped [12,13]. However, the heterogeneity of TME allows for a wide variation in tumor progression between individuals. The degree of T cell infiltration in TME is closely related to the efficacy of immunotherapy. Therefore, a deeper understanding of the tumor immune microenvironment would help us to improve the prognosis of patients with DLBCL [14]. Previous studies have demonstrated the therapeutic potential of costimulatory molecules in various cancers [[15], [16], [17]]. Co-stimulatory molecules play a crucial role in the regulation of tumor immunity by influencing the activation and proliferation of T cells [18]. However, the molecular function of these costimulatory molecules in DLBCL is unknown.
In this study, the expression pattern and prognostic value of co-stimulatory molecules in DLBCL were systematically analyzed. Then, a prognostic profile of DLBCL patients was constructed. This profile was an independent prognostic factor for patient prognosis, presenting different inflammatory profiles. More importantly, we further assessed the immune microenvironment of different DLBCL patients and classified them according to the co-stimulatory molecule-based features.
2. Materials and methods
2.1. Data collection and preprocessing
Two independent diffuse large B-cell lymphomas gene expression profiles, GSE181063 and GSE10846, containing 1712 DLBC samples from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), were collected (Table S1). Of these, GSE181063 was used as the training set, while GSE10846 was used as the validation set for subsequent analysis.
2.2. Identification of costimulatory molecules with prognostic significance in DLBCL
On the GEO dataset, all genes encoding costimulatory molecules were first mapped. The surv_cutpoint function in the R package “survminer” was used to categorize the samples into high- and low-expression groups, and the best cutoff value for each gene in all samples was determined using this method. To choose costimulatory molecular genes linked to survival, univariate Cox regression was used. Using the “survminer” package, Kaplan-Meier curves and log-rank tests are used to determine the prognosis. The most useful genes for predicting survival in DLBC patients were chosen using the LASSO (Least Absolute Shrinkage and Selection Operator) analysis with the “glmnet” software to reduce overfitting. The ideal value for the penalty parameter was chosen using ten-fold cross-validation.
2.3. Consensus clustering of survival-related costimulatory molecule genes
To explore the functional and prognostic value of co-stimulatory molecules in diffuse large B-cell lymphoma, we performed consensus clustering using the “ConsensusClusterPlus” package of R. The present study used the following parameters: 1000 repeats, k = 10, and agglomerative hierarchical clustering with ward criterion (Ward.D2) inner and complete outer linkage. Genes used for consensus clustering analyses are the five selected costimulatory molecule genes. cluster numbers were further confirmed by PCA analysis using the “ggplot2″ package. Kaplan-Meier curves were then drawn to determine the predictive value of cluster classification.
2.4. Construction and validation of a costimulatory molecule-related prognostic signature
Multivariate Cox proportional hazards regression was used to obtain the coefficients of these survival-related costimulatory molecule genes. The costimulatory molecule-related prognostic signature were based on a multivariable Cox regression coefficient weighted with the expression of these genes. The detailed formula is as follows: Risk score = β1 * Exp1 + β2 * Exp2 + β, β and Exp represent multivariable Cox hazard proportional regression coefficients and expression levels of selected genes, respectively. Patients were divided into high-risk and low-risk subgroups based on median risk scores. Kaplan-Meier analysis and logarithmic grade assays were performed for prognostic assessment using the “survminer” package.
2.5. Functional and pathway enrichment analysis
To explore the biological pathways associated with the signature, we obtained genes that were strongly associated with the risk score (correlation coefficient |R| > 0.3). These genes were analyzed for GO and KEGG pathway enrichment using the clusterProfiler package. GSEA was performed to reveal the potential functional mechanisms using the c2.cp.v7.2.symbols.gtm file. False discovery rate (FDR) < 0.2 and normalized P value < 0.05 were set as the threshold values.
2.6. Estimation of the immune microenvironment composition and therapeutic drugs
The infiltration of 22 immune cell species in DLBC tissue from dataset GSE181063 were analyzed in R using the CIBERSORT package. The relative abundance of infiltrating immune cells was obtained based on P < 0.05. Differential infiltration of immune cells between high- and low-risk DLBCL tissues was then explored using the Wilcoxon rank sum test. Finally, Spearman relationships between these five genes and infiltrating immune cells were analyzed and the corresponding network plots were drawn. In addition, tumor purity was compared using the R package estimation package. The results were visualized by the ggplot2 and pheatmap packages of the R software. We used the oncoPredict package to predict DLBCL drug activity and to assess differences in drug activity across risk score groups.
2.7. Statistical analyses
To compare the two variables, we used t-tests or Wilcoxon tests. To evaluate survival differences, Kaplan-Meier curves and log-rank tests were employed. Co-stimulatory molecular genes were evaluated for their prognostic value using univariate and multivariate Cox regression models. To assess variations in the distribution of clinical variables in DLBC patients, Pearson's chi-square test was utilized. The R program was used for all of the methods involved in this investigation. The threshold for statistical significance was P < 0.05.
3. Results
3.1. Identification of costimulatory molecule genes with prognostic value in DLBCL
The workflow of this study is shown in Fig. 1. Expression data for 60 costimulatory molecule genes in DLBCL, including 13 B7-CD28 family genes and 47 TNF family genes, were extracted from the GEO database. A total of 35 costimulatory molecule genes were significantly associated with prognosis in DLBC at P < 0.05 (Table S2). These genes were further screened using LASSO analysis and subsequently validated using the GSE10846 dataset, resulting in five costimulatory molecule genes associated with prognosis (Fig. S1, Fig. S2). the Kaplan-Meier curves further confirmed the prognostic value of each gene (Fig. 2A–B). High expression of these genes (TNFRSF13B and CD70) was associated with poor prognosis in DLBCL, while low expression of these genes (LTBR, CD27, and TMIGD2) was associated with poor prognosis.
Fig. 1.
The flowchart of the present study design.
The mRNA expression data of the tumor were downloaded from the GEO database (GSE181063 and GSE10846). Survival-related costimulatory molecules were obtained and the associations with tumor immune microenvironment were evaluated, and the mutation frequency and clinical relevance of genes were also analyzed.
Fig. 2.
Survival analysis for five costimulatory molecule genes.
(A) The Kaplan-Meier curves for the five costimulatory molecule genes in DLBCL from the GSE181063 dataset, including LTBR, CD27, TNFRSF13B, CD70, and TMIGD2; (B) The Kaplan-Meier curves for the five costimulatory molecule genes in DLBCL from GSE10846 dataset, including LTBR, CD27, TNFRSF13B, CD70, and TMIGD2. Red, representing a high level of expression of the gene; blue, representing a low level of expression of the gene.
3.2. Cluster classification was associated with the malignancy of DLBC
To explore the overall prognostic value of these genes, we performed a consensus clustering analysis to stratify DLBC patients. From the results, we found that k = 2 appeared to be a relatively stable value from k = 2 to 6 (Fig. 3A and B). Principal component analysis (PCA) was then performed to verify the reliability of the cluster numbering (Fig. S3). We found that when k = 3, k = 4 and k = 5, these samples had high similarity and clustered together. Therefore, we divided the DLBC patients into 2 clusters. The Kaplan-Meier curves showed that patients in cluster 1 had a worse prognosis than those in cluster 2 (Fig. 3C). In addition, these 5 genes showed significant differential expression in cluster 1 and cluster 2 (Fig. 3D).
Fig. 3.
Consensus clustering based on the 5 costimulatory molecule genes.
(A) Consensus clustering cumulative distribution function (CDF) for k = 2 to k = 6; (B) The relative change in area under the CDF curve for k = 2 to k = 6; (C) Kaplan–Meier curve for two clusters of diffuse large B-cell lymphoma; (D) Distribution of the 5 costimulatory molecule genes in the two clusters.
3.3. Construction and validation of the prognostic signature based on five costimulatory molecule genes
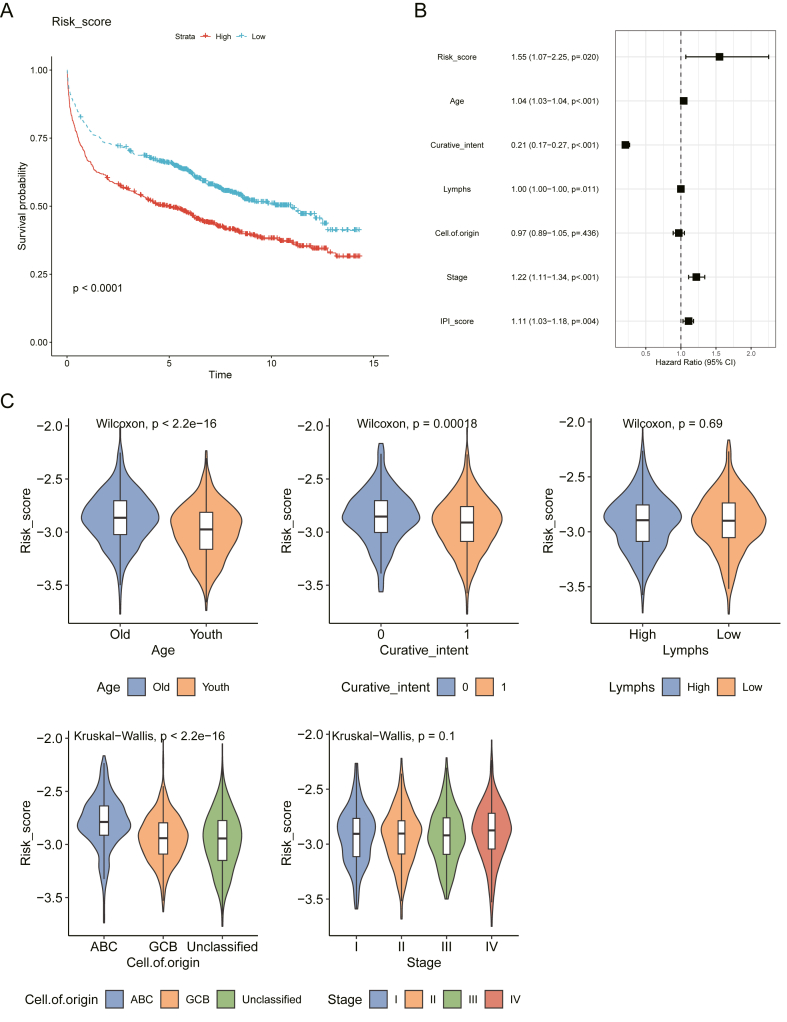
Risk scores for the prognostic characteristics of DLBCL patients were calculated using the expression profiles of five costimulatory molecule genes multiplied by a multivariate Cox proportional hazards coefficient. The detailed formula is given below: Risk score = (−0.52733*LTBR) + (−0.82364*CD27) + (0.68638*TNFRSF13B) + (0.73451*CD70) + (−1.02974*TMIGD2). Patients were divided into high-risk and low-risk groups using the median risk score. The results showed that high-risk patients had a poorer prognosis compared to low-risk patients (Fig. 4A). In addition, the prognostic characteristics were further validated in the GSE10846 dataset. The same formula was used to calculate the risk score and the median risk score was used to classify patients into high and low risk. the Kaplan-Meier curve showed that high-risk patients had a poor prognosis compared to low-risk patients (Fig. S4). These results demonstrate the reliability and stability of the prognostic characteristics.
Fig. 4.
Relationship between the prognostic signature and clinical pathological factors of DLBCL patients.
(A) The multivariate Cox regression analyses of prognosis for the prognostic signature and clinic pathological factors; (B) Kaplan–Meier curve for the risk score of DLBCL; (C) The violin plot showed that the risk score of the prognostic signature in different clinical subgroups. The difference between the two groups was tested using Wilcoxon's test; Differences between three or more groups using Kruskal Wallis test. Curative intent is the goal of treating a disease or condition with the aim of curing it or achieving complete remission. In the case of cancer treatment, the aim is to eliminate all cancer cells from the body and achieve long-term survival. Yes, means that the aim of cancer treatment has been achieved. No, means that the aim of cancer treatment has not been achieved.
3.4. Associations between the prognostic signature and clinicopathological factors of DLBCL
Univariate Cox regression showed that age, Curative intent, Lymphs, Cell of origin, Stage, and risk score were risk factors affecting patient prognosis (Table S2). It is worth noting that although lymphs in multivariate cox regression have a significant effect on patient survival, their high or low level does not always indicate an enhanced or reduced risk of patient survival. Multivariate Cox regression showed that risk score was an independent risk factor for patient prognosis (Fig. 4B and Table S3). Dividing patients into subgroups based on clinical variables, we found that DLBCL patients with age, Curative intent, and Cell of origin had different risk scores (Fig. 4C). These results suggest that our prognostic characteristics are closely related to the clinical factors of DLBCL. We also evaluated the association between cell of origin, Lymphgen class (using LymphGen Classifier) and Risk score and showed that Lymphgen class of mainly NEC and EZB were mainly associated with low risk score. Patients with ABC had a higher risk of survival compared to those with GCB and Unclassified (Figs. S9–10).
3.5. Identification of the prognostic signature-related biological pathways
To explore potential biological pathways for prognostic traits, we selected genes that were strongly associated with risk scores for prognostic traits. A total of 350 positively associated genes and 281 negatively associated genes were selected. The results of the functional enrichment analysis of the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways are shown in (Fig. 5A–C). GO analysis was mainly enriched in DNA replication and regulation of DNA replication, while KEGG analysis was mainly enriched in Neutrophil extracellular trap formation. The results of the GSVA analysis showed significant differences in the reactome nuclear receptor transcription pathway between high and low-risk samples (Fig. 5D).
Fig. 5.
The costimulatory molecule-based signature-related biological pathways.
(A) Enrichment analysis for related genes of costimulatory molecule-based signature; (B) KEGG pathway analysis for related genes of costimulatory molecule-based signature; (C) Network diagram of biological processes for related genes of costimulatory molecule-based signature; (D) Heatmap of GSVA analysis for related genes of costimulatory molecule-based signature. The top 50 pathways that were enriched are shown.
3.6. The associations with the tumor immune microenvironment and drug activity
Box plots show significant differences in immune cell infiltration between high- and low-risk patients, and the differences in the proportions of various immune cells are complex (Fig. 6A). In addition, high-risk patients had higher tumor purity and lower immune scores, while low-risk patients had the opposite (Fig. 6B). Five prognostic genes also remain strongly associated with immune cells, which may have an impact on patient prognosis (Fig. 6C and D). There is a strong correlation between the presence of high frequency mutations in CD70 relative to other co-stimulatory molecules and patient prognosis (Fig. S5). The results of the drug activity analysis showed that BI-2536_1086, BMS-754807_2171, Dactinomycin_1911, Daporinad_1248, Sabutoclax_1849, Sepantronium bromide_1941, Telomerase Inhibitor IX_1930 and Topotecan_1808 showed different drug activities in the high and low risk groups, which can be used as a reference for subsequent drug screening (Fig. S8).
Fig. 6.
Immune cell infiltration analysis, and relationships between 5 co-stimulatory molecular genes, immune cells, and immune score indicators.
(A) Box-plot of the proportion of 22 types of immune cells. (B) Scatter plot between tumor purity, stroma, and immune score, and the difference in immune score and tumor purity at different risk scores. (C) Heatmap of correlations between 5 co-stimulatory molecular genes, immune cells, and immune score indicators. The shape of the points represents the strength of the correlation; the circle represents a positive correlation, and the square represents a negative correlation. Darker color implies a stronger association. (D) Network diagram of interactions between 5 co-stimulatory molecular genes, immune cells, and immune score indicators. The pink circles represent immune cells, the green circles represent 5 co-stimulatory molecular genes and the purple circles represent immune score indicators. The solid lines represent positive correlations, while the dashed lines represent negative correlations. The thicker the line, the stronger the correlation between them; conversely, the weaker the correlation. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns P ≥ 0.05.
4. Discussion
DLBCL has shown durable efficacy in immunotherapy and rituximab has been used as an important component in the treatment of DLBCL [19,20]. However, some DLBCL patients do not respond to immunotherapy. Therefore, identifying biomarkers that predict immunotherapy response and selecting the most sensitive patients is critical to improving response rates [21,22]. Co-stimulatory molecules play an important role in the progression of various cancers, and their role in DLBCL remains to be explored [23]. In this study, we systematically evaluated the role costimulatory molecules in DLBCL and selected five genes with prognostic value for further study. In addition, we constructed and validated novel prognostic features in DLBCL patients. To our knowledge, this study provides the first prognostic marker of a co-stimulatory molecule in DLBCL patients. We found that prognostic features were independent risk factors for DLBCL patients and significantly correlated with patient clinical factors. In addition, we found that our prognostic features are associated with the tumor immune microenvironment, which may provide valuable clues for predicting patient outcomes and selecting immunotherapy patients.
Co-stimulatory molecules play an important role in tumor immunomodulation [24]. Activation of primary T cells relies on signals from costimulatory molecules to ensure that immune responses occur under the required conditions. the more important costimulatory molecules are B7-1 and B7-2. Monoclonal antibodies targeting the PD-1/PD-L1 (B7–H1) or B7-2/CTLA-4 pathways have shown promise in inducing durable tumor regression in a variety of tumors [25,26]. To investigate the expression level and prognostic value of co-stimulatory molecules in DLBCL, we obtained 13 members of the B7-CD28 family and 47 members of the TNF family for use in DLBCL patients [27]. Five co-stimulatory molecule genes with predictive value (LTBR, CD27, TMIGD2, TNFRSF13B, and CD70) were selected. LTBR, CD27, TNFRSF13B, and CD70 are all members of the tumor necrosis factor receptor superfamily and play important roles in B cell activation, natural killer cells cytotoxicity, and immunoglobulin synthesis [28,29]. They are involved in immune-related diseases such as lymphoid tissue proliferation syndrome, combined immunodeficiency, lymphoma and immunoglobulin A deficiency. However, Transmembrane and immunoglobulin domains containing 2 are involved in positive regulation of T cell activation, positive regulation of angiogenesis, and positive regulation of cytokine production, which can enhance T cell proliferation and cytokine production through AKT-dependent signaling cascades, making them potential immunosuppressive target [30].
With the development of immunotherapies, there is an urgent need to identify biomarkers and select drug-sensitive patients to improve response to immunotherapies. In this study, five co-stimulatory molecular genes were selected for co-clustering analysis to explore their overall prognostic value. the Kaplan-Meier curves showed a poor prognosis for these patients in cluster 1. In addition, multiple immune-related pathways were enriched in Category 1, suggesting that these selected genes are highly correlated with the tumor immune microenvironment. The poor prognosis in cohort 1 patients may be due to immune deficiency or limited immune defenses. In addition, risk characterization based on co-stimulatory molecular genes may provide new insights into clinical practice in DLBCL patients. All these prognostic markers are reliable and show good performance. We are believed to be the first to construct a co-stimulatory molecular gene-based risk profile for DLBCL patients. Our prognostic features were validated in an additional GEO dataset and both showed good performance. We further found that prognosis is closely related to clinical factors and can be used as a complement to guide treatment.
Inevitably, the study has several limitations. The study was primarily from a public database and was retrospective. The number of available prognostic information datasets for DLBCL patients is limited, so the clinical parameters analyzed in this study are not comprehensive. Patients with DLBCL need to have real prognostic information to determine the value of prognostic characteristics. Secondly, the genes involved in this study are limited to co-stimulatory molecules, and tumor microenvironment is highly spatially heterogeneous. Therefore, the efficacy of prognostic features was limited. Furthermore, there is no data on expression of co-stimulatory molecule genes in immunotherapy-naïve DLBCL patients. Therefore, assessing the risk profile of immunotherapy responses is indirect. Future prospective studies of DLBCL patients treated with immunotherapy need to confirm the value of our signature for clinical application.
5. Conclusions
In summary, we have performed the first comprehensive analysis of co-stimulatory molecules in DLBCL patients and identified five genes with prognostic and diagnostic values. We have constructed and validated a new prognostic profile of DLBCL patients based on co-stimulatory molecules and explored its underlying molecular mechanisms. Our prognostic signature allows the classification of patients into two subgroups with different prognoses and shows a high correlation with clinical features. Furthermore, patients identified as high risk according to our prognostic signature exhibit lowlevels of immune cell infiltration microenvironment. Therefore, we believe that our signature can provide clinicians with prognostic predictions and treatment guidelines for patients with DLBCL.
Author contribution statement
1 - Conceived and designed the experiments;
2 - Performed the experiments;
3 - Analyzed and interpreted the data;
4 - Contributed reagents, materials, analysis tools or data;
5 - Wrote the paper.
Data availability statement
Gene expression datasets are publicly available (GEO, https://www.ncbi.nlm.nih.gov/geo).
Funding
This study was funded by the Ningbo Natural Science Foundation (2013J017), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commision (2022KY1113), the Traditional Chinese Medicine Administration of Zhejiang Province (2022ZB324), the Natural Science Foundation Project of Ningbo (20221JCGYO10069), Ningbo Science and Technology Plan Project (2022S032) and Zhejiang Provincial Medical Science and Technology Plan Project (2021KY273).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19342.
Contributor Information
Ping Yi, Email: yiping0325@163.com.
Qitian Mu, Email: muqitian@163.com.
Guifang Ouyang, Email: nbhematology@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Li S., Young K.H., Medeiros L.J. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz R., Wright G.W., Huang D.W., et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiappella A., Castellino A., Nicolosi M., Santambrogio E., Vitolo U. Diffuse Large B-cell Lymphoma in the elderly: standard treatment and new perspectives. Expert Rev. Hematol. 2017;10(4):289–297. doi: 10.1080/17474086.2017.1305264. [DOI] [PubMed] [Google Scholar]
- 4.Mondello P., Mian M. Frontline treatment of diffuse large B-cell lymphoma: beyond R-CHOP. Hematol. Oncol. 2019;37(4):333–344. doi: 10.1002/hon.2613. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Barta S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019;94(5):604–616. doi: 10.1002/ajh.25460. [DOI] [PubMed] [Google Scholar]
- 6.He M.Y., Kridel R. Treatment resistance in diffuse large B-cell lymphoma. Leukemia. 2021;35(8):2151–2165. doi: 10.1038/s41375-021-01285-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Li L.R. R-CHOP resistance in diffuse large B-cell lymphoma: biological and molecular mechanisms. Chin. Med. J. 2020;134(3):253–260. doi: 10.1097/CM9.0000000000001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y., Zhou H., Zhang H., et al. Efficacy and safety of the biosimilar IBI301 plus standard CHOP (I-CHOP) in comparison with rituximab plus CHOP (R-CHOP) in patients with previously untreated diffuse large B-cell lymphoma (DLBCL): a randomized, double-blind, parallel-group, phase 3 trial. Adv. Ther. 2021;38(4):1889–1903. doi: 10.1007/s12325-020-01603-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Barta S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019;94(5):604–616. doi: 10.1002/ajh.25460. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D., Mo Q., Sun X., et al. Pyruvate dehydrogenase kinase 4-mediated metabolic reprogramming is involved in rituximab resistance in diffuse large B-cell lymphoma by affecting the expression of MS4A1/CD20. Cancer Sci. 2021;112(9):3585–3597. doi: 10.1111/cas.15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. #N/A. 2015;15(11):669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami A., Khazaei M., Bagherieh F., et al. Targeting stroma in pancreatic cancer: promises and failures of targeted therapies. J. Cell. Physiol. 2017;232(11):2931–2937. doi: 10.1002/jcp.25798. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L., Chan T.A., Kroemer G., Wolchok J.D., López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018;10(459) doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 14.Autio M., Leivonen S.K., Brück O., et al. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica. 2021;106(3):718–729. doi: 10.3324/haematol.2019.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 16.Xu F., Jin T., Zhu Y., Dai C. Immune checkpoint therapy in liver cancer. #N/A. 2018;37(1):110. doi: 10.1186/s13046-018-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi M., Zheng X., Niu M., Zhu S., Ge H., Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer. 2022;21(1):28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlmeier J.E., Benedict S.H. Alternate costimulatory molecules in T cell activation: differential mechanisms for directing the immune response. Histol. Histopathol. 2003;18(4):1195–1204. doi: 10.14670/HH-18.1195. [DOI] [PubMed] [Google Scholar]
- 19.Ma S., Li X., Wang X., et al. Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 2019;15(12):2548–2560. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gouill S., Ghesquières H., Oberic L., et al. Obinutuzumab vs rituximab for advanced DLBCL: a PET-guided and randomized phase 3 study by LYSA. Blood. 2021;137(17):2307–2320. doi: 10.1182/blood.2020008750. [DOI] [PubMed] [Google Scholar]
- 21.Liang X.J., Song X.Y., Wu J.L., et al. Advances in multi-omics study of prognostic biomarkers of diffuse large B-cell lymphoma. Int. J. Biol. Sci. 2022;18(4):1313–1327. doi: 10.7150/ijbs.67892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamil M.O., Mehta A. Diffuse Large B-cell lymphoma: prognostic markers and their impact on therapy. Expert Rev. Hematol. 2016;9(5):471–477. doi: 10.1586/17474086.2016.1146584. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill R.E., Cao X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv. Cancer Res. 2019;143:145–194. doi: 10.1016/bs.acr.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge J.W., Grosenbach D.W., Schlom J. Vector-based delivery of tumor-associated antigens and T-cell co-stimulatory molecules in the induction of immune responses and anti-tumor immunity. Cancer Detect. Prev. 2002;26(4):275–291. doi: 10.1016/s0361-090x(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 25.Cha J.H., Chan L.C., Li C.W., Hsu J.L., Hung M.C. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76(3):359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng H., Ning Y., Zhan Y., Liu S., Wen Q., Fan S. New insights into the important roles of tumor cell-intrinsic PD-1. Int. J. Biol. Sci. 2021;17(10):2537–2547. doi: 10.7150/ijbs.60114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua X., Ge S., Zhang J., et al. A costimulatory molecule-related signature in regard to evaluation of prognosis and immune features for clear cell renal cell carcinoma. Cell Death Dis. 2021;7(1):252. doi: 10.1038/s41420-021-00646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller J., Baeyens A., Dustin M.L. Tumor necrosis factor receptor superfamily in T cell priming and effector function. Adv. Immunol. 2018;140:21–57. doi: 10.1016/bs.ai.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Nie M., Ren W., Ye X., et al. The dual role of CD70 in B-cell lymphomagenesis. Clin. Transl. Med. 2022;12(12):e1118. doi: 10.1002/ctm2.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying H., Xu J., Zhang X., Liang T., Bai X. Human endogenous retrovirus-H long terminal repeat-associating 2: the next immune checkpoint for antitumour therapy. EBioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.103987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene expression datasets are publicly available (GEO, https://www.ncbi.nlm.nih.gov/geo).