Abstract
Objective
Zishen Yutai Pill (ZYP) is a frequently used traditional Chinese medicine (TCM) preparation in women’s health. However, the effects of ZYP on endometrial epithelial response have not been fully explored. Herein, uterine natural killer cell (uNK) secretion medium was used to mimic the uterine microenvironment. Thereafter, an endometrial epithelial cell line (Ishikawa cells) was treated with ZYP-containing serum to elucidate the effects of ZYP on endometrial receptivity.
Methods: uNK cells were isolated from decidual tissues of pregnant women undergoing pregnancy termination surgery, and thereafter, uNK secretion medium was collected. ZYP-containing serum was collected from rats after intragastrical administration of ZYP. Ishikawa cells were divided into three groups, one treated with blank control (control group), one treated with uNK secretion medium (uNK group), and one treated with both uNK secretion medium and ZYP-containing serum (ZYP + uNK group). Total RNAs were extracted. Gene expression profiles of Ishikawa in different groups were determined through microarray analysis. mRNA expressions of selected genes were determined through quantitative real-time polymerase chain reaction (qRT-PCR). Expression of intercellular cell adhesion molecule-1 (ICAM-1) was determined using Western blotting (WB).
Results
Compared with the uNK group, the gene expressions of ZYP group with a total of 1117 genes were significantly altered, among which 510 genes were upregulated and 607 genes were downregulated. Compared with uNK group, expressions of CSF1, CSF2, SPP1, and ICAM1 were upregulated (P < 0.05). Up-regulation of ICAM-1 expression after treatment of ZYP was further confirmed by WB analysis.
Conclusion
In brief, in the presence of uNK cell medium, ZYP could improve the expressions of ICAM1, CSF1, CSF2, TNF, SPP1, etc. However, further exploration should be carried out in in vivo experiments for the validation of the mechanisms of ZYP on endometrial epithelial response.
Keywords: Zishen yutai pill, Uterine natural killer cells, Immunological microenvironment, Endometrial epithelial response
Graphical abstract
Highlight
-
•
The effects of Zishen Yutai Pill on endometrial epithelial response were explored in a simulated uterine microenvironment.
-
•
Gene expression profiles of Zishen Yutai Pill were determined through mRNA sequencing analysis.
-
•
Expressions of genes and protein relating to PI3K/Akt and TNF pathway were validated to confirm its role on endometrial epithelial response.
1. Introduction
Innate immune cells in the uterine microenvironment involve in many aspects of implantation and fetal development, accounting for the major population of leukocytes in the uterus [1]. Natural killer cells (NK) possess anti-tumor and anti-infection functions and can rapidly release pro-inflammatory cytokines and chemokines during early inflammatory reactions [2]. The periodical change of uNK cells is observed among women with normal menstrual cycle. On the 7th to 9th day after LH surge (a period of time coincides with the window of implantation), the proportion of uNK cells among immune cells increases to approximately 40%. In the early stages of pregnancy, NK cells account for approximately 40–70% of decidual lymphocytes and have unique functions in maintaining pregnancy [3]. NK cells in the decidua can secrete cytokines such as vascular endothelial growth factor (VEGF) and stromal cell-derived factor-1 (SDF-1) to promote local angiogenesis and immune regulation [4]. In mice, the absence of NK cells can lead to abnormal spiral artery formation in the decidua and decrease embryo survival rate [5]. Due to the secretion function of these cells [9], the uNK secretion medium is an important part of the uterine microenvironment, contributing to the endometrial receptivity [6]. In addition, a recent report had also indicated uNK, acting as biosensors of low-fitness human embryos, play pivotal role in deciding implantation outcomes via hyaluronan signaling [7,8]. This work provided great insight into the relationship between uNK and embryo implantation.
Traditional Chinese medicine (TCM), during the process of in vitro fertilization (IVF) embryo-transfer (ET), can improve implantation rate among women undergoing their cycles [9]. However, little is known about the mechanisms of TCM on promoting the implantation rate nowadays. Our recent study has also proved that the use of Zishen Yutai Pill (ZYP) could improve implantation rate in in vitro fertilization (IVF) embryo-transfer (ET) [10].
ZYP is a TCM preparation composed of 15 natural medicines (Supplementary Material Table S1). ZYP is well quality-controlled, as the production procedures of ZYP comply with the requirements of Chinese Good Manufacturing Practices (GMP) for Pharmaceutical Products. According to two previous quality control reports, Zishen Yutai Pill exhibited promising consistency across different batches with regards to its chemical profiles [11,12]. However, the underlying mechanism still awaits further validation. Thus, studies on ZYP on the effects of epithelial response were explored in this study.
Previous reports demonstrated that the human endometrial adenocarcinoma Ishikawa cell line is a promising in vitro model in the research of endometrial receptivity [13]. Thus, the effects of ZYP on Ishikawa cells were studied herein. uNK cell secretion medium was applied in the current research to mimic the microenvironment. Gene expression profiles of Ishikawa were determined through mRNA sequencing technique and further confirmed using qRT-PCR.
2. Materials and methods
2.1. Ethics statement
All procedures involving animals were conducted according to the Regulations for the Administration of Affairs Concerning Experimental Animals. Permission for animal studies was approved by the Ethics Committee at Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University (SYSU-IACUC-2020-B0421).
All human tissues were collected after written informed consent of patients were signed. Permission concerning use of human tissues was seek from Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University (Permission NO.18, 2020).
2.2. Animals and serum preparation
Fifteen adult female Sprague Dawley (SD) rats were purchased from Guangdong Provincial Medical Animal Center. Rats were divided into two groups, the normal group (5 rats), and ZYP treated group (10 rats). The clinical dose of ZYP (Baiyunshan Zhongyi Pharmaceutical Co. Ltd, China) is 15 g/day [14]. The equivalent dose for rats is about 1.575 g/kg of body weight per day, as confirmed by a previous report [15]. ZYP or saline solution was administered intragastrically once per day, for one week. Two hours after the last administration of ZYP, blood was collected from the abdominal aorta under sterile conditions. Blood samples were then subjected to centrifugation at 3500 rpm for 15 min, followed by a 56 °C water bath incubation. The samples were pooled and filtered using a microporous membrane (0.22 μm, Millipore, USA), and kept under −80 °C in refrigerator.
2.3. Isolation and identification of uNK cells
Decidual tissues were obtained from normal pregnancies terminated by artificial approaches among 20 patients (at 6–8 weeks of gestation). Those who have any record of hormonal medication within 3 months before the surgical procedure were excluded. All samples were processed under sterile conditions. uNK cells were prepared according to the methods reported in the literature, and the details are as follows [16]. The decidual tissues were minced and digested using 2 ml of 0.1% collagenase type IV (Thermo Fisher, USA) for 60 min at 37 °C under a water bath. The mixture was then filtered through a 100 mesh gauze filter to remove cell debris. Next, the resultant filter suspension was layered over Lymphoprep (STEMCELL Technologies, Canada) at a 1:1 (v:v) ratio. After centrifugation at 400g for 20 min, the lymphocyte layer was aspirated and washed with Ca2+ and Mg2+ free PBS twice.
Decidual CD65bright NK (uNK) cells were purified by incubating the suspension with anti-CD56 microbeads (Miltenyi Biotec, Germany) at 4 °C for 15 min, followed by isolating through MinMACS separator (Miltenyi Biotec, Germany).
2.4. Preparation of uNK cell secretion medium
uNK cells were resuspended in Roswell Park Memorial Institute (RPMI) 1640 Medium (Thermo Fisher, USA) containing 10% FBS and 10 ng/ml interleukin-15 (IL-15) and adjusted to 5 × 105 cells/ml. Cell suspension (1.3 ml) was placed in the upper chamber of 0.4 μm pore transwell insert (Corning, Germany) with 1.5 ml culture medium in the lower chamber. The transwell was incubated at 37 °C with 5% CO2 in a humidified incubator for 24 h, followed by harvesting the cell secretion in the lower well and stored at −80 °C. The proportion of dead cells was evaluated by a live/dead viability kit (Invitrogen). When the uNK cell death rate was below 35%, uNK cell secretion medium was considered successfully extracted and could be used in the following experiments [17].
2.5. Treatment of ishikawa cells with uNK cell secretion medium
Ishikawa cells were purchased from Forevergen Co. Ltd. (China). Cells were maintained on 6-well cell culture plate (Thermo Fisher, USA) under 37 °C with 5% CO2, in RPMI 1640 Medium (containing 100 U/ml Penicillin, 100 μg/ml streptomycin). When the density of Ishikawa cells reached 70–80%, the culture medium was aspirated and washed with PBS for three times. uNK cell secretion medium and rat serum were added.
Ishikawa cells were divided into three groups. One group was treated with 80% blank culture medium and 20% rat serum, being the control group. One group was treated with uNK secretion medium, i.e., 80% uNK secretion medium and 20% rat serum, being uNK group. The remaining one was treated with 80% uNK secretion medium and 20% ZYP-containing serum, being the uNK + ZYP group.
Each group was kept under 37 °C with 5% CO2, with the above-mentioned medium for 24 h. Thereafter, the medium in each group was aspirated. All groups were then washed with PBS twice and treated with 80% RPMI 1640 medium and 20% serum for 6 h. Then, supernatants cells were collected for further analysis. The procedure was performed according to previous published article with small modifications [16].
2.6. RNAseq transcriptomic analysis
The total RNA of Ishikawa cells was extracted using Trizol (Invitrogen, USA). The RNA was purified using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions, quantified by spectrophotometry. Sequencing was performed using the TrueLib mRNA Library Prep Kit for Illumina (ExCell Bio). Briefly, the total RNA was subjected to mRNA isolation and fragmentation, reverse transcription, cDNA synthesis, end repair and poly-A tailing, adapters ligation, size selection and amplification. The obtained DNA library was subjected to sequencing on Novaseq 6000 (Illumina, USA).
2.7. Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Thermo Scientific, USA) following the manufacturer’s protocol. cDNA was synthesized by using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). qRT-PCR was performed using a quantitative SYBR Green Select Master Mix (Applied Biosystems, USA). Triple wells were included for each sample. The expression of the targeted genes was normalized to β-actin and expressed with the method. Sequences of each primer were listed in Table 1.
Table 1.
Sequences of primers relating to endometrial receptivity in qRT-PCR analysis.
| Gene Name | Primer Name | Sequence (5′-3′) |
|---|---|---|
| ICAM1 | H-ICAM1-F | AGCGGCTGACGTGTGCAGTAAT |
| H-ICAM1-R | TCTGAGACCTCTGGCTTCGTCA | |
| CSF1 | H-CSF1-F | TGAGACACCTCTCCAGTTGCTG |
| H-CSF1-R | GCAATCAGGCTTGGTCACCACA | |
| CSF2 | H-CSF2-F | CCGGAAACTTCCTGTGCAAC |
| H-CSF2-R | GTCTCACTCCTGGACTGGCT | |
| TNF | H-TNF-F | CTCTTCTGCCTGCTGCACTTTG |
| H-TNF-R | ATGGGCTACAGGCTTGTCACTC | |
| SPP1 | H-SPP1-F | CGAGGTGATAGTGTGGTTTATGG |
| H-SPP1-R | GCACCATTCAACTCCTCGCTTTC | |
| LIF | H-LIF-F | AGATCAGGAGCCAACTGGCACA |
| H-LIF-R | GCCACATAGCTTGTCCAGGTTG |
2.8. Western blotting
Western blotting was used to detect the expression of ICAM-1 protein. The protein concentration was quantified by BCA protein assay kit (Sigma-Aldrich, USA). The proteins were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, Bio-Rad, USA) and transferred to polyvinylidene fluoride (PVDF) membrane (Bio-Rad, USA). The blot was blocked with 4% BSA at 4 °C overnight and then incubated with anti- ICAM-1 antibody (1:1000, Abcam, UK) or anti-GAPDH (1:5000, Abcam, UK) at room temperature for 2 h. The imaged using ECL Western Blotting Detection Reagents (Amersham, USA). The protein level of ICAM-1 was quantified by ImageJ.
2.9. The gene ontology (GO) functional annotation and kyoto encyclopedia of genes and genomes (KEGG) pathway analysis of different expression genes (DEG)
To elucidate the function of DEGs and their role in signaling transduction, the Database for Annotation, Visualization and Integrated Discovery (DAVID) was used to analyze the GO function and KEGG pathway. P value was calculated using a modified Fisher exact test to evaluate the significance of enrichment. Gene-pathway connection was visualized with Cytoscape 3.2.1.
2.10. Statistical analysis
All data analysis were presented as Means ± SEM. Differences were tested by one-way analysis of variance (ANOVA) test followed by Bonferroni’s multiple comparisons test using GraphPad Prism 8.0 software. P < 0.05 was regarded as significantly different.
3. Results
3.1. Characterization of uNK cells
After isolation of uNK cells, the purity of isolated cells was evaluated using PE conjugated anti-human CD56 antibody. The percentage of CD56 positive cells was 92.01% (Fig. 1A), indicating good reliability of cells. uNK cells contained less than 35% of dead cells after overnight incubation with IL-15 (Fig. 1B), indicating that the uNK cell secretion medium could be used for subsequent experiments.
Fig. 1.
Isolation and identification of primary uNK cells. (A) Representative result of human primary uNK cells subjected to PE-CD56 antibody assessed by flow cytometry. The rate of CD56 natural killer cells was above 92%. The value for the isotype control was also shown on the left panel. (B) Representative flow cytometry result showing the percentage of dead uNK cells after overnight incubation with IL-15. The percentage of live uNK cells was approximately 88%, and the dead uNK cells was approximately 12% (right panel). The flow cytometry result for the blank control was also shown on the left panel.
3.2. Effect of ZYP on mRNA profiles of ishikawa cells in uNK cell secretion medium
To examine the gene expression profile, RNA sequencing technique was used to compare the transcript expression levels in Ishikawa cells in three groups. Profiles between Ishikawa treated with uNK or uNK + ZYP were presented in Fig. 2.
Fig. 2.
Effect of ZYP on mRNA expression profile in Ishikawa cells stimulated by uNK. (A) MA plot representing DEGs in control group and ZYP group. Each plot represents a sample set. The statistical significance of all transcripts (P < 0.05 and fold change ≥1.5) was analyzed. In the plot, green represents downregulated transcripts and red represents the upregulated transcripts. (B) Heat map showing DEGs in control group and ZYP group. The heat map color spectrum corresponds to percentile rank, indicating downregulated transcripts (blue) and upregulated transcripts (red). (C-E) Go enrichment, including biological process (C), molecular function (D), and cellar component (E) were presented. The x-axis presented GO terms, and the y axis presented -log10 P value of each term. P values (all <0.05) were assessed using Fisher’s exact test.
As shown in the MA plot (Fig. 2A), a total of 1117 genes were significantly altered, among which 510 genes were significantly upregulated and 607 genes (Supplementary Material, Table S3-S4) were significantly downregulated. The hierarchical clustering analysis of differentially expressed genes (DEGs) separated into two groups (Fig. 2B), indicating a different expression profile of the two groups.
GO analysis was applied to DEGs, and the top 20 GO terms were presented in Fig. 2C-2E (all P < 0.01). Enrichment analysis indicated that DEGs are responsible for biological functions including extracellular matrix organization, cilium movement, collagen fibril organization, etc. (Fig. 2C). These biological processes are related to molecular functions including platelet-derived growth factor binding, extracellular matrix structural constituents, voltage-gated potassium channel activity, and so on (Fig. 2D). And, these processes occur mainly in the proteinaceous extracellular matrix, extracellular regions, extracellular space, etc. (Fig. 2E).
In addition, the mRNA expression profile comparing Ishikawa cells and Ishikawa cells with uNK medium was also shown in Supplementary Material. The bioinformatic analysis was also shown in Supplementary Material, Figure S4.
3.3. Pathway enrichment analysis
KEGG pathway analysis was conducted for further exploration of DEGs as shown in Fig. 3A. DEGs were highly enriched in phosphatidylinositol 3 kinase - protein kinase B (PI3K-Akt) signaling pathway, focal adhesion, Ras signaling pathway, extracellular matrix (ECM)-receptor interaction, etc. These pathways are highly relevant to the regulation of endometrial receptivity, and ZYP may exert therapeutic effects through the pathways mentioned above. In addition, the DEGs and pathway interactions were visualized in Fig. 3B.
Fig. 3.
Pathway enrichment analysis of DEGs between control group and ZYP group. (A) Bubble plot representing DEGs in control group and ZYP group. The statistical significance of all transcripts (P < 0.05 and fold change ≥1.5) was analyzed. (B) Visualization of the interaction between DEGs and pathways.
3.4. Effects of ZYP on mRNA expression using qRT-PCR analysis
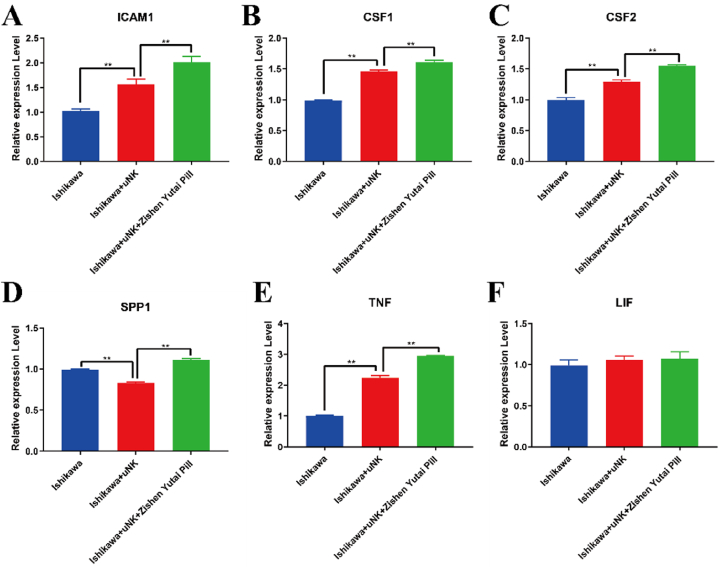
Protein-protein interactions of DEGs in KEGG analysis between uNK and uNK + ZYP group were shown in Supplementary Material, Figure S1. The expressions of the selected genes (ICAM1, CSF1, CFS2, TNF, SPP1 and LIF) were selected due to their central role in the regulation network. As can be seen in Fig. 4, after treatment of uNK secretion medium, the mRNA expressions of ICAM1, CSF1, CFS2, TNF elevated (Fig. 4A–C, 4E), compared with the blank control (P < 0.05). After treatment of ZYP, the mRNA expression of above-mentioned genes underwent an increase compared with the uNK group (P < 0.05). For SPP1 (Fig. 4D), elevated levels of mRNA expression were observed after treatment with ZYP, compared with the uNK group (P < 0.05). However, only slight but not significant differences could be observed in the expression of LIF (Fig. 4F) among the three groups (P > 0.05). RNA-seq results of the above mentioned genes were also presented in Supplement Material, Table S2.
Fig. 4.
Effects of ZYP on genes related to endometrial receptivity using RT-PCR. Gene expressions were quantified, including (A) ICAM-1, (B) CSF1, (C) CSF2, (D) SPP1, (E) TNF, (F) LIF. All data analysis were presented as Means ± SEM. Differences were detected by ANOVA test followed by Bonferroni’s multiple comparisons test. *P < 0.05, vs. Ishikawa + uNK group; **P < 0.01, vs. Ishikawa + uNK group.
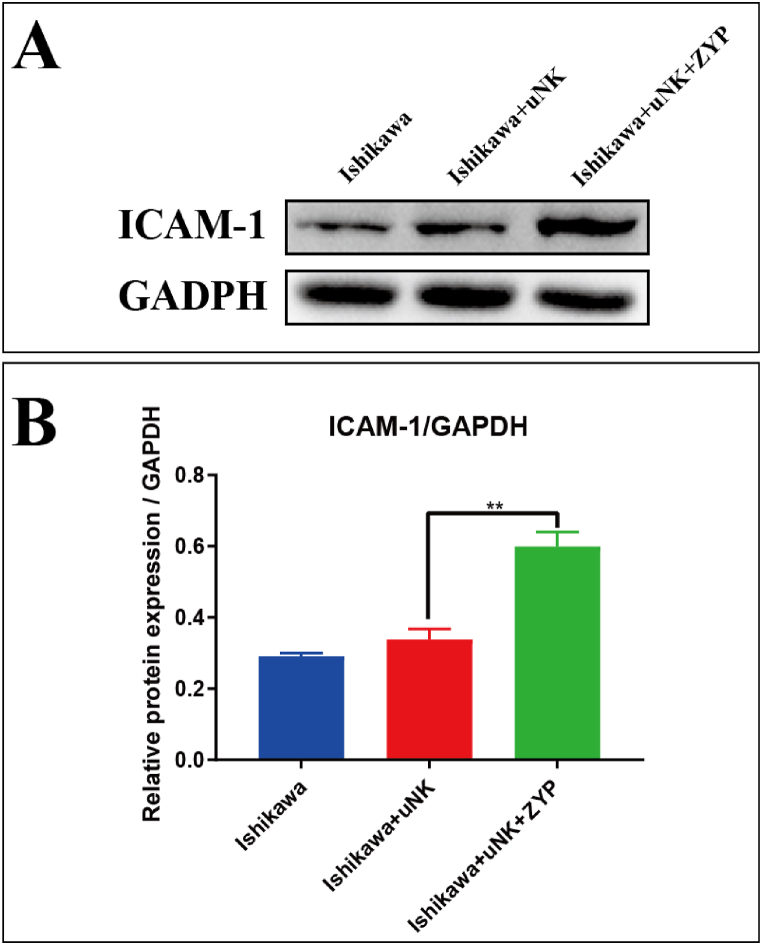
3.5. Effects of ZYP on protein expression of ICAM-1
Representative ICAM-1 panels were shown in Fig. 5A, and the quantitative analysis histogram was shown in Fig. 5B. As can be seen from Fig. 5, the level of ICAM-1 was not significantly altered after uNK treatment, compared with the blank control (P > 0.05). Compared with the uNK group, the level of ICAM-1 was significantly upregulated after ZYP treatment (P < 0.01). The result of protein expression was consistent with that of mRNA expression of ICAM-1.
Fig. 5.
Effects of ZYP on protein expression of ICAM-1 using WB. (A) Representative radiographs. (B) Quantitative analysis of WB assessed with relative intensity (fold difference of ICAM-1 to GADPH). All data analysis were presented as Means ± SEM. Differences were detected by ANOVA test followed by Bonferroni’s multiple comparisons test. *P < 0.05, vs. Ishikawa + uNK group; **P < 0.01, vs. Ishikawa + uNK group. Original photos of Western blotting were shown in Supplementary Material, Figure S2-S3.
4. Discussion
Innate immune cells take part in many aspects during the regulation of endometrial receptivity. uNK promote the formation and remodeling of new blood vessels in the decidual tissue. They also contribute to the establishment of local immune suppression and secrete various embryonic nutritional factors to enhance the receptivity of the uterine endometrium and establish maternal-fetal immunity [18]. This helps maintain immune balance between the mother and fetus, while also participating in the regulation of stromal cell proliferation, differentiation, and the transformation of the endometrium to decidual tissue [8]. During decidualization, the stromal cells in the uterine endometrium undergo a transformation into secretory decidual cells. However, in the current study setting, Ishikawa cells were not decidualized. In our previous clinical setting, ZYP was given from day 21 of the preceding menstrual cycle, through the next menstrual cycle [10]. Considering the intervention period of ZYP, we chose the non-decidualized protocol. In the current study setting, Ishikawa cell was used to observe the effects of ZYP on epithelial response.
After treatment of ZYP, the profiles of transcriptional profiles of Ishikawa cells underwent significant changes. According to the enrichment analysis, DEGs participate in various pathways including PI3K-Akt signaling pathway, focal adhesion, Ras signaling pathway, ECM-receptor interaction.
A previous study has proposed that uNK cells play an important role in angiogenesis in both nonpregnant and pregnant stages [19]. In the secretory stage, the number of uNK cells begins to rise, leading to vessel formation in the uterine endometrium through various pathways [20]. Evidences have shown that vessel formation is regulated by the PI3K/Akt pathway [21,22]. Interesting enough, a metabonomic study suggested that metabolites changed after administration of ZYP related to the upregulation of PI3K-Akt pathway, leading to improvements in endometrial receptivity [23]. Another report suggested that uterine natural killer cells, excreting cellular factors, could lead to spiral artery remodeling [24].
As can be seen in the enrichment analysis (Fig. 3B), the PI3K-Akt signaling pathway, focal adhesion, and ECM-receptor interaction are closely correlated due to the overlap in the genes regulated by these pathways. In addition, uNK cell were correlated with alterations in the PI3K-Akt, focal adhesion signaling pathways. A proteomics study suggested that genes enriched in the focal adhesion pathway may be related to uterine receptivity, partly due to upregulation of creatine kinase B-type [24].
It is generally accepted that the procedure of embryo implantation depends on the capacity of adhesion between cells and cell matrix [25]. And the procedure is closely related to the Ras signaling pathway, as well as the extracellular matrix [26].
For further confirmation of the mechanisms of ZYP on the endometrium under the presence of uNK medium, qRT-PCR was applied to quantify gene expression relating to endometrial receptivity, including ICAM1, CSF1, CSF2, TNF, LIF as well as SPP1. The expressions of the selected genes were selected on the basis of protein-protein-interaction (Supplementary Material, Figure S1). Due to their central role in the regulation network, these genes were selected.
ICAM1, a member of the immunoglobulin super family, is an adhesive molecule which could bind to β2-integrin ligands on leukocytes. ICAM1 is expressed during window of implantation, and could be a potential indicator of endometrial receptivity [27]. In the presence of uNK medium, the gene expression of ICAM1 was significantly upregulated. After administration of ZYP, the gene expression of ICAM1 was further upregulated. Such changes were also observed in the protein expression of ICAM-1 (Fig. 5). The elevation of ICAM1 in uterine cells and its likely involvement in implantation, suggest that ZYP may enhance the adhesion capacity of the embryo to the uterine. Interesting enough, an elevated embryo implantation rate was witnessed among women undergoing assisted reproduction technology (ART) with administration of ZYP in a randomized clinical trial [10]. We could hypophyses that Zishen Yutai Pills could increase endometrial receptivity through regulation of ICAM1.
The family of colony-stimulating factor (CSF) involves several members such as macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) and so on. The M-CSF, GM-CSF, and G-CSF are expressed by genes CSF1, CSF2, and CSF3, respectively [28]. M-CSF is a cytokine involving many aspects during implantation, including development of placenta, growth and differentiation of trophoblasts, etc. In addition, it was proved to be a predictive factor in pregnancy outcomes. Extensive studies have proved that GM-CSF is a key regulator in reproductive function, participating in processes including oocyte maturation, endometrial receptivity, and embryo development [29]. In addition, GM-CSF is widely used in clinical practice, helping women undergoing ART for better pregnancy outcomes [30].
Osteopontin, regulated by the gene SPP1, is reported to be an essential mediator of implantation and receptivity in women. Osteopontin, an estrogen-dependent endometrial gland secretory factor, is reported to participate in the activation of blastocyst adhesion competence [31]. The biological process was proved to be mediated through focal adhesion kinase and PI3K signaling pathways, namely, two major signaling pathways enriched among DEGs. An elevated level of SPP1 gene expression was observed in the RT-PCR.
In RT-PCR analysis, TNF expression was elevated after treatment with uNK cells. When adding ZYP containing serum, TNF expression was raised as compared with the uNK group. In previous cohort studies, elevated level of TNF-α was associated with better pregnancy outcomes [32,33]. This phenomenon may be contributed to the ability of TNF-α in promoting decidualization and stimulating the secretion of cytokine factors. Many factors stimulated by TNF required for implantation are associated with the pancreatic effects of uterine natural killer cells.
There are several limitations of the current work. First, the absence of embryo in the experimental setting could not fully explain the role of ZYP and uNK during the process of implantation. This shortcoming limited our work in the interpretation of experimental results. Second, the process of Ishikawa cell decidualization should also be examined. Third, the rational of biomarkers should be more cautious, especially taking the bioinformatics results and recent research. Due to the limitations of conditions, only the expression of ICAM-1 was measured, which should be improved in our further research.
5. Conclusion
In brief, in the presence of uNK cell medium, ZYP could improve the mRNA expressions, including ICAM1, CSF1, CSF2, TNF, SPP1, etc. However, further exploration should be carried out in vivo experiments for the validation of the mechanisms of ZYP on endometrial receptivity as the procedure of implantation and pregnancy is of great complexity.
Ethics approval and consent to participate
All procedures involving animals were conducted in accordance to Regulations for the Administration of Affairs Concerning Experimental Animals. Permission for animal studies was approved by the Ethics Committee at Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University (SYSU-IACUC-2020-B0421).
Author contribution statement
Xiaoli Chen: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Yanxin Xie, Lin Li, Shumin Chen and Miao Ding Performed the experiments.
Na Ning, Qiuling Huang and Xiufei Pang: Contributed reagents, materials, analysis tools or data.
Jiewen Zhou: Analyzed and interpreted the data; Wrote the paper.
Dongzi Yang: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19395.
Abbreviations
- ANOVA
analysis of variance
- ECM
extracellular matrix
- DEGs
differentially expressed genes
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- ICAM-1
intercellular cell adhesion molecule-1
- IL-15
interleukin-15
- PI3K
phosphatidylinositol 3-kinase
- PI3K-AKT
phosphatidylinositol 3 kinase - protein kinase B
- PVDF
polyvinylidene fluoride
- qRT-PCR
quantitative real-time polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TCM
traditional Chinese medicine
- uNK
uterine natural killer cell
- WB
Western blotting
- ZYP
Zishen Yutai Pill
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Díaz-Hernández I., Alecsandru D., García-Velasco J.A., Domínguez F. Uterine natural killer cells: from foe to friend in reproduction. Hum. Reprod. Update. 2021;27(4):720–746. doi: 10.1093/humupd/dmaa062. [DOI] [PubMed] [Google Scholar]
- 2.Chiossone L., Dumas P.-Y., Vienne M., Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018;18(11):671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 3.Vacca P., Mingari M.C., Moretta L. Natural killer cells in human pregnancy. J. Reprod. Immunol. 2013;97(1):14–19. doi: 10.1016/j.jri.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Vacca P., Cantoni C., Prato C., Fulcheri E., Moretta A., Moretta L., Mingari M.C. Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int. Immunol. 2008;20(11):1395–1405. doi: 10.1093/intimm/dxn105. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann A.P., Gerber S.A., Croy B.A. Uterine natural killer cells pace early development of mouse decidua basalis. Mol. Hum. Reprod. 2014;20(1):66–76. doi: 10.1093/molehr/gat060. [DOI] [PubMed] [Google Scholar]
- 6.Lu X., Cui J., Cui L., Luo Q., Cao Q., Yuan W., Zhang H. The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem Cell Res. Ther. 2019;10(1):214. doi: 10.1186/s13287-019-1313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong C.-S., Ordoñez A.A., Turner S., Tremaine T., Muter J., Lucas E.S., Salisbury E., Vassena R., Tiscornia G., et al. Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation. FASEB (Fed. Am. Soc. Exp. Biol.) J.: Offic. Publicat. Federat. American Societ. Experim. Biol. 2021;35(4) doi: 10.1096/fj.202002217R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muter J., Kong C.-S., Brosens J.J. The role of decidual subpopulations in implantation, menstruation and miscarriage. Front. Reproduct. Health. 2021;3 doi: 10.3389/frph.2021.804921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng J., Wang J., Zhang Y., Zhang Y., Jia L., Zhang D., Zhang J., Han Y., Luo S. The efficacy of complementary and alternative medicine in the treatment of female infertility, evidence-based complementary and alternative medicine. ECAM. 2021;2021 doi: 10.1155/2021/6634309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Hao C., Deng W., Bai H., Li Y., Wang Z., Shi Y., Zhang H., Zhu Y., et al. Effects of the Zishen Yutai Pill compared with placebo on live births among women in a fresh embryo transfer cycle: a randomized controlled trial. Obstet. Gynecol. 2022;139(2):192–201. doi: 10.1097/AOG.0000000000004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao J., Lei T., Wu S., Li H., Deng Y., Lin R., Ning N., Geng C., Wang S., et al. Development of a comprehensive method combining UHPLC-CAD fingerprint, multi-components quantitative analysis for quality evaluation of Zishen Yutai Pills: a step towards quality control of Chinese patent medicine. J. Pharmaceut. Biomed. Anal. 2020;191 doi: 10.1016/j.jpba.2020.113570. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Cao J., Wu X., Deng Y., Ning N., Geng C., Lei T., Lin R., Wu D., et al. Multiple fingerprint profiling for quality evaluation of polysaccharides and related biological activity analysis of Chinese patent drugs: Zishen Yutai Pills as a case study. J. Ethnopharmacol. 2020;260 doi: 10.1016/j.jep.2020.113045. [DOI] [PubMed] [Google Scholar]
- 13.Arnold J.T., Lessey B.A., Seppälä M., Kaufman D.G. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res. 2002;62(1):79–88. [PubMed] [Google Scholar]
- 14.Maharajan K., Xia Q., Duan X., Tu P., Zhang Y., Liu K. Therapeutic importance of Zishen Yutai Pill on the female reproductive health: a review. J. Ethnopharmacol. 2021;281 doi: 10.1016/j.jep.2021.114523. [DOI] [PubMed] [Google Scholar]
- 15.Li M., Ning N., Liu Y., Li X., Mei Q., Zhou J., Huang Q., Xiang W., Zhang L., et al. The potential of Zishen Yutai pills to facilitate endometrial recovery and restore fertility after induced abortion in rats. Pharmaceut. Biol. 2021;59(1):1505–1516. doi: 10.1080/13880209.2021.1993272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong X., Chen Z., Liu Y., Lu Q., Jin Z. Gene expression profiling of the paracrine effects of uterine natural killer cells on human endometrial epithelial cells. Int. J. Endocrinol. 2014;2014 doi: 10.1155/2014/393707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma S., Hiby S.E., Loke Y.W., King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol. Reprod. 2000;62(4):959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 18.Murata H., Tanaka S., Okada H. The regulators of human endometrial stromal cell decidualization. Biomolecules. 2022;12(9) doi: 10.3390/biom12091275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell P., Sacks G., Tremellen K., Gee A. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: further observations and reference ranges. Pathology. 2013;45(4):393–401. doi: 10.1097/PAT.0b013e328361429b. [DOI] [PubMed] [Google Scholar]
- 20.Quenby S., Nik H., Innes B., Lash G., Turner M., Drury J., Bulmer J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum. Reprod. (Oxd. Engl.) 2009;24(1):45–54. doi: 10.1093/humrep/den348. [DOI] [PubMed] [Google Scholar]
- 21.Rätsep M.T., Felker A.M., Kay V.R., Tolusso L., Hofmann A.P., Croy B.A. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction (Cambridge, England) 2015;149(2):R91–R102. doi: 10.1530/REP-14-0271. [DOI] [PubMed] [Google Scholar]
- 22.Li D., Li J. Association of miR-34a-3p/5p, miR-141-3p/5p, and miR-24 in decidual natural killer cells with unexplained recurrent spontaneous abortion. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. : Int. Medi. J. Experim. Clinical Res. 2016;22:922–929. doi: 10.12659/MSM.895459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L., Ning N., Wei J.-a., Huang Q.-L., Lu Y., Pang X.-f., Wu J.-j., Zhou J.-b., Zhou J.-w., et al. Metabonomics study on the infertility treated with zishen Yutai pills combined with in vitro fertilization-embryo transfer. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.686133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Ding L., Diao Z., Yan G., Sun H., Hu Y. CYR61 modulates the vascular endothelial growth factor C expression of decidual NK cells via PI3K/AKT pathway. Am. J. Reprod. Immunol. 2012;67(3):216–223. doi: 10.1111/j.1600-0897.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Zhang A., Yu F., Gao J., Liu Y., Yu C., Zhou H., Xu C. Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J. Proteome Res. 2015;14(4):1831–1842. doi: 10.1021/acs.jproteome.5b00038. [DOI] [PubMed] [Google Scholar]
- 26.Dong F., Zhang Y., Xia F., Yang Y., Xiong S., Jin L., Zhang J. Genome-wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction (Cambridge, England) 2014;148(1):33–41. doi: 10.1530/REP-14-0095. [DOI] [PubMed] [Google Scholar]
- 27.Chen S., Liu B., Li J., Liao S., Bi Y., Huang W., Yuan L., Yang Y., Qin A. Talin 1 regulates endometrial adhesive capacity through the Ras signaling pathway. Life Sci. 2021;274 doi: 10.1016/j.lfs.2021.119332. [DOI] [PubMed] [Google Scholar]
- 28.Camargo-Díaz F., García V., Ocampo-Bárcenas A., González-Marquez H., López-Bayghen E. Colony stimulating factor-1 and leukemia inhibitor factor expression from current-cycle cannula isolated endometrial cells are associated with increased endometrial receptivity and pregnancy. BMC Wom. Health. 2017;17(1):63. doi: 10.1186/s12905-017-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M.T., Kaushansky K., Ralph P., Ladner M.B. Differential expression of M-CSF, G-CSF, and GM-CSF by human monocytes. J. Leukoc. Biol. 1990;47(3):275–282. doi: 10.1002/jlb.47.3.275. [DOI] [PubMed] [Google Scholar]
- 30.Würfel W. Treatment with granulocyte colony-stimulating factor in patients with repetitive implantation failures and/or recurrent spontaneous abortions. J. Reprod. Immunol. 2015;108:123–135. doi: 10.1016/j.jri.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Chaen T., Konno T., Egashira M., Bai R., Nomura N., Nomura S., Hirota Y., Sakurai T., Imakawa K. Estrogen-dependent uterine secretion of osteopontin activates blastocyst adhesion competence. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boomsma C.M., Kavelaars A., Eijkemans M.J.C., Lentjes E.G., Fauser B.C.J.M., Heijnen C.J., Macklon N.S. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. (Oxd. Engl.) 2009;24(6):1427–1435. doi: 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 33.Salama K.M., Alloush M.K., Al Hussini R.M. Are the cytokines TNF alpha and IL 1 Beta early predictors of embryo implantation? Cross sectional study. J. Reprod. Immunol. 2020;137 doi: 10.1016/j.jri.2019.102618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.