Abstract
Objective
This trial was to examine the effect of transcutaneous electrical acupoint stimulation (TEAS) on postoperative cognitive function in older patients who underwent thoracoscopic pulmonary resection.
Methods
This was a prospective, randomized, double-blind, placebo-controlled study. 128 patients scheduled for surgery were randomly assigned to the TEAS group and sham-TEAS group. A standardized intervention of TEAS or sham-TEAS on the acupoints of Baihui (DU20) and bilateral Neiguan (PC6), Hegu (LI4), and Zusanli (ST36) from 30 min before anesthesia induction until the end of the surgery, combined with a general anesthetic protocol performed in the two groups respectively. The primary outcome was the incidence of postoperative cognitive dysfunction (POCD) assessed via the Montreal Cognitive Assessment (MoCA) scale at each time point. The secondary outcomes included the results of the Mini-Mental State Examination (MMSE) score, the Numerical Rating Scale (NRS) on pain and sleep, the European Organization for Research and Treatment of Cancer Quality of Life (EORTC-QLQ-C30), and a chronic pain questionnaire at relative time points.
Results
Participants who completed the 12-month trial of the two groups were well-matched in baseline demographic and clinical parameters. At postoperative day 1, day 7, and day 30 time points, the incidence of POCD in the sham-TEAS group was always significantly higher than in the TEAS group (65.4% vs 20%, 43.6% vs 7.3%, 40% vs 3.6%, all P < 0.001). Also, the TEAS group showed better scores of MMSE, sleep, and pain compared with the sham-TEAS group (all P < 0.001). At 6 and 12 months points, the global health scores of the TEAS group were still significantly higher than the sham-TEAS group, and the prevalence of chronic pain was significantly lower than the sham-TEAS group (all P < 0.05).
Conclusion
TEAS could effectively improve the postoperative cognitive function and long-term life quality of geriatric patients with lung cancer.
Keywords: Transcutaneous electrical acupoint stimulation(TEAS), Postoperative cognitive dysfunction(POCD), Quality of life, Lung cancer, Older patients
1. Introduction
Lung cancer is the second most diagnosed cancer among men and women worldwide and is the leading cause of cancer-related death [1]. With the development of early detection, a growing number of elderly patients diagnosed with stage I or II diseases have the potential to be cured by surgery [2]. Unlike past cancer treatments aimed primarily at survival, the perioperative field is now increasingly recognized for its rapid recovery and high quality of life in surgical patients [3]. Thus, several practical strategies, such as minimally invasive surgery and proper anesthesia management, have been widely adopted to implement the enhanced recovery after surgery (ERAS) pathway [4]. However, given the large number of elderly patients suffering from basic diseases, a relatively high prevalence of postoperative complications remains recognized as a major barrier to their optimal recovery.
Postoperative cognitive dysfunction (POCD) is a neurological complication that frequently occurs in elderly surgical patients and lasts for an indefinite duration (from a few weeks to several years) after surgery [5]. It is defined as an abnormal condition of orientation, attention, perception, consciousness, and judgment that might produce cluster adverse impacts such as prolonged stay in the hospital, delayed functional recovery, and increased mortality [6,7]. Over the past decades, several clinical studies have adopted a broad range of surgical or anesthesia procedures to minimize the impact of POCD [8]. Nevertheless, these perioperative interventions produced negative or inconclusive results due to the complex causes of POCD [9,10]. Therefore, perioperative medicine is encouraged to investigate techniques that would help cognitive function achieve an ideal recovery for geriatric patients after surgery [11].
Transcutaneous electrical acupoint stimulation (TEAS) has been used to facilitate postoperative recovery in various surgical patients. This noninvasive and nonpharmacological adjunctive intervention was born and developed from traditional Chinese acupuncture and modern electroacupuncture. Multiple randomized controlled trials have suggested that TEAS could be effective in preventing nausea and vomiting, alleviating psychological symptoms, lowering anesthetic consumption, and synergizing with sedatives and analgesics among various surgical patients [[12], [13], [14], [15]]. However, the clinical application of this technology was reported limitedly in patients with carcinoma of the lung, and the impact on long-term life quality has not been studied beyond 12 months.
Based on these considerations, the main objective of this study was to determine if TEAS could reduce POCD and promote postoperative recovery in elderly patients undergoing thoracoscopic lung cancer resection. We hypothesized that TEAS could improve the postoperative cognition function of elderly patients with lung cancer in the early stages and further promote their quality of life.
2. Materials and methods
2.1. Study design
This was a prospective, randomized, double-blind, placebo-controlled study. It was approved by the Research Ethics Committee of the Second Affiliated Hospital of Air Force Medical University (NO.202006–03) and registered at the Chinese Clinical Trial Registry (Registration number: ChiCTR2000035552) and the Chinese Medicine Clinical Trial Registry (Registration number: ChiMCTR2000003560). All procedures in this study were performed following the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Before participating, all patients signed the informed consent form without any financial compensation.
2.2. Participants
Those who were scheduled for thoracoscopic pulmonary resection for suspected lung cancer between August 26, 2020, and January 4, 2021, at the Department of Thoracic Surgery, the Second Affiliated Hospital of Air Force Medical University, were consecutively screened and enrolled. The inclusion criteria were: (1) Patients were scheduled for thoracoscopic pulmonary resection, which may last more than 2 h; (2) Patients aged ≥65 years; (3) BMI: 18–30 kg/m2; (4) American Society of Anesthesiologists status was I to III; (5) Patients with primary tumor who never had radiotherapy or chemotherapy; (6) Patients planning to conduct pulmonary isolation with a double-lumen tube (DLT); (7) Patients with normal cognitive function. Patients were excluded as the following criteria: (1) Patients refused to take part in this study; (2) Patients with communication disorders such as language comprehension disorder, mental disorders, epilepsy, a history of Parkinson's disease or Myasthenia Gravis, and other disorders impairing their ability to communicate; (3) History of opioid, alcohol, or other drug abuse and addiction; (4) Patients with TEAS contraindications, including partially damaged skin, infection, or implantable internal electrophysiologic device; (5) History of severe comorbidities in major organ system, including pre-existing coagulopathy, hypertension (systolic pressure ≥180 mmHg and/or diastolic pressure ≥110 mmHg), hepatic or renal insufficiency, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease; (6) History of pulmonary operation; (7) Unilateral pulmonary resection or bilateral pulmonary operation; (8) Massive hemorrhage in operation; (9) Taking pulmonary sequestration ways such as difficult airway, tracheotomy, occluder, artificial pneumothorax excluded DLT; (10) Patients took part in other clinical trials within 3 months; (11) Patients considered inappropriate by the physician in charge (provided that the reasons were detailed and recorded).
2.3. Randomization and blinding
A randomization sequence was generated by Statistical Product and Service Solutions (SPSS 25.0) software, and the allocation code was performed by an independent technician, 128 patients were enrolled and randomly assigned to the TEAS group and the sham-TEAS group (1:1). The patients, anesthesiologists, surgeons, and observers were blinded to group assignment. Until the 12 months trial was completed, the technician didn't share the information about divided groups with the statistician.
2.4. Interventions
This part was performed by an experienced technician who held a Good Clinical Practice Certificate. According to the allocation code of the enrolled patients with the time sequence, they were given TEAS and sham-TEAS interventions, respectively. In detail, electrodes were attached to the acupoints of Baihui (DU20) and bilateral Neiguan (PC6), Hegu (LI4), and Zusanli (ST36) (Supplementary Material 1) in all patients. For the TEAS group, two Han's Acupoint Nerve Stimulators (HANS-200 A; Nanjing Jisheng Medical Technology Co., Ltd; China) were applied to stimulate these combined acupoints with fixed parameters (disperse-dense waves; 2/100 Hz, and 10–30 mA) from 30 min before anesthesia induction until the end of the surgery. In the sham-TEAS group, the stimulators were also connected, but the power was turned off.
To reduce the bias, a standardized anesthetic protocol was elaborated and performed throughout the surgery. No anesthetic was given to the patients before anesthesia induction. The content of intraoperative monitoring included electroencephalogram (EEG), bispectral index (BIS), arterial blood pressure (ABP), pulse oxygen saturation (SPO2), end-tidal carbon dioxide tension (EtCO2), core body temperature (CBT), and urine volume. Dexamethasone (4–5 mg) was used to prevent postoperative nausea and vomiting. The intravenous induction was started with midazolam (0.015–0.03 mg/kg), sufentanil (0.4–0.8 μg/kg), propofol (1.5–2.5 mg/kg), and rocuronium bromide (0.6 mg/kg), and then a double-lumen tube was intubated at an appropriate depth. Anesthesia was maintained by using a target-controlled infusion (TCI) system of propofol, rocuronium, and remifentanil (adjusting the BIS to 40–60). During the procedure, mechanical ventilation was set as follows: tidal volume (6–8 ml/kg), PEEP (5 cm H2O), pause pressure (≤30 cm H2O), respiratory rate (12–16 breaths/minute), and oxygen (50%). The volume of intravenous infusion was managed according to the clinical indication. Patients were given relaxant antagonists (neostigmine and atropine) after extubation. At the end of the surgery, a patient-controlled intravenous analgesia (PCIA) pump (sufentanil: 0.04 μg/kg/h, bolus: 0.01 μg/kg, PCIA dose: 3 ml, background dose: 2 ml/h, interval: 15 min, and duration: 48 h) was connected immediately.
2.5. Measures and data collection
A standardized case report form (Supplementary Material 2) was designed to collect all enrolled patients' information throughout the trial. It consisted of three sections, as follows: (1) On the first preoperative day, the surgeon would share the basic information, physical condition, and surgical schedule of potential subjects with the visitor. All the patients were screened according to the enrolling and excluding criteria. The effects of the preoperative interviews were ensured by workload, operation schedule, nurse talent, and coordination between the operating room and the clinical wards. Preoperative evaluation includes demographic data, past medical history, an assessment of Barthel's index, and cognitive function. Demographic data include age, gender, race, BMI, education level, allergic history, smoking history, drinking history, and surgical history. Chronic diseases such as hypertension, diabetes, and cardiopathy were also collected. All the neuropsychological tests were conducted at the hospital during daily working hours. To obtain cognitive baseline information, the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were used in the preoperative evaluation [16,17]. The MMSE consists of 20 separate items which add up to a maximum score of 30. In the present study, a score >27 was considered indicative of normal cognitive function, and the MMSE values were corrected for age and education. The MoCA has taken the originally recommended 26/30 cutoff score. (2) Perioperative information on surgical and anesthesia management includes the duration of anesthesia and anesthesia recovery, the proportion of lung resected, the dose of propofol, sufentanil, and remifentanil, fluid administration, and the frequency of remedial analgesia in the hospital. (3) Postoperative follow-up of cognitive function, quality of life, and chronic pain was also collected. During the postoperative follow-up, the primary outcome was the incidence of POCD which was diagnosed when the MoCA score declined 1 standard deviation (SD) or more compared with the preoperative MoCA score on postoperative day 1, day 7, and day 30 [18]. Secondary outcomes included the results of MMSE scores, the Numerical Rating Scale (NRS) on pain and sleep on Day 1, Day 7, and Day 30 after surgery [19,20], the quality of life assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30) at 6 months and 12 months [21], and a questionnaire on chronic pain at 12 months.
Adverse events were reported by participants and collected by the investigator throughout the trial. All adverse events were categorized by a discussion between the anesthesiologists and surgeons as related or unrelated to the intervention within 24 h of occurrence. Serious adverse events should be immediately reported to the principal investigator and the ethics committee within 24 h. Any of the reported adverse events should be resolved with proper measures.
2.6. Statistical analysis
According to the previous study, the incidence of POCD was 41.4% in older surgical patients at hospital discharge [22]. We assumed that TEAS could decrease the incidence of POCD to 25.4% in patients who underwent thoracoscopic lung cancer resection. Using an α value of 0.05 and a power (1-β) of 80%, we calculated that a sample size of 102 was required. Considering a 20% dropout rate, the sample size was computed to be 128 by the population correction formula.
All statistical analyses were performed using SPSS 25.0. In this study, the continuous variables were presented as mean ± SD. If the data conforms to the normal distribution, it would be compared by an independent-sample t-test. Otherwise, a Mann-Whitney U test would be used. Categorical data were expressed as numbers and percentages, and compared with χ2 or Fisher's exact test as appropriate. A 2-sided P value less than 0.05 was considered significant.
3. Results
3.1. Participant characteristics
Between August 2020 and January 2021, a total of 412 patients who underwent thoracoscopic pulmonary resection for suspected lung cancer were screened for eligibility. 128 of them were enrolled and randomly assigned to the TEAS or sham-TEAS group, and 110 (85.94%) completed the trial, while 18 (14.06%) patients dropped out of the study, so their data were excluded from the analyses (Fig. 1). No significant difference in baseline characteristics was found between the dropped patients and the completed patients (Supplementary Material 3).
Fig. 1.
Study flow diagram.
3.2. Baseline and demographic characteristics
Overall, patients who completed the trial in the TEAS and the sham-TEAS groups were well-matched for baseline characteristics (Table 1). There were no significant differences in age, sex, education level, chronic diseases, and MoCA, or MMSE scores between the two groups at baseline. In the total sample, the mean age was 70.33 years (SD = 4.27), 42.7% of the participants were female, and 77.3% of them were educated more than 9 years, the mean MoCA score at baseline was 27.85 (SD = 1.52), and the mean MMSE score was 28.17 (SD = 1.23).
Table 1.
Baseline characteristics of patients who completed the trial (n [%], mean ± SD).
| Characteristic | TEAS group (n = 55) | Sham-TEAS group (n = 55) | P-value |
|---|---|---|---|
| Age (years) | 70.09 ± 3.95 | 70.56 ± 4.59 | 0.69a |
| Race | – | ||
| Han | 54 (98.18%) | 54 (98.18%) | |
| Other | 1 (1.82%) | 1 (1.82%) | |
| Gender | 0.18 | ||
| Female | 20 (36.40%) | 27 (49.10%) | |
| Male | 35 (63.60%) | 28 (50.90%) | |
| BMI (kg/m2) | 24.09 ± 2.78 | 23.63 ± 2.85 | 0.52a |
| Education | 0.12 | ||
| <9 years | 12 (21.80%) | 13 (23.60%) | |
| 9–12 years | 36 (65.50%) | 27 (49.10%) | |
| >12 years | 7 (12.70%) | 15 (27.30%) | |
| Allergic history | 8 (14.50%) | 10 (18.20%) | 0.61 |
| Smoking history | 23 (41.80%) | 20 (36.40%) | 0.56 |
| Drinking history | 5 (9.10%) | 7 (12.70%) | 0.54 |
| Surgical history | 33 (60.00%) | 27 (49.10%) | 0.25 |
| Chronic diseases | |||
| Hypertension | 19 (34.50%) | 22 (40.00%) | 0.55 |
| Diabetes | 6 (10.90%) | 3 (5.50%) | 0.49b |
| Cardiopathy | 3 (5.50%) | 7 (12.70%) | 0.19 |
| MMSE Score | 28.27 ± 1.22 | 28.07 ± 1.25 | 0.45a |
| MoCA Score | 27.84 ± 1.21 | 27.86 ± 1.78 | 0.57a |
| NYHA | 0.05 | ||
| Ⅰ | 53 (96.40%) | 47 (85.50%) | |
| Ⅱ | 2 (3.60%) | 8 (14.50%) | |
Abbreviations: BMI, body mass index; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NYHA, New York Heart Association Functional Classification.
Indicates Mann-Whitney U test.
Indicates Fisher's exact test.
According to the perioperative information of the study population (Table 2), it was found that there were no significant differences between the two groups in the duration of anesthesia, the proportion of lung resected, the consumption of propofol and sufentanil, the total of input or output fluid, or the frequency of remedial analgesics. However, the consumption of remifentanil in the sham-TEAS group was significantly higher than in the TEAS group (1.01 ± 0.39 vs 0.87 ± 0.30 mg, P = 0.040).
Table 2.
Perioperative information of the study population (mean ± SD).
| Perioperative information | TEAS group (n = 55) | Sham-TEAS group (n = 55) | P-value |
|---|---|---|---|
| Duration of anesthesia (min) | 159.36 ± 39.68 | 146.82 ± 38.36 | 0.095 |
| Duration of anesthesia recovery (min) | 22.64 ± 9.37 | 24.45 ± 8.80 | 0.297 |
| The proportion of lung resected (%) | 33.82 ± 13.78 | 34.09 ± 15.93 | 0.886a |
| Dose of propofol (mg) | 643.00 ± 213.08 | 626.09 ± 233.47 | 0.680a |
| Dose of sufentanil (ug) | 29.42 ± 7.62 | 30.55 ± 7.94 | 0.433a |
| Dose of remifentanil (mg) | 0.87 ± 0.30 | 1.01 ± 0.39 | 0.040 |
| Fluid administration (ml) | |||
| Input | 1489.09 ± 346.24 | 1431.82 ± 395.62 | 0.417a |
| Output | 508.46 ± 258.96 | 494.27 ± 294.39 | 0.482a |
| Frequency of remedial analgesic in the hospital (times) | 2.29 ± 1.76 | 1.96 ± 1.97 | 0.361 |
Indicates Mann-Whitney U test.
3.3. Primary and secondary outcomes
3.3.1. The incidence of POCD
The primary outcome results are shown in Fig. 2. Following the study procedure, the incidence of POCD in the sham-TEAS group was always significantly higher than in the TEAS group (postoperative day 1: 65.4% vs 20%, postoperative day 7: 43.6% vs 7.3%, and postoperative day 30: 40% vs 3.6%, all P < 0.001). The peak incidence of POCD in the TEAS group and the sham-TEAS group was both on day 1 after surgery, and then a descending trend lasted until 30 days.
Fig. 2.
The incidence rate of POCD. “*” indicates P < 0.001 as the TEAS group compared with the sham-TEAS group at relative time points.
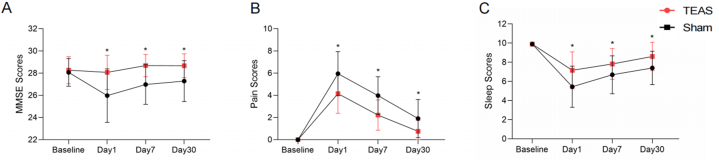
3.3.2. MMSE, pain, and sleep scores
The secondary short-term outcome results are shown in Fig. 3. On day 1, day 7, and day 30 after surgery, the pain scores in the TEAS group were significantly lower than those in the sham-TEAS group. The peak values of pain scores in the TEAS group and the sham-TEAS group were both on day 1 after surgery, and then a reverse trend lasted until 30 days (Fig. 3B). On the contrary, the MMSE and sleep scores of the TEAS group were significantly higher than that of the sham-TEAS group at the three-time points. The lowest values of MMSE and sleep scores both occurred in the two groups on day 1 after surgery and then an increasing trend was observed up to 30 days (Fig. 3A and C).
Fig. 3.
(A) Changes in the MMSE scores. (B) Changes in the NRS on pain scores. (C) Changes in the NRS on sleep scores. “*” indicates P < 0.001 as the TEAS group compared with the sham-TEAS group at relative time points.
3.3.3. Quality of life
EORTC-QLQ-C30 scores were used to assess the long-term quality of life in patients (Table 3). At 6 months after surgery, the mean scores of emotional function and global health in the TEAS group were significantly higher than those in the sham-TEAS group (96.82 ± 9.28 vs 91.67 ± 11.79, P = 0.002; 75.61 ± 10.98 vs 70 ± 10.71, P = 0.007), while the fatigue and pain scores in the TEAS group were lower than in the sham-TEAS group (6.67 ± 12.58 vs 11.92 ± 13.16, P = 0.01; 3.64 ± 8.30 vs 7.58 ± 11.03, P = 0.032). At 12 months after surgery, no difference was found between the two groups except that the mean score of global health and emotional function in the TEAS group was higher than that in the sham-TEAS group (74.70 ± 11.78 vs 69.70 ± 11.82, P = 0.029; 95.45 ± 11.21 vs 92.58 ± 10.60, P = 0.024).
Table 3.
Comparison of the EORTC-QLQ-C30 scores between the two groups at relative time points (mean ± SD).
| Domain | 6 months |
12 months |
||||
|---|---|---|---|---|---|---|
| TEAS group (n = 55) | Sham-TEAS group (n = 55) | P-valuea | TEAS group (n = 55) | Sham-TEAS group (n = 55) | P-valuea | |
| Function scale | ||||||
| Physical function | 93.82 ± 10.01 | 92.73 ± 9.45 | 0.311 | 91.64 ± 10.20 | 92.24 ± 9.50 | 0.772 |
| Role function | 95.15 ± 10.48 | 91.52 ± 13.56 | 0.078 | 92.73 ± 12.32 | 90.61 ± 14.26 | 0.385 |
| Emotional function | 96.82 ± 9.28 | 91.67 ± 11.79 | 0.002 | 95.45 ± 11.21 | 92.58 ± 10.60 | 0.024 |
| Cognitive function | 92.73 ± 14.26 | 90.30 ± 11.87 | 0.09 | 90.91 ± 16.30 | 89.09 ± 12.92 | 0.164 |
| Social function | 96.36 ± 9.99 | 97.58 ± 8.13 | 0.529 | 96.36 ± 9.99 | 97.58 ± 8.13 | 0.529 |
| Symptom scale | ||||||
| Fatigue | 6.67 ± 12.58 | 11.92 ± 13.16 | 0.01 | 10.71 ± 14.18 | 13.94 ± 13.73 | 0.151 |
| Nausea and vomiting | 0 | 2.42 ± 10.35 | 0.043 | 0 | 0.30 ± 2.24 | 0.317 |
| Pain | 3.64 ± 8.30 | 7.58 ± 11.03 | 0.032 | 2.73 ± 7.70 | 5.45 ± 10.17 | 0.092 |
| Dyspnea | 21.82 ± 20.51 | 29.70 ± 34.94 | 0.236 | 16.97 ± 16.82 | 21.21 ± 19.63 | 0.299 |
| Sleep disturbance | 10.91 ± 19.30 | 18.18 ± 27.08 | 0.198 | 12.12 ± 20.65 | 16.36 ± 24.74 | 0.429 |
| Appetite loss | 6.67 ± 17.45 | 12.73 ± 23.56 | 0.106 | 5.45 ± 14.00 | 7.88 ± 16.93 | 0.439 |
| Constipation | 6.06 ± 17.0 | 12.12 ± 22.56 | 0.093 | 4.85 ± 14.93 | 6.06 ± 15.83 | 0.584 |
| Diarrhea | 3.64 ± 12.29 | 3.03 ± 11.61 | 0.734 | 2.42 ± 8.74 | 1.21 ± 6.30 | 0.403 |
| Financial difficulties | 2.42 ± 8.74 | 0.61 ± 4.50 | 0.172 | 2.42 ± 8.74 | 0.60 ± 4.50 | 0.172 |
| Global health | 75.61 ± 10.98 | 70 ± 10.71 | 0.007 | 74.70 ± 11.78 | 69.70 ± 11.82 | 0.029 |
Abbreviations: EORTC-QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Indicates Mann-Whitney U test.
The information on pain persistence and the presence of chronic pain was collected 12 months after surgery (Table 4). Compared with the TEAS group, larger proportions of patients in the sham-TEAS group reported suffering postoperative pain (23.6% vs 7.3%, P = 0.018) with a longer duration (3.46 ± 3.94 vs 1.68 ± 1.52 months; P = 0.043) even more than two months (32.7% vs 14.5%, P = 0.025).
Table 4.
Information on chronic pain collected at 12 months after surgery (n [%] or mean ± SD).
| TEAS group (n = 55) | Sham-TEAS group (n = 55) | P-value | |
|---|---|---|---|
| Postoperative pain duration >2 months | 8 (14.5%) | 18 (32.7%) | 0.025 |
| Pain duration (month) | 1.68 ± 1.52 | 3.46 ± 3.94 | 0.043a |
| Current Pain | 4 (7.3%) | 13 (23.6%) | 0.018 |
| The maximum score of postoperative pain | 7.00 ± 1.14 | 7.31 ± 1.09 | 0.11a |
| Movement disorders on the shoulder or arm of the operation side | 1 (1.8%) | 1 (1.8%) | 1.00 |
| Movement disorders in daily washing activities | 0 | 1 (1.8%) | 1.00 |
| See a doctor for the pain | 0 | 1 (1.8%) | 1.00 |
Indicates Mann-Whitney U test.
3.4. Safety
During the 12-month trial, adverse events were reported in 6 (10.90%) patients of the TEAS group and 9 (16.36%) of the sham-TEAS group. No serious adverse events were reported in either group. Intervention-related adverse events were mild and transient (Table 5).
Table 5.
Adverse events reported after surgery (n [%]).
| Adverse events | TEAS group (n = 55) | Sham-TEAS group (n = 55) |
|---|---|---|
| Any | 6 (10.90%) | 9 (16.36%) |
| Unrelated to the intervention | ||
| Headache | 1 (1.82%) | 0 |
| Anorexia | 0 | 2 (3.63%) |
| Edema of the lower extremity | 0 | 1 (1.82%) |
| Nausea and vomiting | 2 (3.63%) | 5 (9.09%) |
| Related to the intervention | ||
| Localized allergic dermatitis (For the electrode) | 1 (1.82%) | 1 (1.82%) |
| Discomfort feeling (For the electric stimulation) | 2 (3.63%) | 0 |
4. Discussion
This trial investigated the effect of TEAS on postoperative cognitive function and quality of life in elderly patients for up to 12 months. We found that TEAS could significantly decrease the incidence of POCD, and improve pain and sleep after patients underwent thoracoscopic pulmonary resection for lung cancer. In addition, good recovery of brain health (three key intervention targets: cognition, pain, and sleep) may contribute to their long-term quality of life.
In this study, the factors that the average age of the sample was more than 70 years and a certain number of patients had a lower level of education were taken into consideration. To investigate the effect of TEAS on postoperative cognitive function, we performed neuropsychological tests using the MMSE and MoCA scales on the same day. The design could take advantage of the good specificity of the MMSE and the sensitivity of the MoCA in detecting mild cognitive impairment [17]. In addition, several clinical observations demonstrated that anesthesia, surgical stress, inflammation, and neurotoxicity might be conducive to POCD [23]. Therefore, all enrolled patients in this study underwent thoracoscopic pulmonary resection, which was characterized by fewer incisions and rapid recovery that could minimize individual differences. Furthermore, previous studies had shown that old age, preexisting cerebral, cardiac, and vascular diseases, alcohol abuse, a low level of education, and postoperative complications were risk factors for POCD [24]. After strict randomization, these variables were well-matched to reduce the probability of bias in this study. Consequently, we believe that our experimental protocol and data could be powered to support the effect of TEAS on cognitive function.
According to the longitudinal data source in the older patients, the basic tendency of the cognitive trajectories of patients in the two groups was consistent with the same analysis of patients enrolled in the Oxford Project to Investigate Memory and Ageing (OPTIMA) study [25]. Patients in the two groups experienced the worst cognition on the first day after surgery, and then a recovery trend was observed up to 30 days. It should be noted that the incidence of POCD in the TEAS group was dramatically decreased at all the other time points tested, while the values of the sham-TEAS group were always higher than 40%, a value similar to that observed in a previous study [24]. Similarly, we found the MMSE scores in the TEAS group almost returned to the baseline level on postoperative day 7, while the sham-TEAS group still had a gap with the baseline scores on postoperative day 30. Thus, we supposed that the TEAS intervention could help facilitate rapid recovery from cognitive decline in elderly patients who underwent thoracoscopic pulmonary resection.
A recent study highlighted the importance of sleep, pain, and cognition on the risk of perioperative brain health [26]. This study also collected NRS scores for pain and sleep as secondary outcomes within 30 days postoperatively. As a result, the symptoms of sleep and pain in the TEAS group were always better than in the sham-TEAS group at relative time points. Additionally, the present study confirmed that TEAS could significantly reduce the intraoperative consumption of remifentanil, which was in line with the aim of avoidance or minimization of opioids in the ERAS program [27]. These findings extended previous observations and supported the notion that opioids might be linked to disturbed sleep architecture and hyperalgesia postoperatively [28,29]. Alternatively, opioid-related side effects such as sedation or hallucinations might hurt cognitive function [30]. Given that there is a large degree of overlap between sleep disorders, pain, and cognitive decline, our novel findings may provide new insights into reaching an ideal recovery of perioperative brain health.
Over the past decades, clinical observations have demonstrated that pain, insomnia, and fatigue are the most frequently observed symptoms in elderly patients with lung cancer [31]. To explore the potential impacts of early postoperative cognitive function on patients' long-term life quality, we also observed the changes in these symptoms of EORTC-QLQ-C30 scores in patients at 6 months and 12 months. Similar to former studies, our study has shown that the rapid recovery of cognition facilitated by the TEAS intervention can significantly reverse the decline in quality of life, especially in terms of improved global function, emotional function, fatigue, and pain scores, even at 6 months [32]. It was consistent with previous observations that good cognitive function could help patients improve their self-esteem, set goals in their lives, and enjoy activities that help them to achieve better mental health [33]. In addition, this study was in agreement with previous research showing that TEAS could alleviate acute pain and prevent its transition to chronic pain after surgery [34]. Moreover, we speculated that these reciprocally beneficial interactions eventually promoted a better quality of life for the patients who received TEAS.
As is evidenced in previous studies, the potential functional mechanisms of TEAS on the human body depend on selective and specific acupoints. In particular, a recent study revealed the neuroanatomical basis for the acupoints in driving specific autonomic pathways, making the basic theoretical development of TEAS more robust and reliable [35]. It is also proposed by the theory of traditional Chinese medicine and proven in the current literature, the stimulation of the four combined acupoints [Neiguan (PC6), Hegu (LI4), Zusanli (ST36), and Baihui (DU20)] in this study could exhibit a good effect on neurological and mental system diseases [36,37]. Furthermore, the clinical effects of acupoint stimulation on improving postoperative cognitive function could be attributed to inhibiting inflammatory activity and regulating immunity in previous studies [38]. While the present study examined the effect and safety of TEAS on postoperative cognitive function and quality of life by a 12-month trial, future research should also look into potential interventions to avoid or control postoperative complications in elderly individuals as well as the mechanism underlying TEAS's effects.
As society ages, more and more patients require surgical treatment. Particularly with the development of minimally invasive techniques, there has been an expansion of surgical indications for older patients and those with more comorbidities. However, a high rate of postoperative complications, which present a considerable burden to families and caregivers, remains an obstacle to patient recovery. Given the seriousness of postoperative pain, sleep disorders, and cognitive dysfunction, a significant amount of research has focused on investigating effective pharmaceutical agents. However, all commonly used drugs, such as opioids, acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), gabapentin, benzodiazepine, parecoxib, statins, and dexmedetomidine, have various limitations and adverse effects [[39], [40], [41]]. For instance, opioid analgesics could significantly prevent acute pain, but the drugs might cause severe gastrointestinal reactions, cognitive impairment, and psychological dependence [42]. Therefore, interest in non-pharmacological strategies to manage postoperative problems is increasing.
Current non-pharmacological interventions for cognition cover a diverse and broad range of categories, including cognitive training, physical exercise, dietary treatments, art-oriented therapy, and reminiscence therapy [43]. Compared with these interventions, TEAS could not only decrease the incidence of POCD but also improve pain and sleep. The outcome of this paper enriched the basic theoretical framework of non-pharmacological treatment of postoperative complications and directed the future research of TEAS. Developing more personalized TEAS treatments that can be better integrated with patients' family therapy will be an unremitting pursuit of clinical medicine. To reach this goal, future efforts should focus on optimizing the operational simplicity of TEAS devices, implementing TEAS into various home care settings, and conducting longer follow-up periods in trials. Furthermore, improved knowledge of the functional mechanism of TEAS would help set standardized dosing parameters, which is of great significance for promoting its broader application.
4.1. Limitations
Some limitations should be considered in this study. Given the clinical heterogeneity of the collected data in terms of the participants recruited, setting time, and the prevalence of POCD in patients with lung cancer, the applicability of the findings in this study may be limited to patients who underwent minimally invasive approaches. In addition, although we prolonged the interval time between each test point appropriately, patients may develop a learning memory for the content of the MMSE and the MoCA which might influence the accuracy of the assessment. Furthermore, the research method of obtaining a single satisfactory result by controlling variables in small and medium-sized samples was limited in its comprehensiveness and ability to reflect real-world situations in complex medical environments. Future research should use larger, more varied samples and incorporate measurements of potential confounding factors to address the limitations of this study.
5. Conclusions
In conclusion, thoracoscopic surgery is now generally applied to elderly patients with lung cancer, but evidence from previous studies and our results have shown that poor sleep, pain, and cognitive decline might hamper their postoperative recovery. Within this context, the present study suggests that TEAS could be a promising technology for improving the postoperative cognitive function and long-term life quality of geriatric patients.
Ethics statement
This study's design was reviewed and approved by the Research Ethics Committee of the Second Affiliated Hospital of Air Force Medical University (NO.202006–03). All participants provided their written informed consent.
Author contribution statement
Xude Sun; Changjun Gao: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Lanlan Zheng; Yanzheng Wang; Yuting Yan; Chen Liu; Yuan Qin; Chen Yuan: Performed the experiments; Analyzed and interpreted the data.
Shuang Wang: Contributed reagents, materials, analysis tools or data.
Fei Guo; Ruili Han; Li Sun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
1 - Conceived and designed the experiments.
2 - Performed the experiments.
3 - Analyzed and interpreted the data.
4 - Contributed reagents, materials, analysis tools or data.
5 - Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Funding
This research was supported by the National Natural Science Foundation of China (No. Grant number 81971225) and the Clinical Research Project of the Air Force Medical University (No. 2021LC2207).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study would not have been possible without the enduring and passionate commitment of the whole team consisting of graduate students, anesthesiologists, anesthetists, and surgeons. In addition, we sincerely thank all the participating patients and their relatives afford any convenient communication during this trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19386.
Contributor Information
Xude Sun, Email: sunxude@fmmu.edu.cn.
Changjun Gao, Email: gaocj74@163.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Schabath M.B., Cote M.L. Cancer progress and priorities: lung cancer. Cancer Epidemiol. Biomarkers Prev. 2019;28:1563–1579. doi: 10.1158/1055-9965.EPI-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weerink L., van Leeuwen B.L., Gernaat S., Absalom A.R., Huisman M.G., van der Wal-Huisman H., Izaks G.J., de Bock G.H. Vitamin status and the development of postoperative cognitive decline in elderly surgical oncologic patients. Ann. Surg Oncol. 2018;25:231–238. doi: 10.1245/s10434-017-6118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batchelor T., Rasburn N.J., Abdelnour-Berchtold E., Brunelli A., Cerfolio R.J., Gonzalez M., Ljungqvist O., Petersen R.H., Popescu W.M., Slinger P.D., Naidu B. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (eras(r)) society and the european society of thoracic surgeons (ests) Eur. J. Cardio. Thorac. Surg. 2019;55:91–115. doi: 10.1093/ejcts/ezy301. [DOI] [PubMed] [Google Scholar]
- 5.Moller J.T., Cluitmans P., Rasmussen L.S., Houx P., Rasmussen H., Canet J., Rabbitt P., Jolles J., Larsen K., Hanning C.D., Langeron O., Johnson T., Lauven P.M., Kristensen P.A., Biedler A., van Beem H., Fraidakis O., Silverstein J.H., Beneken J.E., Gravenstein J.S. Long-term postoperative cognitive dysfunction in the elderly ispocd1 study. Ispocd investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y., Li X., Yao J. Develop a clinical prediction model for postoperative cognitive dysfunction after major noncardiac surgery in elderly patients: a protocol for a prospective observational study. Gerontology. 2022;68:538–545. doi: 10.1159/000517511. [DOI] [PubMed] [Google Scholar]
- 7.Steinmetz J., Christensen K.B., Lund T., Lohse N., Rasmussen L.S. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 8.Kotekar N., Shenkar A., Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin. Interv. Aging. 2018;13:2267–2273. doi: 10.2147/CIA.S133896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avidan M.S., Maybrier H.R., Abdallah A.B., Jacobsohn E., Vlisides P.E., Pryor K.O., Veselis R.A., Grocott H.P., Emmert D.A., Rogers E.M., Downey R.J., Yulico H., Noh G.J., Lee Y.H., Waszynski C.M., Arya V.K., Pagel P.S., Hudetz J.A., Muench M.R., Fritz B.A., Waberski W., Inouye S.K., Mashour G.A. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390:267–275. doi: 10.1016/S0140-6736(17)31467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radtke F.M., Franck M., Lendner J., Kruger S., Wernecke K.D., Spies C.D. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br. J. Anaesth. 2013;110(Suppl 1):i98–i105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 11.Deiner S., Liu X., Lin H.M., Jacoby R., Kim J., Baxter M.G., Sieber F., Boockvar K., Sano M. Does postoperative cognitive decline result in new disability after surgery? Ann. Surg. 2021;274:e1108–e1114. doi: 10.1097/SLA.0000000000003764. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Wen Q., Lu L., Hu H., He Y., Zhou Y., Wu X., Li N. Transcutaneous electrical acupoint stimulation combined with electroacupuncture for rapid recovery of patients after laparotomy for gastrointestinal surgery: a study protocol for a randomised controlled trial. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-053309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi Y.L., Zhang W.L., Yang F., Su F., Zhou Y.K. Transcutaneous electrical acupoint stimulation for improving postoperative recovery, reducing stress and inflammatory responses in elderly patient undergoing knee surgery. Am. J. Chin. Med. 2019;47:1445–1458. doi: 10.1142/S0192415X19500745. [DOI] [PubMed] [Google Scholar]
- 14.Feng B., Zhang Y., Luo L.Y., Wu J.Y., Yang S.J., Zhang N., Tan Q.R., Wang H.N., Ge N., Ning F., Zheng Z.L., Zhu R.M., Qian M.C., Chen Z.Y., Zhang Z.J. Transcutaneous electrical acupoint stimulation for post-traumatic stress disorder: assessor-blinded, randomized controlled study. Psychiatr. Clin. Neurosci. 2019;73:179–186. doi: 10.1111/pcn.12810. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Tu Q., Miao S., Zhou Z., Hu S. Transcutaneous electrical acupoint stimulation for preventing postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized controlled trials. Int. J. Surg. 2020;73:57–64. doi: 10.1016/j.ijsu.2019.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Braekhus A., Laake K., Engedal K. The mini-mental state examination: identifying the most efficient variables for detecting cognitive impairment in the elderly. J. Am. Geriatr. Soc. 1992;40:1139–1145. doi: 10.1111/j.1532-5415.1992.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Evered L., Silbert B., Knopman D.S., Scott D.A., Dekosky S.T., Rasmussen L.S., Oh E.S., Crosby G., Berger M., Eckenhoff R.G. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 2018;121:1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritmala-Castren M., Lakanmaa R.L., Virtanen I., Leino-Kilpi H. Evaluating adult patients' sleep: an integrative literature review in critical care. Scand. J. Caring Sci. 2014;28:435–448. doi: 10.1111/scs.12072. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez C.S. Pain measurement in the elderly: a review. Pain Manag. Nurs. 2001;2:38–46. doi: 10.1053/jpmn.2001.23746. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., de Haes J.C., Et A. The european organization for research and treatment of cancer qlq-c30: a quality-of-life instrument for use in international clinical trials in oncology. Jnci-J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22.Monk T.G., Weldon B.C., Garvan C.W., Dede D.E., van der Aa M.T., Heilman K.M., Gravenstein J.S. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 23.Ezhevskaya A.A., Ovechkin A.M., Prusakova Z.B., Zagrekov V.I., Mlyavykh S.G., Anderson D.G. Relationship among anesthesia technique, surgical stress, and cognitive dysfunction following spinal surgery: a randomized trial. J. Neurosurg. Spine. 2019:1–8. doi: 10.3171/2019.4.SPINE184. [DOI] [PubMed] [Google Scholar]
- 24.Rundshagen I. Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 2014;111:119–125. doi: 10.3238/arztebl.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel D., Lunn A.D., Smith A.D., Lehmann D.J., Dorrington K.L. Cognitive decline in the elderly after surgery and anaesthesia: results from the oxford project to investigate memory and ageing (optima) cohort. Anaesthesia. 2016;71:1144–1152. doi: 10.1111/anae.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Gara B.P., Gao L., Marcantonio E.R., Sleep B. Subramaniam. pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135:1132–1152. doi: 10.1097/ALN.0000000000004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H., Xie Y., Zhang Q., Xu N., Zhong H., Dong H., Liu L., Jiang T., Wang Q., Xiong L. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: a prospective, randomized, placebo-controlled trial. Br. J. Anaesth. 2014;112:1075–1082. doi: 10.1093/bja/aeu001. [DOI] [PubMed] [Google Scholar]
- 28.Fan Y., Yuan L., Ji M., Yang J., Gao D. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: a randomized controlled trial. J. Clin. Anesth. 2017;39:77–81. doi: 10.1016/j.jclinane.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Yao Y., Wu Y., Dai D., Zhao Q., Qiu L. Transcutaneous electric acupoint stimulation alleviates remifentanil-induced hyperalgesia in patients undergoing thyroidectomy: a randomized controlled trial. Int. J. Clin. Exp. Med. 2015;8:5781–5787. [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X., Gao X., Wang Z., Jiang X., Lu S., Xu J., Qin G., Gu Z., Huang D. Preoperative chronic and acute pain affects postoperative cognitive function mediated by neurotransmitters. J. Mol. Neurosci. 2021;71:515–526. doi: 10.1007/s12031-020-01673-x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman A.J., Given B.A., von Eye A., Gift A.G., Given C.W. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol. Nurs. Forum. 2007;34:785–792. doi: 10.1188/07.ONF.785-792. [DOI] [PubMed] [Google Scholar]
- 32.Hou L., Zhou C., Wu Y., Yu Y., Hu Y. Transcutaneous electrical acupoint stimulation (teas) relieved cancer-related fatigue in non-small cell lung cancer (nsclc) patients after chemotherapy. J. Thorac. Dis. 2017;9:1959–1966. doi: 10.21037/jtd.2017.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Stouwe E., Geraets C., Rutgers M., Veling W. Cognitive behavioral group treatment for low self-esteem in psychosis: a proof of concept study. BMC Psychiatr. 2021;21:567. doi: 10.1186/s12888-021-03579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Z., Wang Q., Sun X., Zhang W., Min S., Zhang J., Zhao W., Jiang J., Wang Y., Zhu Y., Zheng L., Wang Y., Guo Y., Zhang L., Wang L., Lei C., Liu T., Yang X., Zhang J., Li C., Zhang N., Dong H., Xiong L. Transcutaneous electrical acupoint stimulation before surgery reduces chronic pain after mastectomy: a randomized clinical trial. J. Clin. Anesth. 2021;74 doi: 10.1016/j.jclinane.2021.110453. [DOI] [PubMed] [Google Scholar]
- 35.Liu S., Wang Z., Su Y., Qi L., Yang W., Fu M., Jing X., Wang Y., Ma Q. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598:641–645. doi: 10.1038/s41586-021-04001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tida J.A., Catalao C., Garcia C., Dos S.A., Salmon C., Lopes L. Acupuncture at st36 exerts neuroprotective effects via inhibition of reactive astrogliosis in infantile rats with hydrocephalus. Acupunct. Med. 2018;36:386–393. doi: 10.1136/acupmed-2017-011515. [DOI] [PubMed] [Google Scholar]
- 37.Gao F., Zhang Q., Li Y., Tai Y., Xin X., Wang X., Wang Q. Transcutaneous electrical acupoint stimulation for prevention of postoperative delirium in geriatric patients with silent lacunar infarction: a preliminary study. Clin. Interv. Aging. 2018;13:2127–2134. doi: 10.2147/CIA.S183698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J., Peng W., Xu M., Li W., Liu Z. The effectiveness and safety of acupuncture for patients with alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltim.) 2015;94:e933. doi: 10.1097/MD.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Z., Chen F., Li W.A., Geng X., Li C., Meng X., Feng Y., Liu W., Yu F. A review of sleep disorders and melatonin. Neurol. Res. 2017;39:559–565. doi: 10.1080/01616412.2017.1315864. [DOI] [PubMed] [Google Scholar]
- 40.Small C., Laycock H. Acute postoperative pain management. Br. J. Surg. 2020;107:e70–e80. doi: 10.1002/bjs.11477. [DOI] [PubMed] [Google Scholar]
- 41.Liu B., Huang D., Guo Y., Sun X., Chen C., Zhai X., Jin X., Zhu H., Li P., Yu W. Recent advances and perspectives of postoperative neurological disorders in the elderly surgical patients. CNS Neurosci. Ther. 2022;28:470–483. doi: 10.1111/cns.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutlu Y.E., Araujo-Duran J., Turan A. Emerging drugs for the treatment of postsurgical pain. Expet Opin. Emerg. Drugs. 2021;26:371–384. doi: 10.1080/14728214.2021.2009799. [DOI] [PubMed] [Google Scholar]
- 43.Sikkes S., Tang Y., Jutten R.J., Wesselman L., Turkstra L.S., Brodaty H., Clare L., Cassidy-Eagle E., Cox K.L., Chetelat G., Dautricourt S., Dhana K., Dodge H., Droes R.M., Hampstead B.M., Holland T., Lampit A., Laver K., Lutz A., Lautenschlager N.T., Mccurry S.M., Meiland F., Morris M.C., Mueller K.D., Peters R., Ridel G., Spector A., van der Steen J.T., Tamplin J., Thompson Z., Bahar-Fuchs A. Toward a theory-based specification of non-pharmacological treatments in aging and dementia: focused reviews and methodological recommendations. Alzheimers. Dement. 2021;17:255–270. doi: 10.1002/alz.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.