Abstract
Promiscuous enzymes have shown their synthetic abilities in generating various organic compounds with high selectively and efficiency under mild conditions. Therefore, the design and development of conditions to raise promiscuity to the enzymes have been under the spotlight in recent years. Flavin reductase, that reduces flavins by using NADH as a cofactor, has not been studied in promiscuous reactions. In the present study, it was aimed to develop a catalytic promiscuous activity in the recombinant E.coli flavin reductase by removing its cofactor. The flavin reductase demonstrated a promiscuous activity for Knoevenagel condensation and Michael addition reactions individually. The cofactor-independent promiscuous activity of the flavin reductase was further enhanced by altering the reaction conditions to proceed a Knoevenagel-Michael addition cascade for tetraketone synthesis. Yet, the presence of the cofactor blocked the promiscuous Knoevenagel condensation, Michael addition, and therefore the cascade reaction, demonstrating that the removal of NADH was pivotal in inducing the promiscuous activity. Furthermore, molecular docking and MD simulations were performed to obtain more structural and mechanistic details of the transformation. The computational studies identified the most likely catalytic sites of the flavin reductase in the reaction. Additionally, a truncated variant of the enzyme that lacked 28 residues from the C-terminus displayed comparable activity to the wild-type enzyme, indicating the robustness of the enzyme in performing the cascade reaction. In brief, the cofactor-elimination method presented in this work could be considered as a straightforward and economical approach for inducing enzyme promiscuity in promoting organic transformations.
Keywords: Enzyme promiscuity, Biocatalyst, Flavin reductase, Knoevenagel condensation-Michael addition reaction, Tetraketone synthesis
Graphical abstract

1. Introduction
Enzymes are the most considerable proteins, which show their robust catalytic activities in synthetic chemistry as green, cheap, and selective catalysts. Albeit enzymes are well-known for their specificity, over recent years, it has been evidenced that many enzymes are capable of performing non-native reactions, called promiscuous reactions. Enzyme promiscuity, which has played a paramount role in the evolution of novel enzymes [[1], [2], [3], [4]], has been placed in three groups. The first group named catalytic promiscuity, occurs when the enzyme catalyzes a non-native catalytic activity. Many studies clearly have shown this type of promiscuity; for instance, lipases are the most studied enzymes in performing promiscuous reactions. Although the native catalytic activity of lipase is the formation of ester bond or its hydrolysis, lipases have displayed the ability to form carbon-carbon, heteroatom-heteroatom, and carbon-heteroatom bonds [[5], [6], [7], [8], [9], [10], [11], [12], [13]]. The second group, substrate promiscuity, occurs when the enzyme serves a non-native substrate. Oleate hydratase from Elizabethkingia meningoseptica catalyzes the hydration of non-natural substrates by using a decoy fatty acid molecule [14]. N-acetylhexosamine 1-kinase was shown to perform its catalytic activity on various N-acetylglucosamine [15]. When an enzyme performs the catalytic reaction under different conditions, the condition promiscuity takes place. The engineered protease subtilisin E was demonstrated to improve activity in organic media [16]. Besides of this categorization, often various types of promiscuities are merged. Indeed, one approach in developing promiscuity has been altering the cofactor for the cofactor-dependent enzymes. Previous studies have demonstrated that altering the cofactor of enzymes could induce promiscuity in them. As an example, carbonic anhydrase was shown to perform epoxide synthesis by exchanging Fe2+ to Mn2+ [17]. Additionally, it was reported that the replacement of zinc with rhodium could result in a promiscuous reductase activity for the stereoselective hydrogenation of olefins [18]. Catechol oxidase activity was achieved from zinc-dependent aminopeptidase via the introduction of Cu2+ [19]. Although considerable advances have been achieved, they have mostly been limited by substituting metal cofactors.
Reactions with the capability of C–C bond formation are widely used in different industries to synthesize value-added products; therefore, developing new strategies, which could promote this type of transformation in straightforward and eco-friendly manners, are always of great interest among researchers [[20], [21], [22], [23]]. Several enzymes have shown promiscuous activities in generating C–C bonds in recent years [[24], [25], [26]]. The first enzymatic asymmetric Mannich reaction was performed via protease type XIV from Streptomyces griseus. The mentioned enzyme was capable of catalyzing direct three component Mannich reaction with enantioselectivity up to 88% [27]. The three-to six-carbon aldose carbohydrates have been synthesized through tandem aldol reaction by engineered d-fructose-6-phosphate aldolase from Escherichia coli (E. coli) [28]. Recently, Ene-reductase demonstrated its promiscuous catalytic activity for performing the Knoevenagel condensation-reduction cascade; since the enzyme utilizes NADH as a cofactor, the glutamate dehydrogenase was used for in situ generation of the expensive cofactor [29]. Despite the dramatic progress, enzyme promiscuity suffers from limited substrate scope, cofactor dependency, and active site engineering; hence, improved biocatalytic systems which have a broader substrate scope with no cofactor dependency or further engineering and modifications are still in great demand.
Tetraketones are widely used as precursors for synthesizing diverse heterocyclic compounds such as xanthenediones, thioxanthenes, and acridinediones; these compounds are present in many natural and synthetic bioactive molecules [30]. Furthermore, tetraketones have shown some tyrosinase inhibitory properties [31]. Tetraketones are usually synthesized by a Knoevenagel condensation reaction followed by a Michael addition reaction. Various methods using catalysts including taurine [32], HClO4 –SiO2 [33], DABCO-based acidic organic salt [34], eutectic solvents [35], piperidine [36], proline [37], ZnCl2 [38], Fe3O4 @SiO2–SO3H [39], ultrasound [40], ZnAl2O4 [41] have been developed for synthesizing these compounds. Owing to the importance of these compounds, developing novel green approaches are still missing. The concept of enzyme promiscuity equips chemists to design and improve novel catalytic systems.
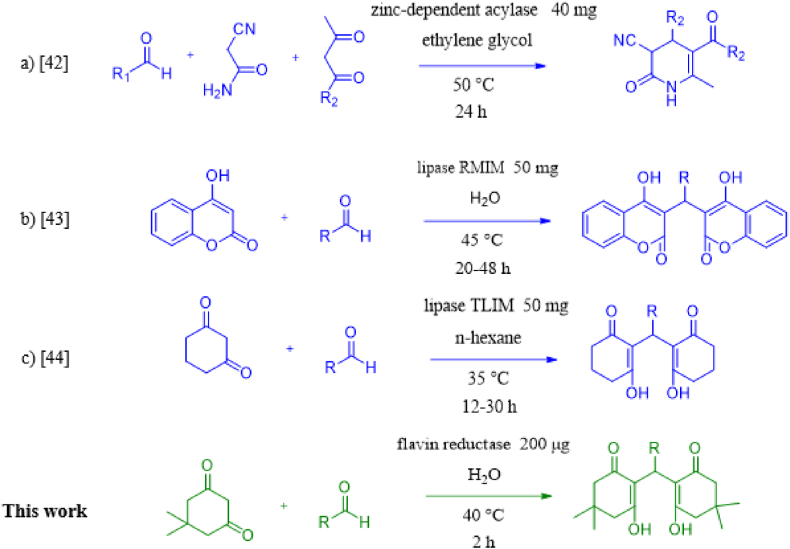
A few biocatalysts have been reported to perform a cascade reaction that entails Knoevenagel condensation and Michael addition in a row. A zinc-dependent acylase was shown to perform the Knoevenagel-Michael addition reactions between cyanoacetamide, aldehydes, and 1,3-dicarbonyl compounds in organic media [42]. A series of enzymes were screened for Knoevenagel-Michael addition cascade, and a Lipase RMIM was identified for catalyzing the enzymatic cascade for 4-hydroxycoumarin and aldehydes [43]. The commercially immobilized lipase TLIM was used to carry out the Knoevenagel-Michael addition cascade reactions of 1,3-diketones with aromatic aldehydes to produce xanthones [44]. The previous reported enzyme-catalyzed Knoevenagel-Michael cascade reactions also suffer from long reaction time, a relatively large amount of enzymes, and the dependency on metal cofactor in the case of the acylase (Scheme 1).
Scheme 1.
Introduction to previous and current work for enzymatic Knoevenagel-Michael addition cascade.
In humans, NAD(P)H flavin reductases are responsible for the reduction of flavins, biliverdins [45], and pyrroloquinoline quinone [46] in the presence of NAD(P)H. In bacteria flavin reductase is composed of two-component systems in which NAD(P)H flavin reductase is coupled with another enzyme such as halogenase [47,48] and monooxygenase [49]. However, in E. coli, flavin reductase participates in the reactivation of ribonucleotide reductase in which the reduced flavin reduces the Fe(III) of ribonucleotide reductase to Fe(II) [50]. Flavin reductase is found as a monomer in solution, but in the crystal form, the enzyme is found as tetramers in the asymmetric unit forming two dimers; the two dimers are linked together by a disulfide bond and each monomer is composed of 232 residues [51]. An ordered sequential mechanism is suggested for the NAD(P)H-dependent flavin reductase [52].
In the present work, catalytic promiscuous activity was induced in the recombinant NADH-dependent flavin reductase by removing the enzyme cofactor. This promiscuous activity led to Knoevenagel condensation and Michael addition reactions. The observed promiscuous activity was further improved for the Knoevenagel-Michael addition cascade, resulting the desired tetraketones from the corresponding C–C bond formation between aldehydes and 1,3-diketones. Furthermore, the promiscuous activity was honed by optimizing the reaction conditions without the need for any catalytic site engineering. Consequently, the promiscuous cascade reaction proceeds in mild and benign conditions, accompanied by an easy work-up process and broad substrate scope including aromatic, heterocyclic, and aliphatic aldehydes. In comparison to the previous enzymatic Knoevenagel-Michael addition cascade, the present work benefits from shorter reaction time, low enzyme-loading, aqueous media, lower temperature, and cofactor independency (Scheme 1). Moreover, molecular docking and molecular dynamics studies revealed the details about the involved residues and structural insights which raised a plausible mechanism. Additionally, due to the high cost of NADH and its regenerating systems, the deletion of such cofactors brought about affordability and simplicity in the reaction. Finally, the elimination of the cofactor could be a novel approach to induce promiscuity to the cofactor-dependent enzyme.
2. Materials and methods
All commercial reagents and solvents were used without further purification. Unless stated otherwise, all reagents and chemicals were purchased from Sigma-Aldrich®. Restriction enzymes, T4 ligase, Taq DNA polymerase, and Pfu DNA polymerase were obtained from Thermo Scientific. The DNA gel extraction and purification kit was acquired from Macherey-nagel. Unless otherwise mentioned, all NMR spectra were recorded on a Bruker 500 spectrometer (for 1H NMR 500 MHz and 13C NMR 125 MHz) and chemical shifts were reported as ppm. The 4-chlorobenzaldehyde derivative NMR spectra were recorded via Bruker 300 spectrometer (for 1H NMR 300 MHz and for 13C NMR 75 MHz). UV–visible analysis was carried out via Carey 100 Spectrophotometer. Sanger sequencing was performed at the sequencing lab at Microsynth's AG (Balgach, Switzerland).
2.1. Wild-type flavin reductase cloning
The gene encoding flavin reductase was amplified via polymerase chain reaction (PCR) using genomic DNA of the E. coli BL21 as a template and Pfu DNA polymerase. The used primers were designed based on the nucleotide sequence of the E. coli's flavin reductase (Accession No. M61182) :
Forward primer (NdeI): CGC CAT ATG ACA ACC TTA AGC TGT AAA
Reverse primer (XhoI): CCG CTC GAG TCA GAT AAA TGC AAA CGC
The amplified PCR fragments and vector pET-26b-(+) were first purified via a purification kit (Figure S2) then digested with NdeI and XhoI restriction enzymes at 37 °C for 2.5 h for each digestion step. 1 μL of alkaline phosphatase was then added to the mixture and incubated at 37 °C for 1 h. Subsequently, the fragments were extracted using a purification kit (Figure S1). The purified PCR product ligated to the NdeI and XhoI sites of the pET-26b-(+) via T4 ligase at 16 °C for 12 h, then transformed to the E. coli DH5α. Afterwards, the construct pET-26b-(+)/flavin reductase transferred to the E. coli BL21 as an expression strain. The DNA sequencing demonstrated no mutations in the cloned gene (Figure S4).
2.2. General procedure for tetraketone synthesis
Generally, 0.2 mmol of dimedone and 0.1 mmol of the aldehyde compound were transferred to the 10 ml round bottom flask and 1 ml di-H2O was added. 200 μg of the enzyme (dissolved in water) was added to the reaction mixture. The reaction stirred for 2 h at 40 °C. In the workup process, 3 ml di-H2O was added to the reaction mixture and was stirred for 10 min. The product was filtered and washed with di-H2O to remove the impurities. The obtained solid was then placed to dry in an oven at 60 °C.
3. Results and discussions
3.1. Screening for promiscuous activities using the flavin reductase
Since the native catalytic activity of the flavin reductase belongs to the oxidoreductase family of enzymes, promiscuous activity screening for the flavin reductase was first examined for a number of non-native organic redox reactions (Table S1). Each reaction was performed in the presence of NADH under different conditions. The recombinant enzyme showed no promiscuous activity towards examined redox reactions. Therefore, as a second strategy, eliminating the enzyme cofactor from the reaction condition, to reduce the steric hindrance of the cofactor in the catalytic pocket of the enzyme, was employed. Due to an unoccupied catalytic pocket, the enzyme would have enough space for the non-native substrates to enter the catalytic pocket. Assuming the effective interaction between the substrate and catalytic residues, the chance of promiscuous activity attainment would be increased. Based on the aforementioned concept, a number of non-redox organic reactions were examined in the absence of NADH (Table S1). Eventually, the E. coli flavin reductase showed promiscuous activity towards catalyzing the Michael addition and the Knoevenagel condensation. To study the effect of the enzyme on the Michael addition and Knoevenagel condensation reactions, some control reactions were performed, and it was shown that the enzyme catalyzed both reactions (Table 1, Table 2).
Table 1.
Evaluation of the effect of the flavin reductase as biocatalyst on the Knoevenagel condensation reaction. a.
| Entry | Catalyst | Buffer | Yield (%) b |
|---|---|---|---|
| 1 | Flavin reductase | No buffer | 56 |
| 2 | Uninduced cell lysate soup | Tris-HCl pH 8 | N.R. |
| 3 | Denaturated flavin reductase | No buffer | N.R |
| 4 | BSA | No buffer | N.R. |
| 5 | No catalyst | No buffer | N.R. |

Reaction condition: 4a (0.1 mmol), 2a (0.1 mmol), and flavin reductase (400 μg) in 2 ml dioxane at 40 °C for 24 h.
Isolated yield.
Table 2.
Evaluation of the effect of the flavin reductase as biocatalyst on the Michael addition reaction. a.
| Entry | Catalyst | Buffer | Yield (%) b |
|---|---|---|---|
| 1 | Flavin reductase | No buffer | 100 |
| 2 | Uninduced cell lysate soup | Tris-HCl pH 8 | 33 |
| 3 | Denaturated flavin reductase | No buffer | 35 |
| 4 | BSA | No buffer | 32 |
| 5 | No catalyst | No buffer | 33 |
Reaction condition: 1a (0.1 mmol), 6a (0.1 mmol), and flavin reductase (200 μg) in 2 ml ethanol at 40 °C for 15 h.
isolated yield.
As regards to the ability of the enzyme to catalyze both Knoevenagel condensation and Michael addition reactions separately, the ability of the enzyme in catalyzing both reactions in a cascade was questioned. However, the promiscuous activity of the flavin reductase towards tetraketone synthesis through the Knoevenagel-Michael addition cascade was investigated, and it was realized that the tetraketone formation reaction between the active methylene and an aldehyde compound was catalyzed by the E. coli flavin reductase.
3.2. Promiscuous activity of the flavin reductase: tetraketone synthesis
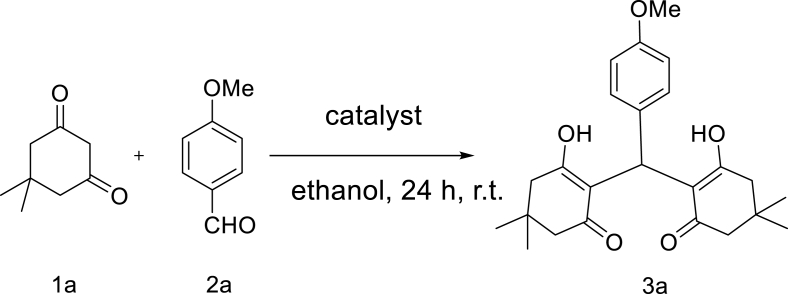
The promiscuous activity of the enzyme towards tetraketones synthesis was further investigated. The reaction between 4-methoxybenzaldehyde and dimedone was chosen as a model reaction. Surprisingly the enzymatic reaction resulted in a 36% yield. To explore the effect of the enzyme, the selected model reaction was examined under different conditions as shown in Table 3, the control reaction in the absence of the enzyme resulted in a much lesser amount of the product (Table 3, entry 6). To verify whether the 3D shape of the enzyme (enzyme native folding) was responsible for catalyzing the reaction , a reaction in the presence of the denatured enzyme was conducted. In comparison with the active enzyme, the deactivated enzyme resulted in a much lesser amount of the product which was almost as same as the control reaction (Table 3, entries 4 and 6), making it clear that the native folding of the enzyme plays a pivotal role in proceeding the reaction. However, in order to see the effect of the residues which are on the surface of the enzyme or any other contaminants in the solution, two more control reactions (Table 3, entries 3 and 5) were performed, and the results demonstrated that possible contaminants had no catalytic effect on the reaction.
Table 3.
Evaluation of the effect of the flavin reductase as biocatalyst. a.
| Entry | Catalyst | Buffer | Yield (%) b |
|---|---|---|---|
| 1 | Flavin reductase | Tris-HCl pH 7 | 36 |
| 2 | Flavin reductase | No buffer | 35 |
| 3 | Uninduced cell lysate soup | Tris-HCl pH 8 | 23 |
| 4 | Denaturated flavin reductase | No buffer | 17 |
| 5 | BSA | No buffer | 15 |
| 6 | No catalyst | No buffer | 18 |
reaction condition: 1a (0.2 mmol), 2a (0.1 mmol) and 100 μg catalyst in 2 ml solvent at room temperature for 24 h.
isolated yield.
3.3. Optimization of reaction conditions: solvent, temperature, time and catalyst-loading
The model reaction was used to investigate the effect of various solvents on the enzymatic reaction. As seen in Table 4, polar solvents afforded higher yields. Nonetheless, water seemed to be the best solvent for the model reaction, giving an 81% reaction yield (Table 4, entry 6). The significant increase in the enzymatic reaction yield, in water, probably is due to the better activation of the enzyme even though it might decrease the reactants' solubility.
Table 4.
Evaluation of the effect of the solvent on the yield of the reaction. a.
| Entry | Catalyst | solvent | Yield (%) b |
|---|---|---|---|
| 1 | Flavin reductase | DMSO | 49 |
| 2 | Flavin reductase | Ethylene glycol | 61 |
| 3 | Flavin reductase | Ethanol | 34 |
| 4 | Flavin reductase | Methanol | 63 |
| 5 | Flavin reductase | Octanol | Trace |
| 6 | Flavin reductase | H2O | 81 |
| 7 | Flavin reductase | PEG-400 | 59 |
| 8 | Flavin reductase | Acetonitrile | 51 |
| 9 | – | H2O | 23 |
reaction condition: 1a (0.2 mmol), 2a (0.1 mmol) and 100 μg catalyst in 2 ml solvent at room temperature for 24 h.
isolated yield.
The effect of the temperature on the model reaction was also investigated. Three different temperatures: room temperature, 40 °C, and 55 °C were examined for the progression of the model reaction. The best result was achieved at 40 °C (Table 5, entry 2). The yield for the control reaction at 40 °C, in the absence of the enzyme, turned out to be only 20% (Table 5, entry 4).
Table 5.
Evaluation of the effect of the temperature on the yield of the reaction. a.
| Entry | Catalyst | Temperature | Yield (%) b |
|---|---|---|---|
| 1 | Flavin reductase | Room temperature | 80 |
| 2 | Flavin reductase | 40 °C | 88 |
| 3 | Flavin reductase | 55 °C | 67 |
| 4 | – | 40 °C | 20 |
reaction condition: 1a (0.2 mmol), 2a (0.1 mmol) and 100 μg catalyst in 2 ml H2O at mentioned temperature for 24 h.
isolated yield.
Furthermore, the model reaction was studied to determine the optimal time for the reaction. The best yield was achieved in 15 h (Table 6, entry 7); however, in 2 h (Table 6, entry 4) a notable yield (76%) was obtaied. Therefore, due to the high yield in this short time; the 2 h reaction time was selected as the optimal time. The reaction with no catalyst resulted in only 17% of the product in 2 h (Table 6, entry 8). It was noteable that alternating reaction conditions largely affected the enzyme performance with no need for active site modification or any protein engineering.
Table 6.
Evaluation of the effect of the time on the yield of the reaction. a.
| Entry | Catalyst | Time | Yield (%) b |
|---|---|---|---|
| 1 | Flavin reductase | 15 min | 19 |
| 2 | Flavin reductase | 45 min | 20 |
| 3 | Flavin reductase | 1.5 h | 35 |
| 4 | Flavin reductase | 2 h | 76 |
| 5 | Flavin reductase | 5 h | 76 |
| 6 | Flavin reductase | 8 h | 77 |
| 7 | Flavin reductase | 15 h | 88 |
| 9 | – | 2 h | 17 |
reaction condition: 1a (0.2 mmol), 2a (0.1 mmol) and 100 μg catalyst in 2 ml H2O at 40 °C for mentioned time.
isolated yield.
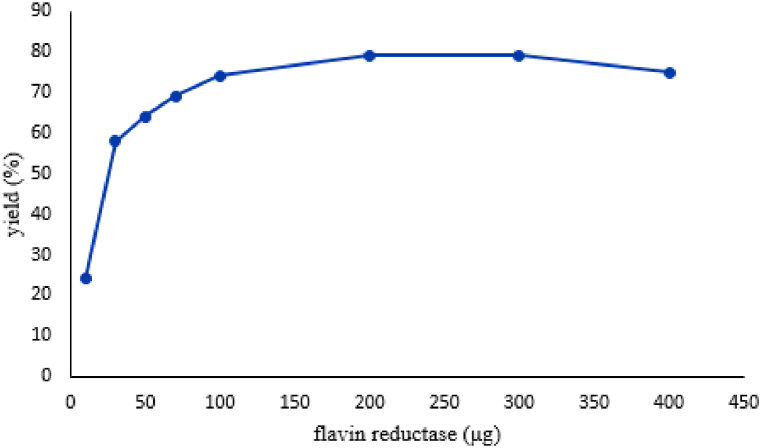
Afterwards, the effect of E. coli flavin reductase loading was studied in the reaction (Fig. 1). To this end, the model reaction was performed with different amounts of the enzyme. The best result was achieved when 200 μg of flavin reductase was used (Table 7, entry 2). A further increase in the catalyst loading not only did not improve the yield but also decreased it. Surprisingly, the model reaction progressed with a negligible amount of enzyme in a significant yield. It must be mentioned that when comparing the loading of the E. coli flavin reductase to other biocatalysts that perform similar reactions [[42], [43], [44]], the reductase catalyzed the tetraketone synthesis almost 100 times less loading.
Fig. 1.
The effect of the flavin reductase loading on the yield of the cascade reaction. Reaction condition: 1a (0.2 mmol), 2a (0.1 mmol) and catalyst in 2 ml H2O at 40 °C for 2 h.
Table 7.
Evaluation of the effect of the catalyst loading on the yield of the reaction. a.
| Entry | Catalyst loading (μg) | Yield (%) b |
|---|---|---|
| 1 | 10 | 24 |
| 2 | 30 | 58 |
| 3 | 50 | 64 |
| 4 | 70 | 69 |
| 5 | 100 | 74 |
| 6 | 200 | 79 |
| 7 | 300 | 79 |
| 49 | 400 | 75 |
reaction condition: 1a (0.2 mmol), 2a (0.1 mmol) and catalyst in 2 ml H2O at 40 °C for 2 h.
isolated yield.
3.4. Substrate scope for the reaction
To investigate the scope of the promiscuous reaction performed by the flavin reductase, a wide range of aldehydes including aliphatic, heterocyclic, and aromatic compounds bearing both electron-withdrawing and electron-donating groups were scrutinized in the reaction (Table 8). It was determined that flavin reductase could embrace various aldehydes as the non-native substrates in the reaction, suggesting a great potential for promiscuity of the enzyme in organic reactions. As expected, when electron-donating groups (Table 8, entries 1 and 2) were used, the yield increased in comparison with electron-withdrawing groups (Table 8, entries 5 and 7). A noticeable decrease in the yield of the reaction was observed when chlorinated benzaldehyde was used as an electrophile in the reaction (Table 8, entries 8 and 9). Likewise, in the case of aliphatic aldehyde, showing 40% yield, the reaction furnished moderate amounts of the corresponding product (Table 8, entry 14). Furthermore, to study the capability of enzyme in catalyzing double-site aldehydes, terephthalaldehyde was used in the reaction and the biocatalytic transformation yielded only the symmetric product with both sites involved in the reaction (Table 8, entry11), and in comparison with a previous report [53], a mixture of products was not detected under our condition. Heterocyclic aldehydes as important building blocks in synthetic organic chemistry were also explored in the transformation and proceeded with moderate to excellent yield (Table 8, entries 12 and 13). Furfural, in particular, as a known molecule in biomass upgrading conversions, turned into the corresponding product with an acceptable high yield in the reaction (Table 8, entry 13). The exact structure of the product from salicylaldehyde (Table 8, entry 4) as the substrate of the reaction has been left in doubt (Scheme S1). A recent report [54], has suggested the formation of the corresponding tetraketone derivative although its NMR pattern was completely different from other obtained derivatives. To clarify this ambiguity, X-ray crystallography of this product (3d) was obtained (Fig. 2). The resulting structure confirmed the formation of the corresponding xanthone skeleton instead of teraketone derivatives. When the linear diketone (acetylacetone) was used, the enzyme only catalyzed the Knoevenagel condensation reaction and did not give the desired tetraketone product.
Table 8.
Reaction condition: 1a (0.2 mmol), 2 (0.1 mmol) and catalyst (200 μg) in 2 ml H2O at 40 °C for 2 h.
Isolated yield. The products were confirmed by H-NMR and C-NMR (Figures S13-S42).
Fig. 2.
Molecular structure obtained from X-ray crystallography of the compound 3d with atom numbering scheme.
3.5. Investigating the mechanism for the model reaction
To explore the effect of the NADH on the enzymatic cascade, the model reaction was performed in the presence of equimolar quantities of NADH, dimedone, and 4-methoxybenzaldehyde (Table 9). When the enzymatic promiscuous reaction was performed in the presence of the NADH, a trace amount of the product was afforded (Table 9, entry 1). Since the yield of this reaction turned out to be much lower than the reaction with no enzyme and NADH (entry 4), a control reaction was set in the presence of NADH and the absence of the enzyme in order to elucidate the inhibitory role of the NADH (Table 9, entry 3); only 8% yield for the reaction was obtained. By comparing entries 3 and 4, it may be deduced that the cofactor inhibits the non-enzymatic tetraketone formation as well as the enzymatic tetraketone formation. In other words, the inhabitation of enzymatic tetraketone formation reaction most probably pertains to the binding of the cofactor in the binding site of the flavin reductase, excluding the reactants binding to the catalytic pocket.
Table 9.
The effect of NADH on the reaction.
| Entry | Cofactor | Catalyst | Yield (%) d |
|---|---|---|---|
| 1a | NADH | Flavin reductase | trace |
| 2b | – | Flavin reductase | 79 |
| 3c | NADH | – | 8 |
| – | 15 |
Reaction condition: 1a (0.1 mmol), 2a (0.05 mmol) in 1 ml H2O at 40 °C for 2 h.
NADH (0.05 mmol) and flavin reductase (200 μg).
Flavin reductase (200 μg).
NADH (0.05 mmol).
isolated yield.
To investigate the mechanism of the promiscuous enzymatic cascade, the ability of the enzyme to catalyze Knoevenagel condensation and Michael addition reactions separately, was studied in the presence of the NADH. As shown in Table 10, the Knoevenagel condensation reaction was catalyzed by the E. coli flavin reductase resulting in 28% yield (Table 10, entry 1) whereas in the absence of the enzyme, no product was observed (Table 10, entry 2). Interestingly, when NADH was included in the enzymatic Knoevenagel reaction, no product was detected (Table 10, entry 3), alluding to the inhibitory role of NADH. Furthermore, the Michael addition , using dimedone and an α,β-unsaturated compound was studied (Table 11). Intriguingly, the enzymatic Michael addition reaction produced the desired product with a 100% yield (Table 11, entry 1). However, the enzymatic reaction carried out in the presence of NADH and followed by TLC, similar to the control reaction 33% yield was obtained and no side product was detected (Table 11, entry 3). These findings could demonstrate that NADH plays an inhibitory role for both reactions.
Table 10.
The Knoevenagel condensation reaction. a.
Reaction comdition: 4a (0.1 mmol), 2a (0.1 mmol), and flavin reductase (200 μg) in 2 ml dioxane at 40 ◦ C for 15 h.
NADH (0.1 mmol).
no cofactor and no enzyme.
isolated yield.
Table 11.
The Michael addition reaction. a.
Reaction condition: 1a (0.1 mmol), 6a (0.1 mmol), and flavin reductase (200 μg) in 2 ml ethanol at 40 °C for 15 h.
NADH (0.1 mmol).
no cofactor and no enzyme.
isolated yield.
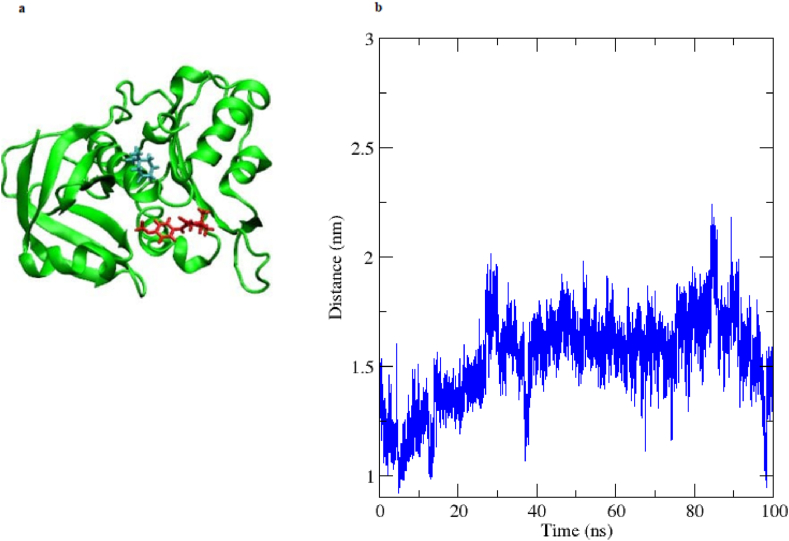
To further explore the NADH inhibitory mechanism of the enzymatic tetraketone synthesis, molecular docking was performed. The substrates and NADH were docked separately onto the E. coli flavin reductase. The docking illustrated that the two dimedone molecules and the aldehyde molecule were found to bind in the cavity to which NADH was also bound (Fig. 3a and b). These overlapping binding sites can explain the inhibitory effect of NADH on the tetraketone formation reaction and confirm the experimental results.
Fig. 3.
a) Two dimedone molecules and 4-methoxybenzaldehyde b) The NADH cofactor molecule docked into the E. coli flavin reductase structure (PDB ID: 1QFJ).
The docking results also indicated that the two dimedone molecules were located at both sides of the 4-methoxybenzaldehyde with a distance of 8.3 Å and 7.8 Å (Fig. 4a). Indeed, a hydrogen bonding at 2.7 Å between Ser 49 and dimedone carbonyl oxygen, could stabilize the dimedone in the binding site. 4-methoxybenzaldehyde was found to be stabilized and activated by three hydrogen bonds with Tyr 146, Ser 115 (side chain), and Ser 115 (main chain) (Fig. 4b).
Fig. 4.
Two dimedone and 4-methoxybenzaldehyde molecules docked into the flavin reductase structure (PDB ID: 1QFJ). a) The distance between α-carbon of the dimedone and the electrophilic carbon of 4-methoxybenzaldehyde. b) The interactions between substrates and catalytic pocket.
To gain more details on the mechanism of the model reaction, Molecular Dynamics (MD) simulation was employed. The simulations, were performed for 100 ns for the free enzyme, enzyme docked with two dimedone molecules and 4-methoxybenzaldehyde, and the enzyme docked with Knoevenagel adduct and a dimedone molecule. As shown in Figure S8, the root mean square deviation (RMSD) for enzyme-substrates complex displayed less variation, alluding to more stability in the presence of the substrate. The protein gyration, in the presence of substrates, changed more significantly indicating better folding (Figure S9). The number of H-bonding between the enzyme and substrates were also examined during the simulation. The results demonstrated a consistent pattern of H-bonding between the enzyme and the substrates throughout the simulation (Figure S10). Indeed, a snapshot at 100 ns demonstrated the same pattern of H-bonding as at the beginning of the simulation, underpinning the role of stable H-bonding between the enzyme and substrates. As shown in Fig. 5b, the average distance between the electrophilic carbon (from the aldehyde) and the nucleophilic carbon (from dimedone) decreased to 1.1 Å as simulation progressed, indicating a meaningful approach for forming the desired bond. During the 100 ns MD simulation, the second dimedone molecule came out from the cavity of the flavin reductase at 10 ns and the first dimedone molecule and 4-methoxbenzaldehyde stayed in the cavity (Fig. 5a), suggesting that at first probably only one dimedone and 4-methoxybenzaldehyde were bound to the protein so that the Knoevenagel reaction could take place. When the MD simulation for the Knoevenagel adduct and dimedone was analyzed, both substrates stayed in the cavity of the flavin reductase during the 100 ns simulation (Fig. 6a). It was determined that duing the simulation, the Knoevenagel adduct and dimedone had an average distance of about 1.53 Å (Fig. 6b).
Fig. 5.
a) Snapshot at 100 ns of simulation for the dimedone-4-methoxybenzaldehde-dimedone-flavin reductase complex, first dimedone molecule (pink), second dimedone molecule (cyan) and 4-methoxybenzaldehyde (purpule) (PDB ID: 1QFJ). b) The distance between the nucleophilic C of the dimedone and the electrophilic C of the 4-methoxybenzaldehyde during 100 ns simulation.
Fig. 6.
a) Snapshot at 100 ns of simulation for the Knoevenagel adduct-dimedone-flavin reductase complex, dimedone molecule (cyan) and Knoevenagel adduct (red) (PDB ID: 1QFJ). b) The distance between the nucleophilic C of the dimedone and the electrophilic C of the Knoevenagel adduct during 100 ns simulation.
Based on experiments reported in the present study, molecular docking and MD simulation, H-bond assisted enol formation mechanism is the most plausible mechanism for tetraketone synthesis (Scheme 2). The mechanism suggests that two H-bonds between the first dimedone molecule and Ser 49 assist the enol formation. Additionally, the oxygen atom of the Asp 227, located at a distance of 4.3 Å, could aid the dimedone deprotonating. On the other hand, the carbonyl of the aldehyde molecule is activated by the three H-bonds. and it becomes capable of accepting the nucleophile. The protonated Asp 227 probably donates its proton to the compound (III), producing compound (IV). After protonation and dehydration of compound (IV), the Knoevenagel adduct (V) would be generated and is stabilized by the three H-bonds (Figure S11). Furthermore, the H-bonds with Thr 16 and Thr 112 activate the Knoevenagel adduct for the 1,4-additition reaction. The second dimedone molecule enters the catalytic pocket of the flavin reductase and is stabilized by its two H-bonds as similar to the first dimedone molecule. The H-bonds once again could assist the enol formation. The dimedone in enol form would attack the β-carbon of the Knoevenagel adduct giving rise to the desired compound.
Scheme 2.
Proposed mechanism for tetraketone formation by E. coli flavin reductase.
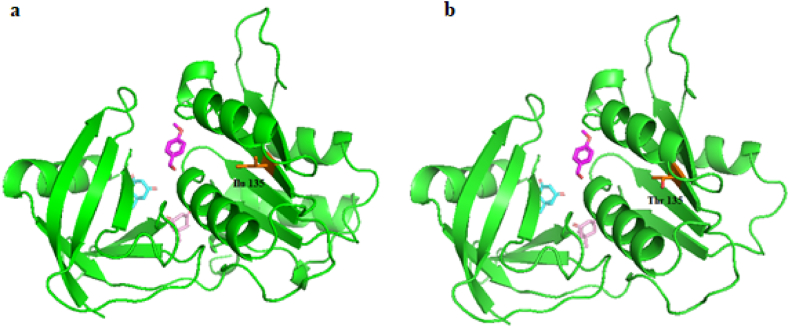
During the process of cloning of the E. coli reductase, using Taq polymerase accidentally, a mutated version of the reductase was cloned (Fig. 7). After DNA sequencing (Figure S5), it was determined that the cloned gene had one point mutation Ile135T and a premature stop codon resulting in a truncated enzyme lacking the last 28 amino acids (Fig. 7a and b). The mutated enzyme was expressed in E. coli as inclusion bodies. After solubilization and activation by using urea gradient, its size and activity were examined. As shown in Figure S12, the truncated enzyme as compared to the wild-type moved faster in electrophoresis, demonstrating its truncated size. Fig. 8 compares a turnover frequency (TOF) of the wild-type and the truncated flavin reductase; interestingly, the activity of truncated enzyme was similar to the wild-type enzyme, indicating that such a severe truncation and a point mutation did not affect the activity of the enzyme in performing the promiscuous reaction.
Fig. 7.
The first dimedone (pink), second dimedone (cyan) and 4-methoxyenzaldehyde (purple) docked into the a) the wild-type flavin reductase. b) The mutated flavin reductase (PDB ID: 1QFJ).
Fig. 8.
The TOF of the wild-type and truncated flavin reductase.
Some of the limitations of the present study relate to the production and the activity of the enzyme. The production of the recombinant enzyme was time-consuming process, and the structural stability of the enzyme was influenced with respect to the operating temperature. Furthermore, the activity of the enzyme decreased over time. The homogenous character of the recombinant enzyme challenged the recycling of the biocatalyst.
4. Conclusion
In the present study, E. coli flavin reductase was used to catalyze the Knoevenagel condensation and the Michael addition reactions, and the promiscuous activity of the flavin reductase was further explored in cascade tetraketone synthesis reactions that entailed Knoevenagel condensation and Michael addition reactions. The above cascade reaction was only possible when NADH was omitted from the reaction. The scope of substrate includes aromatic, heterocyclic, and aliphatic aldehydes. The product of the reaction with salicylaldehyde, confirmed by X-ray crystallography, as a xanthone derivative. Using computational approaches, the roles that the binding residues could play in the mechanism of the reaction, were put forward. A truncated version of the reductase , lacking 28 amino acids at the C-terminus, did not lose activity as compared to the wild-type, indicating the robustness of the enzyme in performing the promiscuous reaction. Omitting the natural cofactor of enzymes in order to induce promiscuity could be a novel approach based on the present study. Since the approach taken in this study proceeds in a green reaction condition and under moderate temperature it can be considered a highly eco-friendly method for synthesizing tetraketon derivatives.
Author contribution statement
Amene Navaser: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fatemeh Hayati: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Hamid R. Kalhor: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Hamid R. Kalhor reports administrative support was provided by Sharif University of Technology. Amene Navaser reports administrative support was provided by Sharif University of Technology. Fatemeh Hayati reports administrative support was provided by Sharif University of Technology. Hamid R. Kalhor reports a relationship with Sharif University of Technology that includes: employment. NO other activity.
Acknowledgments
The authors would like to thank Morteza Hasani for his critical reading of the manuscript. They also thank the Sharif University of Technology Research Council for financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19315.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Copley S.D. Shining a light on enzyme promiscuity. Curr. Opin. Struct. Biol. 2017;47:167–175. doi: 10.1016/j.sbi.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bornscheuer U.T., Kazlauskas R.J. Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. 2004;43(45):6032–6040. doi: 10.1002/anie.200460416. [DOI] [PubMed] [Google Scholar]
- 3.Babtie A., Tokuriki N., Hollfelder F. What makes an enzyme promiscuous? Curr. Opin. Chem. Biol. 2010;14(2):200–207. doi: 10.1016/j.cbpa.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Lukowski A.L., et al. Substrate promiscuity of a paralytic shellfish toxin amidinotransferase. ACS Chem. Biol. 2020;15(3):626–631. doi: 10.1021/acschembio.9b00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhake K.P., et al. Promiscuous Candida Antarctica lipase B-catalyzed synthesis of β-amino esters via aza-Michael addition of amines to acrylates. Tetrahedron Lett. 2010;51(33):4455–4458. [Google Scholar]
- 6.Hua X., Xing Y., Zhang X. Enhanced promiscuity of lipase-inorganic nanocrystal composites in the epoxidation of fatty acids in organic media. ACS Appl. Mater. Interfaces. 2016;8(25):16257–16261. doi: 10.1021/acsami.6b05061. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor M., Gupta M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012;47(4):555–569. [Google Scholar]
- 8.Giorno L., et al. Influence of OR ester group length on the catalytic activity and enantioselectivity of free lipase and immobilized in membrane used for the kinetic resolution of naproxen esters. J. Catal. 2007;247(2):194–200. [Google Scholar]
- 9.Koszelewski D., Ostaszewski R. Biocatalytic promiscuity of lipases in carbon‐phosphorus bond formation. ChemCatChem. 2019;11(10):2554–2558. [Google Scholar]
- 10.Svedendahl M., Hult K., Berglund P. Fast carbon− carbon bond formation by a promiscuous lipase. J. Am. Chem. Soc. 2005;127(51):17988–17989. doi: 10.1021/ja056660r. [DOI] [PubMed] [Google Scholar]
- 11.Svedendahl M., et al. Suppressed native hydrolytic activity of a lipase to reveal promiscuous Michael addition activity in water. ChemCatChem. 2009;1(2):252–258. [Google Scholar]
- 12.Wikmark Y., Svedendahl Humble M., Bäckvall J.E. Combinatorial library based engineering of Candida Antarctica lipase A for enantioselective transacylation of sec‐alcohols in organic solvent. Angew. Chem. 2015;127(14):4358–4362. doi: 10.1002/anie.201410675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwivedee B.P., et al. Promiscuity of lipase‐catalyzed reactions for organic synthesis: a recent update. ChemistrySelect. 2018;3(9):2441–2466. [Google Scholar]
- 14.Demming R.M., et al. Optimized reaction conditions enable the hydration of non‐natural substrates by the oleate hydratase from Elizabethkingia meningoseptica. ChemCatChem. 2017;9(5):758–766. [Google Scholar]
- 15.Li Y., et al. Substrate promiscuity of N-acetylhexosamine 1-kinases. Molecules. 2011;16(8):6396–6407. doi: 10.3390/molecules16086396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K., Arnold F.H. Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide. Proc. Natl. Acad. Sci. USA. 1993;90(12):5618–5622. doi: 10.1073/pnas.90.12.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández‐Gacio A., et al. Transforming carbonic anhydrase into epoxide synthase by metal exchange. Chembiochem. 2006;7(7):1013–1016. doi: 10.1002/cbic.200600127. [DOI] [PubMed] [Google Scholar]
- 18.Jing Q., Okrasa K., Kazlauskas R.J. Stereoselective hydrogenation of olefins using rhodium‐substituted carbonic anhydrase—a new reductase. Chem.--Eur. J. 2009;15(6):1370–1376. doi: 10.1002/chem.200801673. [DOI] [PubMed] [Google Scholar]
- 19.da Silva G.F., Ming L.-J. Catechol oxidase activity of di-Cu2+-substituted aminopeptidase from Streptomyces griseus. J. Am. Chem. Soc. 2005;127(47):16380–16381. doi: 10.1021/ja056034u. [DOI] [PubMed] [Google Scholar]
- 20.Sheldon R.A., Woodley J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018;118(2):801–838. doi: 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- 21.Hu C., et al. Carbon‐based metal‐free catalysts for energy storage and environmental remediation. Adv. Mater. 2019;31(13) doi: 10.1002/adma.201806128. [DOI] [PubMed] [Google Scholar]
- 22.Yadav C., Payra S., Moorthy J.N. Ionic porous organic polymer (IPOP) based on twisted biphenyl Scaffold: green and efficient heterogeneous catalytic synthesis of β-Arylthioketones and biscoumarins. J. Catal. 2022;413:769–778. [Google Scholar]
- 23.Liu X., Dai L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016;1(11):1–12. [Google Scholar]
- 24.Miao Y., et al. Recent developments in enzyme promiscuity for carbon–carbon bond-forming reactions. Curr. Opin. Chem. Biol. 2015;25:115–123. doi: 10.1016/j.cbpa.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Guengerich F.P., Yoshimoto F.K. Formation and cleavage of C–C bonds by enzymatic oxidation–reduction reactions. Chem. Rev. 2018;118(14):6573–6655. doi: 10.1021/acs.chemrev.8b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zetzsche L.E., Narayan A.R. Broadening the scope of biocatalytic C–C bond formation. Nat. Rev. Chem. 2020;4(7):334–346. doi: 10.1038/s41570-020-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue Y., et al. Protease-catalysed direct asymmetric Mannich reaction in organic solvent. Sci. Rep. 2012;2(1):1–4. doi: 10.1038/srep00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szekrenyi A., et al. Asymmetric assembly of aldose carbohydrates from formaldehyde and glycolaldehyde by tandem biocatalytic aldol reactions. Nat. Chem. 2015;7(9):724–729. doi: 10.1038/nchem.2321. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., et al. Biosynthesis of α-substituted β-ketoesters via the tandem Knoevenagel condensation–reduction reaction using a single enzyme. ACS Sustain. Chem. Eng. 2020;8(22):8206–8213. [Google Scholar]
- 30.Yu J.-J., et al. Synthesis of tetraketones in water and under catalyst-free conditions. Green Chem. 2010;12(2):216–219. [Google Scholar]
- 31.Khan K.M., et al. Tetraketones: a new class of tyrosinase inhibitors. Bioorg. Med. Chem. 2006;14(2):344–351. doi: 10.1016/j.bmc.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Shirini F., Daneshvar N. Introduction of taurine (2-aminoethanesulfonic acid) as a green bio-organic catalyst for the promotion of organic reactions under green conditions. RSC Adv. 2016;6(111):110190–110205. [Google Scholar]
- 33.Kantevari S., Bantu R., Nagarapu L. HClO4–SiO2 and PPA–SiO2 catalyzed efficient one-pot Knoevenagel condensation, Michael addition and cyclo-dehydration of dimedone and aldehydes in acetonitrile, aqueous and solvent free conditions: scope and limitations. J. Mol. Catal. Chem. 2007;269(1–2):53–57. [Google Scholar]
- 34.Ziyaei Halimehjani A., Barati V., Karimi M. Synthesis of a novel tetracationic acidic organic salt based on DABCO and its applications as catalyst in the Knoevenagel condensation reactions in water. Synth. Commun. 2019;49(5):724–734. [Google Scholar]
- 35.Azizi N., Dezfooli S., Hashemi M.M. Chemoselective synthesis of xanthenes and tetraketones in a choline chloride-based deep eutectic solvent. Compt. Rendus Chem. 2013;16(11):997–1001. [Google Scholar]
- 36.Chung T.-W., et al. Multicomponent synthesis of functionalized tetrahydroacridinones: insights into a mechanistic route. Org. Lett. 2015;17(21):5368–5371. doi: 10.1021/acs.orglett.5b02705. [DOI] [PubMed] [Google Scholar]
- 37.Ramachary D.B., Kishor M. Organocatalytic sequential one-pot double cascade asymmetric synthesis of Wieland− Miescher ketone analogues from a Knoevenagel/hydrogenation/Robinson annulation sequence: scope and applications of organocatalytic biomimetic reductions. J. Org. Chem. 2007;72(14):5056–5068. doi: 10.1021/jo070277i. [DOI] [PubMed] [Google Scholar]
- 38.Jung D.-H., et al. New and general methods for the synthesis of arylmethylene bis (3-hydroxy-2-cyclohexene-1-ones) and xanthenediones by EDDA and in (OTf) 3-catalyzed one-pot domino Knoevenagel/Michael or Koevenagel/Michael/cyclodehydration reactions. Bull. Kor. Chem. Soc. 2009;30(9):1989–1995. [Google Scholar]
- 39.Nemati F., Heravi M.M., Rad R.S. Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for highly efficient Knoevenagel condensation and Michael addition reactions of aromatic aldehydes with 1, 3-cyclic diketones. Chin. J. Catal. 2012;33(11–12):1825–1831. [Google Scholar]
- 40.Li J.-T., et al. Improved synthesis of 2, 2′-arylmethylene bis (3-hydroxy-5, 5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea under ultrasound. Ultrason. Sonochem. 2012;19(1):1–4. doi: 10.1016/j.ultsonch.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Mandlimath T.R., Umamahesh B., Sathiyanarayanan K.I. Rapid one pot synthesis of xanthene derivatives by an efficient and reusable nano-ZnAl2O4–An insight into a new process. J. Mol. Catal. Chem. 2014;391:198–207. [Google Scholar]
- 42.Liu Z.-Q., et al. Diastereoselective enzymatic synthesis of highly substituted 3, 4-dihydropyridin-2-ones via domino Knoevenagel condensation–Michael addition–intramolecular cyclization. Tetrahedron. 2011;67(50):9736–9740. [Google Scholar]
- 43.Fu Y., et al. Promiscuous enzyme-catalyzed cascade reaction in water: synthesis of dicoumarol derivatives. Bioorg. Med. Chem. Lett. 2019;29(10):1236–1240. doi: 10.1016/j.bmcl.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y., et al. Promiscuous enzyme-catalyzed cascade reaction: synthesis of xanthone derivatives. Bioorg. Chem. 2018;80:555–559. doi: 10.1016/j.bioorg.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 45.Shalloe F., et al. Evidence that biliverdin-IX β reductase and flavin reductase are identical. Biochem. J. 1996;316(2):385–387. doi: 10.1042/bj3160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu F., et al. Pyrroloquinoline quinone acts with flavin reductase to reduce ferryl myoglobin in vitro and protects isolated heart from reoxygenation injury. Biochem. Biophys. Res. Commun. 1993;193(1):434–439. doi: 10.1006/bbrc.1993.1642. [DOI] [PubMed] [Google Scholar]
- 47.Andorfer M.C., et al. Aromatic halogenation by using bifunctional flavin reductase–halogenase fusion enzymes. Chembiochem. 2017;18(21):2099–2103. doi: 10.1002/cbic.201700391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith D.R., et al. An unusual flavin-dependent halogenase from the metagenome of the marine sponge Theonella swinhoei WA. ACS Chem. Biol. 2017;12(5):1281–1287. doi: 10.1021/acschembio.6b01115. [DOI] [PubMed] [Google Scholar]
- 49.Ellis H.R. The FMN-dependent two-component monooxygenase systems. Arch. Biochem. Biophys. 2010;497(1–2):1–12. doi: 10.1016/j.abb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Fontecave M., Covès J., Pierre J.-L. Ferric reductases or flavin reductases? Biometals. 1994;7:3–8. doi: 10.1007/BF00205187. [DOI] [PubMed] [Google Scholar]
- 51.Ingelman M., et al. Crystal structure of NAD (P) H: flavin oxidoreductase from Escherichia coli. Biochemistry. 1999;38(22):7040–7049. doi: 10.1021/bi982849m. [DOI] [PubMed] [Google Scholar]
- 52.Fieschi F., et al. The mechanism and substrate specificity of the NADPH: flavin oxidoreductase from Escherichia coli (∗) J. Biol. Chem. 1995;270(51):30392–30400. doi: 10.1074/jbc.270.51.30392. [DOI] [PubMed] [Google Scholar]
- 53.Gilanizadeh M., Zeynizadeh B. Synthesis and characterization of the immobilized Ni–Zn–Fe layered double hydroxide (LDH) on silica-coated magnetite as a mesoporous and magnetically reusable catalyst for the preparation of benzylidenemalononitriles and bisdimedones (tetraketones) under green conditions. New J. Chem. 2018;42(11):8553–8566. [Google Scholar]
- 54.Sharma H., Srivastava S. Anion functionalized ionic liquid from artificial sugar: a sustainable pathway for diverse bis-enol derivatives. New J. Chem. 2019;43(30):12054–12058. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.