Abstract
Purpose
Preventing fragility fractures by treating osteoporosis may reduce disability and mortality worldwide. Algorithms combining clinical risk factors with bone mineral density have been developed to better estimate fracture risk and possible treatment thresholds. This systematic review supported panel members of the Italian Fragility Fracture Guidelines in recommending the use of best-performant tool. The clinical performance of the three most used fracture risk assessment tools (DeFRA, FRAX, and FRA-HS) was assessed in at-risk patients.
Methods
PubMed, Embase, and Cochrane Library were searched till December 2020 for studies investigating risk assessment tools for predicting major osteoporotic or hip fractures in patients with osteoporosis or fragility fractures. Sensitivity (Sn), specificity (Sp), and areas under the curve (AUCs) were evaluated for all tools at different thresholds. Quality assessment was performed using the Quality Assessment of Diagnostic Accuracy Studies-2; certainty of evidence (CoE) was evaluated using the Grading of Recommendations Assessment, Development and Evaluation approach.
Results
Forty-three articles were considered (40, 1, and 2 for FRAX, FRA-HS, and DeFRA, respectively), with the CoE ranging from very low to high quality. A reduction of Sn and increase of Sp for major osteoporotic fractures were observed among women and the entire population with cut-off augmentation. No significant differences were found on comparing FRAX to DeFRA in women (AUC 59–88% vs. 74%) and diabetics (AUC 73% vs. 89%). FRAX demonstrated non-significantly better discriminatory power than FRA-HS among men.
Conclusion
The task force formulated appropriate recommendations on the use of any fracture risk assessment tools in patients with or at risk of fragility fractures, since no statistically significant differences emerged across different prediction tools.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-023-02082-8.
Keywords: Fracture risk assessment, Fragility fracture, Secondary prevention, Systematic review
Introduction
Osteoporosis is a chronic disease characterized by bone fragility, which leads to an increased risk of fractures [2]. As fragility fractures are a leading cause of disability and mortality worldwide, osteoporosis treatment should primarily aim at preventing fractures [1].
Low bone mineral density (BMD) is a major determinant of risk; it has been demonstrated that an increase in BMD is associated with fracture risk reduction in a quasi-linear manner [3]. However, BMD combined with clinical risk factors predicts fracture risk better than BMD alone [4]; these include: comorbidities, treatment with glucocorticoids, or a history of previous fractures. These factors are independent predictors of fracture and are associated with deterioration of bone quality [2]. Algorithms that combine clinical risk factors with BMD have been developed to better estimate fracture risk and determine possible thresholds for treatment [5–7].
The most widely used algorithm is the Fracture Risk Assessment Tool (FRAX), which was originally developed in 2008 by the World Health Organization collaborating center of the University of Sheffield, UK [6]. In Italy, other FRAX-derived tools (DeFRA and FRA-HS) are widely used for calculating fracture risk. The DeFRA was developed in 2010 by the Italian Society for Osteoporosis, Mineral Metabolism, and Bone Diseases (SIOMMMS) and the Italian Society of Rheumatology (SIR) [5]. The FRA-HS was developed and published by the Italian Society of General Practitioners (SIMG) [8]. Both algorithms have been validated against FRAX in post-menopausal women with osteoporosis [8, 9]. DeFRA considers the following patients’ clinical and densitometric characteristics for fracture risk calculation: age, weight, height, number and site of prior fragility fracture, parental history of hip and clinical vertebral fractures, glucocorticoid intake (semi-quantitative variable), treatment with adjuvant hormone therapy for breast cancer, the presence of various comorbidities (including rheumatoid arthritis, multiple sclerosis, psoriatic arthritis, systemic lupus erythematosus, other connective tissue disease), calcium intake from diet and supplements, vitamin D intake, falls, exposure to sunlight and both lumbar spine and femoral neck BMD [5].
FRA-HS estimate the fracture risk upon these characteristics: age, sex, history of osteoporotic fractures (dichotomic variable), secondary osteoporosis (dichotomic variable), long-term use of corticosteroids (dichotomic variable, at least 180 defined daily dose within the year prior to assessment), rheumatoid arthritis diagnosis, body mass index, smoking (dichotomic variable), and alcohol abuse/alcohol-related diseases (dichotomic variable) [8].
The Italian National Institute of Health (Istituto Superiore di Sanità) recently published the Italian guidelines “Diagnosis, risk stratification and continuity of care of Fragility Fractures” [10]. In regard to risk stratification, the task force focused on the three most commonly used fracture risk assessment tools in Italy (DeFRA, FRAX, and FRA-HS). A systematic review was conducted for each of these tools with the aim of assessing their clinical performance in patients at risk of fractures; the review also aimed to accumulate all relevant literature for formulating evidence-based recommendations. Herein, we present the results of the systematic review and meta-analysis on the performance of fracture risk assessment tools in patients at risk of fracture. The present meta-analysis informed the guidelines of the Italian National Institute of Health on fragility fractures.
Materials and methods
A systematic review was performed to support the panel members of the Italian Fragility Fracture Guidelines (published on the platform of the Italian National Institute of Health [11]) in formulating recommendations. In accordance with the GRADE-ADOLOPMENT methodology [12] and the standards elaborated by the Sistema Nazionale Linee Guida (SNLG) [13, 14], the multidisciplinary panel aimed to answer the following clinical question: “Which risk assessment tools are the most accurate in predicting the risk of fragility fractures in adults, including those without known osteoporosis or previous fragility fractures?”. The recommendations from the CG146 guideline of the National Institute for Clinical Excellence (NICE) (which assessed fragility fracture risk in patients with osteoporosis) were updated and adapted for this review.
Inclusion and exclusion criteria
Observational studies were selected if they met the following criteria: (1) population: patients with osteoporosis or those who had experienced a fragility fracture, according to the diagnostic criteria for osteoporosis and the definition of fragility given by different studies’ authors. In the vast majority of studies osteoporosis was defined based on T-score levels, fragility fracture was defined as: any asymptomatic morphometric vertebral fractures and/or any clinical bone fracture resulting from a fall from standing height or less or for a low-energy trauma; (2) risk assessment tools: FRAX [15], DeFRA [16], and FRA-HS [17]; reference standard: risk threshold for major osteoporotic fractures (MOF) (3%, 5%, 10%, 20%, and 30%) and hip fractures (3% and 5%), either with or without the BMD criterion; (3) outcome: (i) primary outcome measures of sensitivity (Sn) (capacity to correctly detect the fracture risk) and specificity (Sp) (exclusively identified fracture-free patients) for the risk assessment tools (studies were required to have Sn and Sp values, an adequate 2 × 2 table, or adequate data for creating the 2 × 2 table). Moreover, (ii) secondary outcomes were the receiver operating characteristic curve and the area under the curve (AUC) for Sn and Sp and, to easier interpret their goodness of fit, values were expressed in percentages by multiplying per 100.
Studies were excluded if they: (i) were not published in the English language, (ii) did not report original findings (i.e., letters and case reports), (iii) did not identify patients affected by fragility fractures or osteoporosis, or (iv) did not consider the risk assessment tools of interest (FRAX, DeFRA, or FRA-HS).
Data source and search strategy
PubMed, Embase, and the Cochrane Library were searched (between September 2011 and December 2020) by updating the search strategy of the NICE guidelines for the FRAX tool; a new search was conducted for the DeFRA and FRA-HS tools. Publications on the risk assessment tools were identified in patients with fragility fractures or osteoporosis. The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [18]; the statement has been provided in Supplemental Table S1. The search strategy (Supplemental Material, A) included specific keywords and/or corresponding Medical Subject Headings terms related to fragility fracture/osteoporosis AND risk assessment tools. The reference lists of the studies were checked and systematic reviews were identified during the search process.
Study selection and data extraction
Three independent authors (AB, GP, and RR) screened the titles and abstracts based on the search strategy and then assessed the full text of potentially relevant studies. Discrepancies between readers were resolved in conference.
The following data were extracted for each included observational study: (i) first author, year, and country of publication; (ii) study setting; (iii) duration of study; (iv) type of population; (v) intervention; and (vi) outcome (Supplemental Material, B).
Study quality
The methodological quality of the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies version 2 (QUADAS-2) checklist [19]. The QUADAS-2 assessment was structured in four key domains: patient selection, index test, reference standard, flow and timing (Supplemental Table S2).
Quality of evidence
The quality of evidence for each primary outcome was assessed based on five dimensions (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) using the GRADE approach [20]. If serious or very serious limitations were found for each of the 5 dimensions, the evidence was downgraded from “high quality” by 1 and 2 levels, respectively.
Statistical analysis
The following operating characteristics were evaluated for analysis of the risk assessment tool: the Sn and Sp (at different thresholds) and the AUC. Specific thresholds were used to differentiate between individuals with or without the target condition. In this context, the development group of the NICE guidelines established risk thresholds for MOF (3%, 5%, 10%, 20%, and 30%) and hip fractures (3%, 5%, and 10%). A low Sn implied that the tool did not recognize a proportion of MOFs or hip fractures; conversely, a low Sp indicated that the tool could lead to false positive cases and overestimate the incidence of these fractures. Analyses were therefore performed when studies reported different cut-off values for the same risk assessment tool.
The Sn and Sp estimates were used to realize coupled forest plots with 95% confidence intervals (CIs) across studies (at various thresholds); RevMan V.5.4 (Nordic Cochrane Center) software was used for evaluation. The AUC was used to evaluate the overall diagnostic accuracy of each risk assessment tool. Diagnostic meta-analysis was conducted when 3 or more studies were available per threshold. This measure was also plotted on a graph using RStudio software version 1.4.1717. Heterogeneity or inconsistency among studies was visually inspected using the forest plots for MOF or hip fractures, both with and without BMD.
Results
Study selection
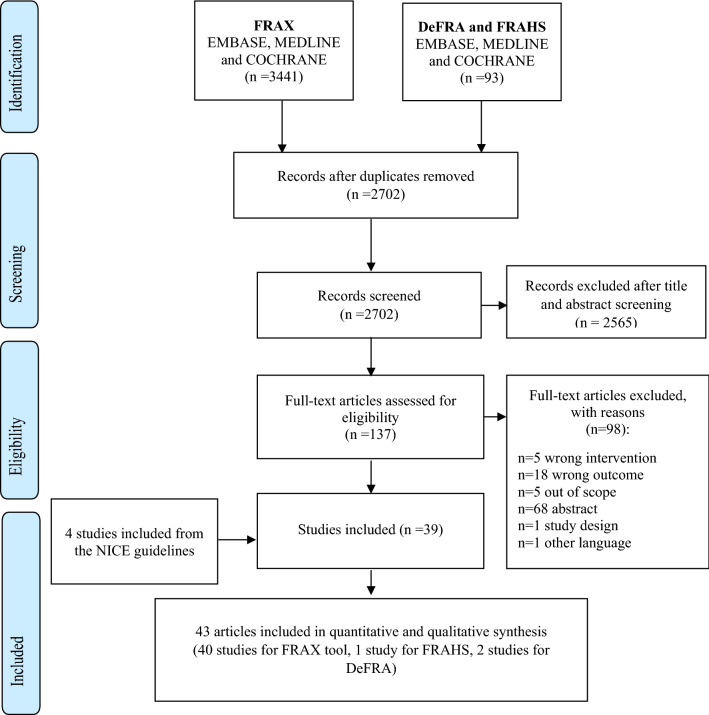
As shown in Fig. 1, a total of 2702 publications were identified; 2565 studies were excluded after title and abstract screening. Among the remaining 137 articles which were assessed for full-text review, 98 were excluded owing to the following reasons: (i) the intervention (n = 5) or outcome (n = 18) was considered to be incorrect, (ii) they were out of scope (n = 5) or only abstract (n = 68), (iii) the study design was not eligible for inclusion (n = 1), and (iv) the studies were not published in the English language (n = 1). Finally, 43 articles were considered for the present analysis; these included 40, 1, and 2 studies pertaining to the FRAX [21–60], FRA-HS [8], and DeFRA [9, 61] tools, respectively.
Fig. 1.
Flow chart
Characteristics of included studies
Among the selected articles, 14, 20, and 8 studies had a retrospective [8, 23, 26–28, 33, 35, 36, 43, 53, 59–61], prospective [22, 25, 30–32, 38–42, 47–52, 55–58], and cross-sectional [9, 24, 29, 34, 37, 44–46] designs, respectively; 1 article described a randomized clinical trial [21]. Among the publications, 14 [23–25, 33, 34, 36, 40, 44, 46, 49, 54, 55, 57, 60] were from Asia (Israel, China, Japan, India, Palestine, and Thailand), 21 [8, 9, 22, 26, 28, 29, 31, 35, 37–39, 41–43, 45, 48, 50, 52, 56, 59, 61] were from European countries (Italy, Spain, Poland, France, Denmark, Norway, Portugal, United Kingdom and the Netherlands), 5 [27, 30, 47, 51, 58] were from America, and 3 [21, 32, 53] were from Oceania (Australia, New Zealand). Eleven studies [8, 25, 26, 33, 38–40, 48, 52, 56, 57] considered subjects aged more than 40 years, 2 studies [34, 41] had participants aged over 45 years, 18 publications [9, 23, 24, 27–29, 35, 36, 43, 44, 46, 47, 49–51, 59–61] had individuals aged over 50 years, 4 studies [21, 22, 37, 45] had participants aged over 55 years, 4 publications [32, 42, 53, 58] had subjects aged over 60 years, and 4 studies [30, 31, 54, 55] had participants aged over 65 years. The general characteristics have been presented in Supplemental Material B.
The majority of studies considered subjects with fractures in less than (i) 5% [8, 9, 27, 38, 48, 49, 61], (ii) 10% [23, 24, 36, 40, 41, 43, 46, 47, 52], (iii) 20% [25, 26, 35, 39, 42, 50, 51, 53–55, 60], (iv) 30% [31, 44, 45, 57, 58], and (v) 40% [21, 22, 28–30, 37] cases. One study included participants with previous fractures [59], while four publications did not select subjects with a history of fracture [32–34, 56].
Risk of bias assessment and certainty of the evidence
Unclear risk of bias was generally present across the studies (Supplemental Table S2). In the entire population, the FRAX tool demonstrated high certainty of evidence: (i) with or without BMD for MOF (at 30% threshold), (ii) MOF (at 20% or 30% cut-off), and (iii) hip fractures (at 3% cut-off, only for Sp) (Supplemental Table S3). A moderate certainty of evidence (Supplemental Table S3) was detected for MOF: (i) without BMD (cut-off at 5% or 20%), (ii) with BMD (at 3% threshold, only for Sn), and (iii) hip fracture with BMD (cut-off at 5%). The remaining Sn and Sp values had low or very low certainty of evidence.
Sensitivity (Sn) and specificity (Sp)
Sn and Sp evaluation was only performed for the FRAX tool. The results showed a reduction of Sn and an increase of Sp with cut-off augmentation (Table 1, Supplemental Material C).
Table 1.
Sensitivity (Sn) and specificity (Sp) for major osteoporotic (a) and hip (b) fractures by considering the FRAX tool (with or without BMD) and different cut-off (3%, 5%, 10%, 20%, 30%)
| (a) Major osteoporotic fractures | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off | 3% | 5% | 10% | 20% | 30% | |||||
| FRAX | With BMD (95% CI) | without BMD (95% CI) | With BMD (95% CI) | Without BMD (95% CI) | With BMD (95% CI) | Without BMD (95% CI) | With BMD (95% CI) | Without BMD (95% CI) | With BMD (95% CI) | Without BMD (95% CI) |
| Women | ||||||||||
| Sn | 67 [30–93] | 57–85 [49–90] | 66 [57–73] | 34 [27–42] | 42–97 [28–98] | 46–100 [31–100] | 8–41 [2–44] | 8 [2–20] | – | 4 [0–14] |
| Sp | 75 [63–84] | 34–79 [23–82] | 71 [67–74] | 89 [86–91] | 15–84 [14–88] | 0–77 [0–81] | 81–97 [80–98] | 95 [93–97] | 99 [97–100] | 99 [98–100] |
| Total | ||||||||||
| Sn | – | 52–85 [42–90] | – | 34–35 [26–44] | 42–97 [28–98] | 24–100 [16–100] | 8–41 [2–44] | 8–29 [2–31] | 0–9 [0–11] | 4–10 [0–14] |
| Sp | 34–79 [23–82] | 81–89 [71–91] | 15–84 [14–88] | 0–93 [0–97] | 81–97 [80–98] | 88–95 [87–97] | 98–99 [97–100] | 97–99 [97–100] | ||
| (b) Hip fractures | |||||
|---|---|---|---|---|---|
| cut-off | 3% | 5% | 10% | ||
| FRAX | With BMD (95% CI) | Without BMD (95% CI) | With BMD (95% CI) | Without BMD (95% CI) | With BMD (95% CI) |
| Women | |||||
| Sn | 43–62 [28–64] | 8–77 [0–82] | 29–76 [19–80] | 42–78 [41–82] | 33 [28–39] |
| Sp | 72–87 [69–89] | 39–100 [36–100] | 63–91 [61–94] | 50–92 [49–92] | 86 [85–87] |
| Total | |||||
| Sn | 43–77 [28–81] | 8–78 [0–82] | 29–76 [19–80] | 22–78 [14–82] | – |
| Sp | 72–87 [69–89] | 39–100 [36–100] | 63–91 [61–94] | 50–97 [49–99] | |
We reported the minimum and the maximum Sn/Se value, the lower and the upper limit of the 95% confidence interval (CI)
Major osteoporotic fractures
In women, the Sn and Sp for FRAX without BMD (and 3% threshold) ranged between 57 and 85% and 34% and 79%, respectively (2 studies [29, 43]). The Sn and Sp for 30% threshold were approximately 4% (95% CI 0–14%) and 99% (95% CI 98–100%), respectively (1 study [42]).
The discriminatory values for FRAX without BMD were lower compared to the predictive values for FRAX with BMD. For FRAX with BMD (and 3% threshold), the estimated Sn and Sp were 67% (95% CI 30–93%) and 75% (95% CI 63–84%), respectively (1 study [23]); the Sp for the 30% threshold was 99% (95% CI 97–100%) (1 study [42]). As showed in Table 1a, the same trend was confirmed in the entire population.
Hip fractures
Three studies [21, 33, 56] evaluated the diagnostic accuracy in women (Table 1b); for FRAX without BMD (and 3% threshold), they detected a Sn and Sp ranging from 8 to 77% and 39 to 100%, respectively. For the 5% cut-off value, the Sn and Sp ranged from 42 to 78% and 50 to 92%, respectively (3 studies [21, 30, 56]).
For FRAX with BMD and 3% threshold, the Sn varied from 43 to 62% while the Sp was estimated to be 78–87% (4 studies [21, 23, 27, 42]). For the 10% cut-off value, the Sn was 33% (95% CI 28–39%; 1 study) and the Sp was 86% (95% CI 85–87%; 1 study [27]). As shown in Table 1b, these trends for Sn and Sp (FRAX with or without BMD) were confirmed in the entire population.
Area under the curve
The meta-analytic summary of the AUCs for the risk assessment tools is shown in Supplemental Material C and Table 2. The diagnostic accuracy of the FRAX (with and without BMD) and FRA-HS tools (without BMD) was evaluated in women, men, and the entire population. The AUC for DeFRA (with BMD) in cases of MOF was evaluated and compared to that of the FRAX instrument in women as well as in diabetic patients.
Table 2.
Area under the curve (AUC) for major osteoporotic (a) and hip (b) fractures by considering the FRAX, FRA-HS, DeFRA tools (with or without BMD)
| (a) Popolation | FRAX (95% CI) | FRA-HS (95% CI) | DeFRA (95% CI) | |
|---|---|---|---|---|
| Women | MOF with BMD | 59–88 [54–88] | 74 [69–80] | |
| MOF without BMD | 50–78 [57–80] | 58 [54–62] | ||
| HIP with BMD | 70–93 [61–100] | |||
| HIP without BMD | 60–86 [56–100] | 74 [67–81] | ||
| Men | MOF with BMD | 57–85 [41–88] | ||
| MOF without BMD | 55–81 [55–85] | 48 [42–54] | ||
| HIP with BMD | 75–90 [72–93] | |||
| HIP without BMD | 57–93 [57–95] | 54 [39–69] | ||
| Total | MOF with BMD | 57–88 [41–88] | ||
| MOF without BMD | 55–81 [55–85] | 65 [61–69] | ||
| HIP with BMD | 70–93 [61–100] | |||
| HIP without BMD | 57–93 [56–100] | 73 [66–80] | ||
| (b) Popolation | FRAX | DeFRA | |
|---|---|---|---|
| Diabetics | MOF with BMD | 73 [60–87] | 89 [78–100] |
We reported the minimum and the maximum AUC value, the lower and the upper limit of the 95% confidence interval (CI)
In women (Table 2a), the summary AUC of the FRAX (MOF without BMD) indicated a better diagnostic performance (50–78%; 19 studies [21, 22, 25, 29–32, 34, 36, 38–43, 48, 52, 55, 58]) compared with the FRA-HS tool (58%; 1 study [8]); this was reflected in men (55–81% in 5 studies [44, 46, 48, 52, 55] vs. 48% in 1 study [8]) and in the entire population (55–81% in 24 studies [21, 22, 25, 29–32, 34, 36, 38–44, 46–49, 51, 52, 55, 58] vs. 65% in 1 study [8]).
Thus, the summary AUC of the FRAX (hip without BMD) was higher compared to that of the FRA-HS tool in men (57–93% in 6 studies [46, 48, 50, 52, 55, 56] vs. 54% in 1 study [8]); however, no differences were observed in women (60–86% in 17 studies [21, 24, 27, 29–31, 33, 34, 36, 40, 43, 48, 50, 52, 55, 56, 58] vs. 74% in 1 study [8]) and in the entire population (57–93% in 21 studies [21, 24, 27, 29–31, 33, 34, 36, 40, 43, 46–52, 55, 56, 58] vs. 73% in 1 study [8]).
In women, the predictive value of FRAX (MOF with BMD) was similar to that of DeFRA (59–88% in 23 studies [9, 21–23, 25, 26, 28–30, 32, 34, 35, 37, 39–42, 52–55, 57, 59] vs. 74% in 1 study [9]).
In individuals with diabetes, the Italian DeFRA demonstrated a major but non-significant discriminatory value (AUC 89%, 95% CI 78–100%; 1 study [61]) for MOF with BMD with respect to the FRAX tool (AUC: 73%, 95% CI 60–87%; 1 study [61]) (Table 2b).
Inconsistencies, classified as not serious, serious, and very serious, have been presented in Supplementary Table S3.
Discussion
This systematic review evaluated one clinical question of the Italian Guidelines [11], and a multidisciplinary panel of experts formulated recommendations through a structured, transparent, and evidence-based process. This systematic review and meta-analysis was particularly conducted to evaluate the accuracy of three fracture risk assessment tools (DeFRA, FRAX, and FRA-HS). A total of 43 studies that assessed the performance of tools in identifying at-risk patients were included. Overall, FRAX and DeFRA appeared to perform better than FRA-HS in terms of discriminatory power. All three tools generally performed better for hip fractures than for MOF. As expected, the AUC was higher in women compared to men, mostly with the addition of BMD in the algorithm.
The results of this meta-analysis allowed determination of a recommendation, which suggests the use of risk assessment tools for predicting fractures in patients with or at risk of fragility fractures (moderate quality of evidence).
Other meta-analyses have been conducted on this topic. In 2019, Beaudoin and colleagues published a systematic review and meta-analysis that assessed 14 tools including the FRAX and FRA-HS. The authors analyzed 53 validation studies and found results similar to those of the present meta-analysis. For instance, Beaudoin et al. showed that the tools performed better in predicting hip fractures than fractures at other sites. They also found that the Q-Fracture and Garvan risk tools slightly outperformed the FRAX in predicting hip fractures; this concurs with the findings of an older meta-analysis by Marques and colleagues [62]. In the present meta-analysis, we also found that the DeFRA had slightly higher discriminatory power compared to the FRAX. Indeed, the Garvan, Q-Fracture, and DeFRA tools resolve certain critical issues of the FRAX. Although the FRAX tool represents a crucial milestone in the management of osteoporosis, the algorithm has significant limitations; this may undermine its predictive value. For example, the FRAX does not consider lumbar spine BMD data, which are considered by the DeFRA and Garvan tools. In addition, clinical risk factors (e.g., prior fractures, glucocorticoids, and smoking habits, among others) are scaled down to dichotomous variables in FRAX. However, small differences in prediction ability between FRAX and other more complex algorithms may only have minimal relevance.
Limitations and strengths
The findings of this study should be interpreted considering its strength and limitations. First, the task force decided to include only three fracture risk assessment tools in the Italian Guideline on the management of Fragility Fracture, because these instruments have been translated into the Italian language. Second, there are certain concerns as to whether findings from selected studies can be combined to draw one conclusion; this is because all the aforementioned results had high levels of heterogeneity depending on the baseline characteristics of the validation cohorts and the quality of the included studies (fracture diagnosis, and length of follow-up, among others). Third, an unclear risk of bias was detected across the included studies. Thus, the certainty of evidence for the assessed outcomes was judged to be “very low” or “moderate” owing to very serious inconsistencies and serious imprecision of the estimates. Fourth, most of the studies included in the meta-analysis were conducted outside Italy and the results might not be directly applicable to the Italian population. However, the vast majority of the population of the meta-analysis was of European ancestry possibly reducing such bias.
Despite these limitations, this study had certain strengths. In view of the discriminatory power of the risk assessment tools, the exhaustive search strategy provided a reliable overview of the studies. In addition, the internal validity of the included studies was assessed using the QUADAS-2 checklist for diagnostic accuracy studies.
Conclusion
The present meta-analysis evaluated the diagnostic accuracy of three (FRAX, FRA-HS, and DeFRA) fracture risk prediction tools. The task force formulated recommendations on the use of any of these algorithms but did not identify a better performing tool. Although, our systematic review identified some outcomes (Sn and Sp) that were affected by “very low” to “moderate” quality evidence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank prof. Silvia Migliaccio and Maria Luisa Brandi who are members of Society of Endocrinology (SIE). We thank the Charlesworth Author Services for the English Academic Editing.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. The Italian guideline was funded by ALTIS Omnia Pharma Service, which did not affect the content of the document.
Declarations
Conflict of interest
GA declares personal fees from Theramex, Amgen, BMS, Lilly, Fresenius Kabi and Galapagos. LC declares personal fees from UCB Pharma, Abiogen Pharma, Bruno Farmaceutici, Sandoz, Metagenics. DG has received honoraria as consultant for Eli-Lilly, Organon, MSD Italia. SG has received honoraria as consultant for UCB Pharma. SM has received honoraria as consultant for UCB, Eli-Lilly, Amgen. MLB has received (i) honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB, (ii) grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma, (iii) consultant: Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB Pharma. GC received research support from the European Community (EC), the Italian Agency of Drug (AIFA), and the Italian Ministry for University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN and BMS). He also received honoraria as member of Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MR declares personal fees from Amgen, ABBvie, BMS, Eli Lilly, Galapagos, Menarini, Novartis, Pfizer, Sandoz, Theramex and UCB outside the submitted work. RM took part to a project funded by Abiogen Pharma. GI received honoraria as speaker by Eli-Lilly, Menarini, UCB Pharma. The other authors declare that they have no conflict of interest. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflicts of interest.
Research involving human participants and/or animals
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Data sharing
No additional data is available.
Transparency declaration
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Informed consent
Informed consent was waived, as this study conducted a meta-analysis of anonymised data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16:82. doi: 10.1007/s11657-020-00871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 3.Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632–642. doi: 10.1002/jbmr.3641. [DOI] [PubMed] [Google Scholar]
- 4.Watts NB, Manson JE. Osteoporosis and fracture risk evaluation and management: shared decision making in clinical practice. JAMA. 2017;317:253–254. doi: 10.1001/jama.2016.19087. [DOI] [PubMed] [Google Scholar]
- 5.Adami S, Bianchi G, Brandi ML, Di Munno O, Frediani B, Gatti D, et al. Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: project rationale and description. Clin Exp Rheumatol. 2010;28:561–570. [PubMed] [Google Scholar]
- 6.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adami S, Bertoldo F, Gatti D, Minisola G, Rossini M, Sinigaglia L, et al. Treatment thresholds for osteoporosis and reimbursability criteria: Perspectives associated with fracture risk-assessment tools. Calcif Tissue Int. 2013;93:195–200. doi: 10.1007/s00223-013-9748-0. [DOI] [PubMed] [Google Scholar]
- 8.Francesco L, Elisa B, Raffaella M, Alessandro P, Iacopo C, Giampiero M, et al. Assessing risk of osteoporotic fractures in primary care: development and validation of the FRA-HS algorithm. Calcif Tissue Int. 2017;100:537–549. doi: 10.1007/s00223-016-0230-7. [DOI] [PubMed] [Google Scholar]
- 9.Bonaccorsi G, Fila E, Cervellati C, Romani A, Giganti M, Rossini M, et al. Assessment of fracture risk in a population of postmenopausal italian women: a comparison of two different tools. Calcif Tissue Int. 2015;97:50–57. doi: 10.1007/s00223-015-0009-2. [DOI] [PubMed] [Google Scholar]
- 10.Milan Bicocca University and Italian National Institute of Health (2021) Diagnosi, stratificazione del rischio e continuità assistenziale delle Fratture da Fragilità. SNLG Sistema Nazionale per le Linee Guida. https://snlg.iss.it/wp-content/uploads/2023/01/LG-392_Fratture-da-Fragilit%C3%A0_v3.pdf. Accessed 3 May 2022
- 11.Italian National Institute of Health. Platform for the Italian Guidelines SNLG Sistema Nazionale per le Linee Guida. https://www.iss.it/en/lineeguida-snlg. Accessed 3 May 2022
- 12.Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–110. doi: 10.1016/j.jclinepi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Centro Nazionale per l’Eccellenza Clinica, la Qualità e la Sicurezza delle Cure (2019) Manuale metodologico per la produzione di linee guida di pratica clinica. Italian National Institute of Health. https://snlg.iss.it/wp-content/uploads/2021/08/MM_v1.3.2_apr_2019.pdf. Accessed 3 May 2022
- 14.Programma Nazionale per le Linee guida (2019) Come produrre, diffondere e aggiornare raccomandazioni per la pratica clinica. Manuale metodologico. Italian National Institute of Health. http://www.snlg-iss.it/manuale_metodologico_SNLG. Accessed 3 May 2022
- 15.National Osteporosis Society (2008) Fracture Risk Assessment Tool (FRAX). World Health Organization collaborating center of the University of Sheffield, UK. http://www.shef.ac.uk/FRAX. Accessed 3 May 2022
- 16.Italian Society for Osteoporosis, Mineral Metabolism, and Bone Diseases (SIOMMMS) and the Italian Society of Rheumatology (SIR) (2010). DeFRA - l’algoritmo per la stima del rischio di frattura. https://defra-osteoporosi.it/. Accessed 3 May 2022
- 17.Italian Society of General Practitioners (SIMG) (2016) FRAHS: Il nuovo Score per la valutazione del rischio di frattura osteoporotica. https://www.simg.it/sicilia/frahs-il-nuovo-score-per-la-valutazione-del-rischio-di-frattura-osteoporotica. Accessed 3 May 2022
- 18.Page MJ, Moher D. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and extensions: a scoping review. Syst Rev. 2017;6:263. doi: 10.1186/s13643-017-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Bolland MJ, Siu AT, Mason BH, Horne AM, Ames RW, Grey AB, et al. Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res. 2011;26:420–427. doi: 10.1002/jbmr.215. [DOI] [PubMed] [Google Scholar]
- 22.Briot K, Paternotte S, Kolta S, Eastell R, Felsenberg D, Reid DM, et al. FRAX®: prediction of major osteoporotic fractures in women from the general population: the OPUS study. PLoS One. 2013;8:e83436. doi: 10.1371/journal.pone.0083436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandran M, McCloskey EV, Thu WPP, Logan S, Hao Y, Tay D, et al. FRAX® based intervention thresholds for management of osteoporosis in Singaporean women. Arch Osteoporos. 2018;13:130. doi: 10.1007/s11657-018-0542-5. [DOI] [PubMed] [Google Scholar]
- 24.Cherian KE, Kapoor N, Shetty S, Naik D, Thomas N, Paul TV. Evaluation of different screening tools for predicting femoral neck osteoporosis in rural south Indian postmenopausal women. J Clin Densitom. 2018;21:119–124. doi: 10.1016/j.jocd.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Cheung E, Cheung CL, Kung AWC, Tan KCB. Possible FRAX-based intervention thresholds for a cohort of Chinese postmenopausal women. Osteoporos Int. 2014;25:1017–1023. doi: 10.1007/s00198-013-2553-9. [DOI] [PubMed] [Google Scholar]
- 26.Tebé Cordomí C, Del Río LM, Di Gregorio S, Casas L, Estrada MD, Kotzeva A, et al. Validation of the FRAX predictive model for major osteoporotic fracture in a historical cohort of Spanish women. J Clin Densitom. 2013;16:231–237. doi: 10.1016/j.jocd.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Crandall CJ, Larson J, Gourlay ML, Donaldson MG, LaCroix A, Cauley JA, et al. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US preventive services task force strategy and two traditional strategies in the women’s health initiative. J Bone Miner Res. 2014;29:1661–1666. doi: 10.1002/jbmr.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czerwiński E, Borowy P, Kumorek A, Amarowicz J, Górkiewicz M, Milert A. Fracture risk prediction in outpatients from Krakow Region using FRAX tool versus fracture risk in 11-year follow-up. Ortop Traumatol Rehabil. 2013;15:617–628. doi: 10.5604/15093492.1091517. [DOI] [PubMed] [Google Scholar]
- 29.El Maghraoui A, Sadni S, Jbili N, Rezqi A, Mounach A, Ghozlani I. The discriminative ability of FRAX, the WHO algorithm, to identify women with prevalent asymptomatic vertebral fractures: a cross-sectional study. BMC Musculoskelet Disord. 2014;15:365. doi: 10.1186/1471-2474-15-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ensrud KE, Lui LY, Taylor BC, Schousboe JT, Donaldson MG, Fink HA, et al. A comparison of prediction models for fractures in older women: is more better? Arch Intern Med. 2009;169:2087–2094. doi: 10.1001/archinternmed.2009.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González-Macías J, Marin F, Vila J, Díez-Pérez A. Probability of fractures predicted by FRAX® and observed incidence in the Spanish ECOSAP Study cohort. Bone. 2012;50:373–377. doi: 10.1016/j.bone.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Henry MJ, Pasco JA, Merriman EN, Zhang Y, Sanders KM, Kotowicz MA, et al. Fracture risk score and absolute risk of fracture. Radiology. 2011;259:495–501. doi: 10.1148/radiol.10101406. [DOI] [PubMed] [Google Scholar]
- 33.Indhavivadhana S, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Tanmahasamut P, Leerasiri P. Validation of osteoporosis risk assessment tools in middle-aged Thai women. Climacteric. 2016;19:588–593. doi: 10.1080/13697137.2016.1231176. [DOI] [PubMed] [Google Scholar]
- 34.Kharroubi A, Saba E, Ghannam I, Darwish H. Evaluation of the validity of osteoporosis and fracture risk assessment tools (IOF One Minute Test, SCORE, and FRAX) in postmenopausal Palestinian women. Arch Osteoporos. 2017;12:6. doi: 10.1007/s11657-016-0298-8. [DOI] [PubMed] [Google Scholar]
- 35.Kral R, Osima M, Borgen TT, Vestgaard R, Richardsen E, Bjørnerem Å. Increased cortical porosity and reduced cortical thickness of the proximal femur are associated with nonvertebral fracture independent of Fracture Risk Assessment Tool and Garvan estimates in postmenopausal women. PLoS One. 2017;12:e0185363. doi: 10.1371/journal.pone.0185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Chen R, Ding N, Wang Q, Huang M, Liu H, et al. Setting the new FRAX reference threshold without bone mineral density in Chinese postmenopausal women. J Endocrinol Invest. 2021;44:347–352. doi: 10.1007/s40618-020-01315-4. [DOI] [PubMed] [Google Scholar]
- 37.Pluskiewicz W, Adamczyk P, Franek E, Leszczynski P, Sewerynek E, Wichrowska H, et al. Ten-year probability of osteoporotic fracture in 2012 Polish women assessed by FRAX and nomogram by Nguyen et al.-Conformity between methods and their clinical utility. Bone. 2010;46:1661–1667. doi: 10.1016/j.bone.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Rubin KH, Abrahamsen B, Friis-Holmberg T, Hjelmborg JVB, Bech M, Hermann AP, et al. Comparison of different screening tools (FRAX®, OST, ORAI, OSIRIS, SCORE and age alone) to identify women with increased risk of fracture. A population-based prospective study. Bone. 2013;56:16–22. doi: 10.1016/j.bone.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Sornay-Rendu E, Munoz F, Delmas PD, Chapurlat RD. The FRAX tool in French women: how well does it describe the real incidence of fracture in the OFELY cohort? J Bone Miner Res. 2010;25:2101–2107. doi: 10.1002/jbmr.106. [DOI] [PubMed] [Google Scholar]
- 40.Tamaki J, Iki M, Kadowaki E, Sato Y, Kajita E, Kagamimori S, et al. Fracture risk prediction using FRAX®: a 10-year follow-up survey of the Japanese Population-Based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2011;22:3037–3045. doi: 10.1007/s00198-011-1537-x. [DOI] [PubMed] [Google Scholar]
- 41.Trémollieres FA, Pouillès JM, Drewniak N, Laparra J, Ribot CA, Dargent-Molina P. Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J Bone Miner Res. 2010;25:1002–1009. doi: 10.1002/jbmr.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Geel TACM, Eisman JA, Geusens PP, van den Bergh JPW, Center JR, Dinant GJ. The utility of absolute risk prediction using FRAX® and Garvan Fracture Risk Calculator in daily practice. Maturitas. 2014;77:174–179. doi: 10.1016/j.maturitas.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Villa P, Lassandro AP, Moruzzi MC, Amar ID, Vacca L, Di Nardo F, et al. A non-invasive prevention program model for the assessment of osteoporosis in the early postmenopausal period: a pilot study on FRAX(®) and QUS tools advantages. J Endocrinol Invest. 2016;39:191–198. doi: 10.1007/s40618-015-0341-4. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Yang Y, Fei Q, Zhang X, Ma Z, Wang Q, et al. Validation of three tools for identifying painful new osteoporotic vertebral fractures in older Chinese men: bone mineral density, Osteoporosis Self-Assessment Tool for Asians, and fracture risk assessment tool. Clin Interv Aging. 2016;11:461–469. doi: 10.2147/CIA.S101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pluskiewicz W, Adamczyk P, Franek E, Sewerynek E, Leszczynski P, Wichrowska H, et al. FRAX calculator and Garvan nomogram in male osteoporotic population. Aging Male. 2014;17:174–182. doi: 10.3109/13685538.2013.875991. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Lin J, Yang Y, Wu H, Li Y, Yang X, et al. Comparison of three tools for predicting primary osteoporosis in an elderly male population in Beijing: a cross-sectional study. Clin Interv Aging. 2018;13:201–209. doi: 10.2147/CIA.S145741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser LA, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, et al. Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int. 2011;22:829–837. doi: 10.1007/s00198-010-1465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friis-Holmberg T, Rubin KH, Brixen K, Tolstrup JS, Bech M. Fracture risk prediction using phalangeal bone mineral density or FRAX(®)?-A Danish cohort study on men and women. J Clin Densitom. 2014;17:7–15. doi: 10.1016/j.jocd.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Goldshtein I, Gerber Y, Ish-Shalom S, Leshno M. Fracture risk assessment with FRAX Using real-world data in a population-based cohort from Israel. Am J Epidemiol. 2018;187:94–102. doi: 10.1093/aje/kwx128. [DOI] [PubMed] [Google Scholar]
- 50.Hoff M, Meyer HE, Skurtveit S, Langhammer A, Søgaard AJ, Syversen U, et al. Validation of FRAX and the impact of self-reported falls among elderly in a general population: the HUNT study, Norway. Osteoporos Int. 2017;28:2935–2944. doi: 10.1007/s00198-017-4134-9. [DOI] [PubMed] [Google Scholar]
- 51.Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25:2350–2358. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 52.Marques A, Lucas R, Simões E, Verstappen SMM, Jacobs JWG, da Silva JAP. Do we need bone mineral density to estimate osteoporotic fracture risk? A 10-year prospective multicentre validation study. RMD Open. 2017;3:e000509. doi: 10.1136/rmdopen-2017-000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandhu SK, Nguyen ND, Center JR, Pocock NA, Eisman JA, Nguyen TV. Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int. 2010;21:863–871. doi: 10.1007/s00198-009-1026-7. [DOI] [PubMed] [Google Scholar]
- 54.Su Y, Leung J, Hans D, Lamy O, Kwok T. The added value of trabecular bone score to FRAX® to predict major osteoporotic fractures for clinical use in Chinese older people: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int. 2017;28:111–117. doi: 10.1007/s00198-016-3741-1. [DOI] [PubMed] [Google Scholar]
- 55.Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc. 2014;15:918–923. doi: 10.1016/j.jamda.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ. 2009;339:b4229. doi: 10.1136/bmj.b4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka S, Yoshimura N, Kuroda T, Hosoi T, Saito M, Shiraki M. The fracture and immobilization score (FRISC) for risk assessment of osteoporotic fracture and immobilization in postmenopausal women–a joint analysis of the Nagano, Miyama, and Taiji Cohorts. Bone. 2010;47:1064–1070. doi: 10.1016/j.bone.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook PN, Flahive J, Hooven FH, Boonen S, Chapurlat R, Lindsay R, et al. Predicting fractures in an international cohort using risk factor algorithms without BMD. J Bone Miner Res. 2011;26:2770–2777. doi: 10.1002/jbmr.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cummins NM, Poku EK, Towler MR, O’Driscoll OM, Ralston SH. clinical risk factors for osteoporosis in Ireland and the UK: a comparison of FRAX and QFractureScores. Calcif Tissue Int. 2011;89:172–177. doi: 10.1007/s00223-011-9504-2. [DOI] [PubMed] [Google Scholar]
- 60.Dagan N, Cohen-Stavi C, Leventer-Roberts M, Balicer RD. External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort study. BMJ. 2017;356:i6755. doi: 10.1136/bmj.i6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonaccorsi G, Messina C, Cervellati C, Maietti E, Medini M, Rossini M, et al. Fracture risk assessment in postmenopausal women with diabetes: comparison between DeFRA and FRAX tools. Gynecol Endocrinol. 2018;34:404–408. doi: 10.1080/09513590.2017.1407308. [DOI] [PubMed] [Google Scholar]
- 62.Marques A, Ferreira RJO, Santos E, Loza E, Carmona L, da Silva JAP. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1958–1967. doi: 10.1136/annrheumdis-2015-207907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.