Abstract
Background
Glioblastoma is the most aggressive primary brain cancer with a poor prognosis. Despite numerous studies in the past 17 years, effective treatment options for glioblastoma remain limited. In this study, we aimed to identify and compare phase III clinical trials for glioblastoma in terms of efficacy and baseline characteristics.
Methods
A systematic literature search was conducted using PubMed and ClinicalTrials.gov to identify phase III clinical trials for glioblastoma in adult patients. The target population included adult patients aged 18 years and above (younger cohort) and patients ≥60 years of age (elderly cohort). The search results were screened based on predefined inclusion criteria, and the included trials were analyzed for their study design, baseline characteristics, and survival results.
Results
Eleven trials met the inclusion criteria in the younger cohort. Of these, three reported a statistically significant improvement in overall survival (OS), including the EORTC/NCIC study (NCT00006353), EF-14 (NCT00916409), and CeTeG (NCT01149109). Of the 11 trials, eight were open-label randomized trials, including all of the positive ones, while three negative trials employed treatment blinding and a placebo control. The baseline characteristics of the trials [such as extent of resection, age, gender, and O(6)-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status] did not significantly differ between positive and negative trials. Isocitrate dehydrogenase (IDH) mutation status was analyzed in only two trials, with a small percentage of IDH-mutated tumors in each. Additionally, three more trials in the elderly cohort showed a statistically significant improvement of OS, the NOA-08 trial, the ISRCTN81470623-trial by Malmström et al. and NCT00482677-trial by Perry et al. Their baseline characteristics and implications are also analyzed.
Conclusion
This analysis of phase III clinical trials for glioblastoma conducted since 2005 showed that the majority of trials did not result in a significant improvement in OS. Among the trials included in this analysis, only the EORTC/NCIC, EF-14, and CeTeG studies demonstrated a positive OS outcome in the younger cohort.
Keywords: CeTeG, EF-14, glioblastoma, meta-analysis, phase III trials
Key Points.
Despite numerous studies in the past 17 years, effective treatment options for GBM remain limited.
Literature search was performed to identify phase III GBM trials in adults from 2005 to 2022.
Only Stupp et al. trial of 2005, EF-14 and CeTeG trial demonstrated positive survival outcomes in a cohort including young patients.
Importance of the Study.
Plenty of glioblastoma studies have been performed over the last 2 decades but only some of them led to therapy improvement for patients. This study is a structured meta-analysis of relevant phase III studies in last 2 decades and their value in the field of neuro-oncology. Only three phase III trials (Stupp et al. trial of 2005, EF-14 and CeTeG trial) allowing younger patients demonstrated positive survival outcomes. Based on these findings, we advocate for the integration of these therapies into clinical practice and future clinical trials, also if various limitations must be noted.
Glioblastoma is the most aggressive primary brain cancer with a median overall survival (mOS) ranging from 8 to 48 months, depending on various prognostic factors.1,2 The first-line treatment for adult glioblastoma patients younger than 70 years of age involves maximum-safe tumor resection, concurrent radiotherapy with temozolomide (TMZ) for 6 weeks, followed by six courses of adjuvant TMZ treatment.3–5 The addition of Tumor Treating Fields (TTFields) to adjuvant TMZ in the first-line therapy has significantly increased the median OS of glioblastoma patients from 16 to 20.9 months.6 TTFields are alternating electric fields at an intermediate frequency (200 kHz for glioblastoma) that are delivered to the tumor through arrays placed on the shaved scalp of patients. TTFields disrupt the cell division of glioblastoma cells, leading to an increase in mitotic arrest and cell death.7–9 In patients older than 60 years of age the existing body of evidence remains weak.
In addition, the CeTeG trial suggested that a combination chemotherapy of TMZ and lomustine (CCNU) can increase the median survival of MGMT promoter methylated glioblastoma patients.1 However, this combined chemotherapy did not show any differences in progression-free survival (PFS) compared to TMZ alone.
To provide a comprehensive overview of high-level evidence findings since 2005, we conducted a systematic literature search for prospective phase III randomized clinical trials investigating the potential for OS benefits from treatment interventions in adult patients with newly diagnosed glioblastoma.
Methods
Search and Selection Strategy
On September 19, 2022, we conducted a search on ClinicalTrials.gov and PubMed.gov for clinical trials on glioblastoma, using the terms “glioblastoma” and “glioblastoma OR gbm,” respectively, (see Figure 1). We applied filters to the search results in PubMed.gov to narrow them down to phase III clinical trials with a publication or primary completion date between 2005 and 2022. On ClinicalTrials.gov, we also filtered for interventional, completed, terminated, adult, older adult, and phase III trials. The target population included adult patients aged 18 years and above (younger cohort). Additionally, for completeness, we have included clinical trials where patients were aged ≥60 years (elderly cohort).
Figure 1.
Search terms and filters used to conduct the literature search for publications and trials completed from 2005/01/01 to 2022/19/09.
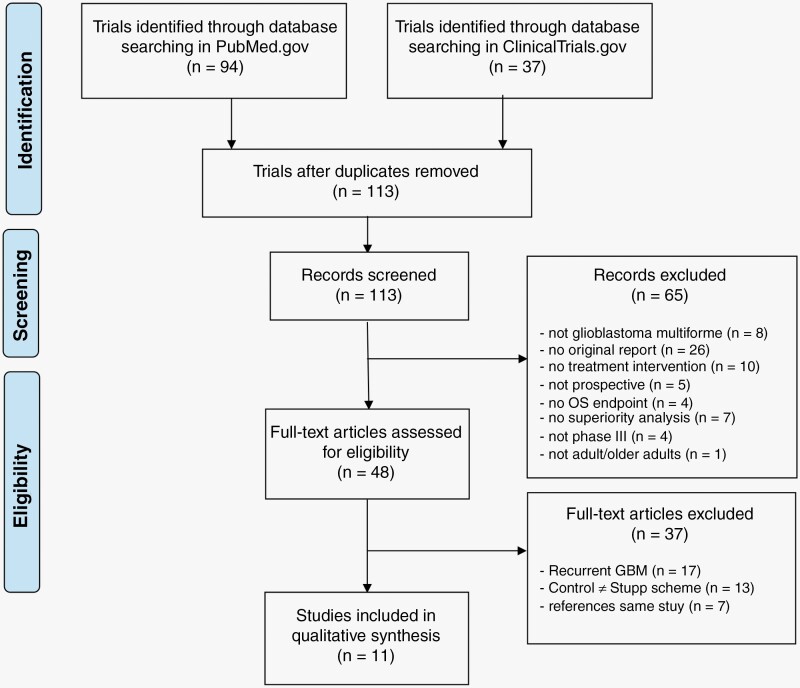
After reconciling the results from the two databases and eliminating duplicates, we further narrowed down the list of trials by reviewing the titles and abstracts and applying the following criteria: the investigation of glioblastoma, original reports, interventional or prospective studies, endpoint of OS, superiority analysis of investigated interventions, and phase III trial status. We then reviewed the remaining trials investigating newly diagnosed glioblastoma for their control treatment, and excluded those that did not use the Stupp scheme of radiochemotherapy with temozolomide (TMZ) followed by adjuvant TMZ chemotherapy in the control arm (Figure 2).
Figure 2.
Search and selection strategy to identify phase III studies investigating survival benefit in glioblastoma from 2005 to 2022. Exclusion criteria and strategy to identify clinically relevant interventional trials on newly diagnosed glioblastoma using current standard of care (Stupp protocol) as control.
We carefully examined the trials in this final set, collecting data on study design, treatment interventions, blinding, patient baseline characteristics, and survival efficacy results in a pre-specified form. These data were selected and used to make the studies comparable and not to determine prognostic markers. A second investigator independently verified the data.
Statistical Analysis
We conducted a comprehensive comparative analysis to investigate the relationship between patient baseline characteristics and blinding and survival outcomes in clinical trials. Our study focused on variables that have been established as having prognostic significance and are well-recognized in the scientific community, such as age, extent of resection, performance status, and molecular features. To compare the baseline characteristics between positive and negative trials in our final trial set, we calculated weighted medians and means for continuous variables, and, if applicable, categories for binary variables. This statistical analysis allowed us to evaluate the effect of these variables on the blinding and survival outcomes of the trials in our study.
Results
How Many Trials Demonstrated Significant Improvements in OS?
After applying our inclusion criteria, we conducted a systematic review of the literature and clinical trial database and identified 11 large phase III studies allowing younger patients aged 18 years and above, which are listed in Table 1. The published data for these trials, including information on study design, patient characteristics (such as age, gender, MGMT promoter methylation status, IDH mutation status, and extent of resection), and efficacy outcomes (such as OS, PFS, and hazard ratios [HRs]), are summarized in Table 1. Only three of these trials demonstrated significant improvements in OS: the EORTC/NCIC trial4 investigating temozolomide, the EF-14 trial6 evaluating TTFields, and the CeTeG trial1 studying CCNU/TMZ. Additionally, three trials only allowing patients older than 60 years, NOA-08 trial, ISRCTN81470623-trial by Malmström et al. and NCT00482677-trial by Perry et al., showed benefit in OS.10–12
Table 1.
Overview of the filtered trials’ study design, efficacy endpoints, and prognostic patient characteristics in the target population including adult patients aged 18 years and above (younger cohort)1,4,6,13,15–17,19,22–24,26
| NCTID (primary completion date) | First author, year, journal | Eligibility criteria | Treatment arms Arm 1: control Arm 2: intervention |
Blind | # Pat | Gender m (%) | Age in years | MGMT-prom meth (%) | IDH-mut (%) | Extent of resection (%) | mOS HR (P-value) |
mPFS HR (P-value) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EORTC/NCIC NCT00006353 (2002–03) |
Stupp 2005 NEJM, Stupp 2009 |
ndGBM, 18–70 years, WHO PS 0-2 | Arm 1:RT Arm 2: RT+TMZ (Stupp) |
No | 573 | Arm 1: 61% Arm 2: 64% |
Arm 1: 57 (23–71) Arm 2: 56 (19–70) |
Arm 1: +MGMT: 46% −MGMT: 54% Arm 2: +MGMT: 43.4% −MGMT: 56.6% |
NA | Arm 1: Biopsy: 16%, Partial: 45% Complete: 40% Arm 2: Biopsy: 17%, Partial: 44% Complete: 39% |
Arm 1: 12.1 mo [−MGMT: 11.8 mo; +MGMT: 15.3 mo] Arm 2: 14.6 mo [−MGMT: 12.6 mo; +MGMT: 23.4 mo] HR 0.63 (P < .001) |
Arm 1: 5.0 mo Arm 2: 6.9 mo HR 0.54 (P < .001) |
| EF-14 NCT00916409 (2016–12) |
Stupp 2017 JAMA |
ndGBM, supratentorial, ≥18 years, KPS ≥70 | Arm 1: adjuvant TMZ (Stupp) Arm 2: adjuvant TMZ (Stupp) + TTFields |
No | 695 | Arm 1: 69% Arm 2: 68% |
Arm 1: 57 (19–80) Arm 2: 56 (19–83) |
Arm 1: +MGMT: 42% −MGMT: 51% Arm 2: +MGMT: 36% −MGMT: 54% |
IDH1-R132H: Arm 1: tested 52%, mut 5%, negative 95% Arm 2: tested 56%, mut 7%, negative 92% |
Arm 1: Biopsy: 13%, Partial: 33% GTR: 54% Arm 2: Biopsy: 13%, Partial: 34% GTR: 53% |
Arm 1: 16.0 mo [−MGMT:14.7 mo, +MGMT: 21.2 mo] Arm 2: 20.9 mo [−MGMT: 16.9 mo, +MGMT: 31.6 mo] HR 0.63 (P < .001) −MGMT: HR 0.66, +MGMT: HR 0.62 |
Arm 1: 4.0 mo Arm 2: 6.7 mo HR 0.63 (P < .001) |
| CeTeG NCT01149109 (2017–04) |
Herrlinger 2019 Lancet | ndGBM/gliosarcoma, +MGMT, 18–70 years, KPS ≥70 | Arm 1: RT+TMZ (Stupp) Arm 2: RT+TMZ+CCNU |
No | 141 | Arm 1: 48% Arm 2: 71% |
Arm 1: 56 (28–70) Arm 2: 59 (31–71) |
+MGMT: 100% | IDH1/2: total: mut 6%, wt 80%, oligo 5%; Arm 1: mut 8%, wt 83%, oligo 5%; Arm 2: mut 5%, wt 77%, oligo 5% |
Arm 1: Biopsy: 2%, Partial: 35% Complete: 63% Arm 2: Biopsy: 5%, Partial: 36% Complete: 59% |
Arm 1: mITT 31.4 mo; ITT 30.4 mo, PPP 30.4 mo Arm 2: mITT 48.1 mo; ITT 46.9 mo, PPP 40.3 mo HR 0.60 (P = .0492) |
Arm 1: 16.7 mo Arm 2: 16.7 mo HR 0.91 (P = .6775) |
| ASPECT EudraCT 2004-000464-28 | Westphal M 2013 Lancet Oncology | ndGBM, completely resectable, unifocal, supratentorial, KPS ≥70, 18–70 years | Arm 1: Standard care Arm 2: Standard care + sitimagene ceradenovec |
No | 250 | Arm 1: 65% Arm 2: 59% |
Arm 1: 57.0 (26–70) Arm 2: 58.0 (20–70) |
Arm 1: +MGMT: 24%, −MGMT: 76% Arm 2: +MGMT: 35%, −MGMT: 65% |
NA | Arm 1: radical: 81%, partial: 19% Arm 2: radical: 83%, partial: 17% |
Arm 1: 452 days Arm 2: 497 days HR 1.18 (P = .31) |
Not available |
|
NCT00304031 (2011–02) |
Gilbert MR 2013 JCO | ndGBM, ≥18 years, KPS ≥60 | Arm 1: RT+TMZ (Stupp 6–12 m) Arm 2: RT+TMZ (dose dense 6–12 m) |
No | 833 | Arm 1: 58% Arm 2: 56% |
Arm 1: <50: 27%, ≥50: 73% Arm 2: <50:26%, ≥50: 74% |
Arm 1: +MGMT: 30%, −MGMT: 62% Arm 2: +MGMT: 29%, −MGMT: 62% |
NA | Arm 1: biopsy 3%, Partial 41%, Total: 56%; Arm 2: biopsy 3%, Partial: 45%, Total: 52% |
Arm 1: 16.6 mo [−MGMT: 14.6 mo, +MGMT: 21.4 mo] Arm 2: 14.9 mo [−MGMT: 13.3 mo, +MGMT: 20.2 mo] HR 1.03 (P = .63) −MGMT: HR 0.99 (P = .44) +MGMT: HR 1.19 (P = .86) |
Arm 1: 5.5 mo; −MGMT: 5.1 mo, +MGMT: 6.5 mo Arm 2: 6.7 mo; −MGMT: 6.0 mo, +MGMT:10.1 mo HR 0.87 (P = .06); −MGMT: HR 0.88 (P = .15), +MGMT:HR 0.87 (P = .33) |
|
NCT00807027 (2012–10) |
Kong DS 2017 Oncotarget | ndGBM, 18–70 years, KPS ≥60 | Arm 1: RT+TMZ (Stupp) Arm 2: RT+TMZ (Stupp)+(CIK) cell immunotherapy |
No | 180 | Arm 1: 57.3% Arm 2: 56.0% |
Arm 1: 54 (23–68) Arm 2: 55 (19–69) |
NA | NA | Arm 1: biopsy 11.2%, Partial: 6.7%, Subtotal: 28.1%, Gross Total: 53.9% Arm 2: biopsy 13.2%; Partial: 8.8%, Subtotal: 29.7%, Gross Total: 48.4% |
Arm 1: 16.88 mo Arm 2: 22.47 mo HR 0.693 (P = .5237) |
Arm 1: 5.4 mo Arm 2: 8.1 mo HR 0.745 (P = .0401) |
| CENTRIC NCT00689221 (2012–11) |
Stupp R 2014 Lancet Oncol | ndGBM, supratentorial, ≥18 years, +MGMT, ECOG PS 0–1 | Arm 1: RT+TMZ (Stupp) Arm 2: RT+TMZ (Stupp)+cilengitide |
No | 545 | Arm 1: 52% Arm 2: 54% |
Arm 1: 58 (22–79) Arm 2: 58 (22–81) |
+MGMT: 100% | NA | Arm 1: biopsy 3%, Partial: 47%, Gross Total: 50% Arm 2: biopsy 3%, Partial: 48%, Gross Total: 49% |
Arm 1: 26.3 mo Arm 2: 26.3 mo HR 1.02 (P = .86) |
Arm 1: 10.7 mo Arm 2: 13.5 mo HR 0.93 (P = .46) |
| AVAglio NCT00943826 (2013–02) |
Chinot OL 2014 NEJM | ndGBM, supratentorial, ≥18 years, WHO PS 0–2 | Arm 1: RT+TMZ (Stupp)+placebo (concomitant) Arm 2: RT+TMZ (Stupp)+bevacizumab (concomitant) |
Double | 921 | Arm 1: 64.4% Arm 2: 61.6% |
Arm 1: 56 (18-79) Arm 2: 57 (20–84) |
Arm 1: +MGMT: 25.9%, −MGMT: 51.0% Arm 2: +MGMT: 25.5%, −MGMT: 49.1% |
NA | Arm 1: biopsy 9.5%, Partial: 48.2%, Complete: 42.3% Arm 2: biopsy 13.1%, Partial: 45.9%, Complete: 41.0 |
Arm 1: 16.7 mo Arm 2: 16.8 mo HR 0.88 (P = .10) |
Arm 1: 6.2 mo Arm 2: 10.6 mo HR 0.64 (P < .001) |
| RTOG 0825 NCT00884741 (2013–03) |
Gilbert MR 2014 NEJM | ndGBM, ≥18 years, KPS ≥70 |
Arm 1: RT+TMZ (Stupp 6–12 m) + placebo (adjuvant) Arm 2: RT+TMZ (Stupp 6–12 m)+bevacizumab (adjuvant) |
Double | 637 | Arm 1: 63% Arm 2: 57% |
Arm 1: <50: 21%, ≥50:79% Arm 2: <50: 18%, ≥50: 82% |
Arm 1: +MGMT: 28%, −MGMT: 69% Arm 2: +MGMT: 29%, −MGMT: 69% |
NA | Arm 1: Partial: 38%, Total: 59%, other: 3% Arm 2: Partial: 34%, Total: 63%, other: 3% |
Arm 1: 16.1 mo Arm 2: 15.7 mo HR 1.13 (P = .21) |
Arm 1: 7.3 mo Arm 2: 10.7 mo HR 0.79 (P = .007) |
| ACT IV NCT01480479 (2016–11) |
Weller M 2017 Lancet Oncology | ndGBM, +EGFRvIII, maximal resected, supratentorial, ECOG PS 0–2, ≥18 years | Arm 1: RT+TMZ (Stupp ≥6 m)+placebo Arm 2: RT+TMZ (Stupp ≥6 m)+Rindopepimut |
Double | 745 (MRD 405) | Arm 1: 58% Arm 2: 68% |
Arm 1: 57 (51–64) Arm 2: 59 (51–64) |
Arm 1: +MGMT: 35%, −MGMT: 57% Arm 2: +MGMT: 35%, −MGMT: 55% |
NA | Maximal resection for eligibility | Arm 1: MRD 20.0 mo; ITT 17.4 Arm 2: MRD 20.1 mo; ITT 17.4 MRD HR 1.01 (P = .93) ITT: HR 0.89 (P = .22) |
Arm 1: MRD 7.4 mo; ITT 5.6 Arm 2: MRD 8.0 mo; ITT 7.1 MRD HR 1.01 (P = .91) ITT: HR 0.94 (P = .51) |
| OSAG 101—BSA-05 NCT00753246, EudraCT 2005–003101-85 | Westphal M 2015 EJC | ndGBM, 18–70 years, KPS ≥70 | Arm 1: RT+TMZ (Stupp) Arm 2: RT+TMZ (Stupp) + nimotuzumab |
No | 149 | Arm 1: 63.4% Arm 2: 59.2% |
Arm 1: 56 (30–70) Arm 2: 55 (25–71) |
Arm 1: +MGMT: 22.5%, −MGMT: 45.1% Arm 2: +MGMT: 21.1%, −MGMT: 46.5% |
NA | Arm 1: residual tumor: 57.7%; no residual tumor: 42.3% Arm 2: residual tumor: 56.3% no residual tumor: 43.7%; |
Arm 1: 19.6 mo [−MGMT 15.5 mo, +MGMT: 33.8 mo] Arm 2: 22.3 mo [−MGMT 19.5 mo, +MGMT: nr] HR 0.86 (P = .49) [−MGMT HR 0.81 (P = .46), +MGMT: HR 0.86 (P = .80)] |
Arm 1: 5.8 mo; −MGMT: 5.8 mo, +MGMT: 12.7 mo, Arm 2: 7.7 mo; −MGMT: 8.3 mo, +MGMT: 8.9 mo HR 0.95 (P = .79) |
ECOG PS, ECOG Performance Score; HR, hazard ratio; ITT, intention-to-treat population; KPS, Karnofsky Performance Score; +MGMT, methylated MGMT promoter; −MGMT, unmethylated MGMT promoter; mITT, modified intention-to-treat population; mo, months; mut, mutated; NA, not available; nr, not reached; ppp, per protocol population; RT, radiotherapy; TMZ, temozolomide; WHO PS, WHO Performance Score; wt, wildtype. Green font marks significant results (P <.05), red font marks non significant results (P ≥.05). Positive studies are provided in blue.
Description of the EORTC/NCIC Trial
The EORTC/NCIC trial randomly assigned 573 patients aged 18–70 years to receive either radiotherapy alone or radiotherapy in combination with temozolomide (TMZ) followed by adjuvant TMZ.4 Following confirmation of histological diagnosis, patients were randomized over a period of 6 weeks. Of these, 286 patients were assigned to the control arm, while 287 were treated with temozolomide (TMZ) in the experimental arm. Patients in the TMZ group received a daily dosage of TMZ at 75 mg/m2 body surface during radiotherapy, which lasted no more than 49 days. After a 4-week hiatus, patients resumed TMZ treatment on the first 5 days of a 28-day cycle. This was repeated for a total of six cycles. The median OS was 12.1 months in the control arm, compared to 14.6 months in the experimental arm. The median OS was 12.1 months in the control arm and 14.6 months in the experimental arm, respectively, resulting in an unadjusted HR of 0.63 (P < .001). The median PFS was improved by almost 2 months in the treatment group (5.0 vs 6.9 months, HR 0.54, P < .001). A survival analysis based on prognostic factors showed an improvement in nearly all subgroups, with the exception of patients with biopsy only and those with a poor performance status.4 The mOS was significantly improved in patients with a methylated MGMT promoter, from 15.3 months with radiotherapy alone to 23.4 months with the addition of TMZ (P = .004). In patients with an unmethylated MGMT promoter, the increase in median OS was smaller, at 11.8 vs 12.6 months (P = .035).13
Description of the EF-14 Trial
The EF-14 study enrolled 695 patients aged 18 years or older with supratentorial glioblastoma multiforme, regardless of O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation status.6 Patients were stratified and randomly assigned to treatment arms consisting of temozolomide (TMZ) alone or alternating electric fields (Tumor Treating Fields; TTFields) in addition to adjuvant TMZ (TTFields/TMZ). Randomization was performed after surgery and concomitant radiotherapy with TMZ. Patients with progressive disease were excluded. Primary endpoint was median PFS. Secondary endpoint was OS but the used statistical methods sufficed to power both endpoints, OS and PFS. Final analysis of the intention-to-treat (ITT) population showed significantly improved OS (OS; 16.0 vs 20.9 months, HR 0.63, P < .001) and PFS (PFS; 4.0 vs 6.7 months, HR 0.63, P < .001) with the addition of TTFields to TMZ. A pre-specified subgroup analysis identified the OS benefit to be independent of canonical prognostic factors such as Karnofsky Performance Score (KPS), age, MGMT status, and extent of resection. Patients with methylated MGMT promoter showed a median OS of 31.6 months from randomization with TTFields/TMZ vs 21.2 months with TMZ alone (HR 0.62).6
Description of the CeTeG Trial
In the CeTeG trial, 141 patients aged 18–70 years with newly diagnosed glioblastoma or gliosarcoma and MGMT promoter methylation were randomized to receive either lomustine (CCNU) plus TMZ (CCNU/TMZ) or TMZ alone.1 Patients in both arms received standard radiotherapy (59–60 Gy). In the control arm, patients were treated according to the Stupp regimen. Meanwhile, the experimental arm received CCNU at 100 mg/m2 body surface on day 1 and temozolomide (TMZ) at 100–200 mg/m2 body surface from day 2 to day 6, following a 42-day cycle. This regimen was repeated for six cycles, with the second cycle commencing on day 43, thus forgoing the typical 4-week break after radiotherapy. Results were reported based on a pre-specified modified ITT analysis stratified for study center and recursive partitioning analysis (RPA) class (n = 109). A significantly improved median OS (31.4 vs 48.1 months, HR 0.60, P = .049) was reported, while PFS was not improved in comparison to the control group (16.7 vs 16.7 months, HR 0.91, P = .678). Adverse event rates were found to be higher in the experimental arm, with 59.1% of patients experiencing adverse events (including 26.4% with hematotoxicity), as compared to 50.8% in the control arm (including 28.6% with hematotoxicity). Despite these results, no deaths were reported due to toxicity. Furthermore, the rate of infectious diseases did not increase in the experimental arm.1
Studies in Elderly (≥60 Years of Age)
Although the NOA-08 trial, the ISRCTN81470623-trial and the NCT00482677 trial did not meet the full inclusion criteria for this review due to the absence of the Stupp protocol in the control arm, they are worth mentioning. This is because the Stupp protocol is not considered the standard of care for this cohort. Furthermore, these are the only Phase III glioblastoma trials that met all the other inclusion criteria of this review and demonstrated a survival benefit for elderly patients. The Japan Clinical Oncology Group is conducting a trial to determine whether short-course radiotherapy, delivering 25 Gy in increments of 5 Gy per fraction, coupled with concurrent (150 mg/m2 body surface, 5 days) and adjuvant temozolomide, is non-inferior to the current standard. The standard is 40 Gy delivered in 15 fractions with concurrent (daily 75 mg of temozolomide per square meter body surface) and adjuvant temozolomide. This trial is focused on elderly patients who are at least 71 years of age.14 Results are pending.
Description of NOA-08-Trial
The NOA-08 trial enrolled 412 patients aged 65 years and older, with confirmed diagnoses of anaplastic astrocytoma or glioblastoma. One group received temozolomide at a dosage of 100 mg/m2 body surface in alternating weekly cycles, while the other group underwent radiotherapy at 60 Gy with 1.8–2.0 Gy per fraction. A KPS lower than 60 was not considered a relevant exclusion factor for the per protocol population. The median overall survival was 8.6 months in the temozolomide group vs 9.6 months in the radiotherapy group, indicating a HR of 1.09 with a non-inferiority P-value of .03. The median event-free survival, defined as the time from surgery to the first progression for patients whose disease progressed, or to death for patients without progression, was 3.3 months in the temozolomide group vs 4.7 months in the radiotherapy group (HR 1.15, P = .043). Among MGMT-methylated patients, median event-free survival was longer when treated with temozolomide compared to radiotherapy (8.4 vs 4.6 months). Conversely, MGMT-unmethylated patients experienced a longer median event-free survival in the radiotherapy arm than in the temozolomide arm (4.6 vs 3.3 months). Intervention-related adverse events ranging from grade 3 to 4 were more frequent in the temozolomide group across all categories, except for cutaneous adverse events.12
Description of the ISRCTN81470623-Trial
The ISRCTN81470623 trial, conducted by Malmström et al., engaged 342 participants, each at least 60 years old, from seven European nations. They were subjected to one of three treatments: temozolomide (200 mg/m2 body surface for 5 days in a 28-day cycle, for up to six cycles), hypofractionated radiotherapy (34.0 Gy, 3.4 Gy per fraction over a span of 2 weeks), or standard radiotherapy (60 Gy, 2 Gy per fraction over 6 weeks).
Among the participants, 291 were randomly assigned to the three treatment groups (93 to temozolomide, 98 to hypofractionated radiotherapy, and 100 to standard radiotherapy), whereas 51 were randomly distributed between only two groups (26 to temozolomide and 25 to hypofractionated radiotherapy). In the trivariate randomization, the median OS was markedly prolonged in the temozolomide group compared to the standard radiotherapy group (8.3 vs 6.0 months, HR 0.70, P = .01). However, there was no significant difference between the hypofractionated radiotherapy and standard radiotherapy groups (7.5 vs 6.0 months, HR 0.85, P = .24). When considering all patients who received either temozolomide or hypofractionated radiotherapy (n = 242), no significant variance in OS was observed (8.4 vs 7.4 months, HR 0.82, P = .12). Participants older than 70 years demonstrated improved survival rates with either temozolomide or hypofractionated radiotherapy compared to standard radiotherapy (HR 0.35, P < .0001 for temozolomide vs standard radiotherapy; HR 0.59, P = .02 for hypofractionated radiotherapy vs standard radiotherapy). In the temozolomide group, patients with methylated MGMT exhibited increased survival rates (9.7 vs 6.8 months, HR 0.56, P = .02). MGMT methylation had no significant effect on OS in the radiotherapy groups (HR 0.97, P = .81). Across all groups, 18 patients suffered from grade 3–5 infections, two of which were fatal (one in the temozolomide group and one in the standard radiotherapy group). The most frequently observed adverse events in the temozolomide group were neutropenia and thrombocytopenia.10
Description of NCT00482677-Trial
The NCT00482677 trial, conducted by Perry et al., enrolled 562 glioblastoma patients aged 65 or older. Eligible patients had an ECOG score of 2 or lower. Participants received either radiotherapy alone, delivered as 40.05 Gy in 15 fractions, or a combination of radiotherapy with concomitant (75 mg/m2 body surface daily) and maintenance temozolomide (150–200 mg/m2 body surface, for 5 days out of a 28-day cycle, up to 12 cycles). OS was longer in the temozolomide group than in the radiotherapy-alone group (9.3 vs 7.6 months, with a HR of 0.67 and P < .001). PFS was also greater in the temozolomide group (5.3 vs 3.9 months, HR 0.5, P < .001). MGMT methylation was identified as a predictor for longer survival in the temozolomide group. In MGMT-methylated patients, the OS was 13.5 months in the temozolomide group compared to just 7.7 months in the radiotherapy-alone group (HR 0.53, P < .001). Meanwhile, in MGMT-unmethylated patients, OS was 10.0 months in the temozolomide group vs 7.9 months in the radiotherapy-alone group (HR 0.75, P = .055). The combination of radiotherapy and temozolomide was linked to a higher rate of toxicity.11
Description of Negative Phase III Trials
The ASPECT trial randomized 250 patients aged between 20 and 70 years with newly diagnosed glioblastoma amenable to complete resection. They were randomized to either undergo surgical resection of the tumor and receive an intraoperative perilesional injection of the prodrug converting enzyme sitimagene ceradenovec (1 × 1012 viral particles), followed by ganciclovir (administered postoperatively at 5 mg/kg intravenously twice a day), in conjunction with standard care, or to have the resection and standard care alone. The ganciclovir was phosphorylated by the thymidine kinase produced by transgene-expressing cells, creating ganciclovir triphosphatase, a cytotoxic nucleotide analogue that destroys dividing cells. The study found no significant survival benefit, yet noted an increase in treatment-related adverse events in the experimental arm (median OS, control arm: 452 days vs experimental arm: 497 days, HR 1.18, P = .31).15
In the NCT00304031 trial, 833 patients with newly diagnosed glioblastoma were enrolled. The experimental arm underwent “dose-dense” schedules of temozolomide, designed to diminish MGMT levels and activity, which in turn is supposed to enhance sensitivity towards chemotherapy with temozolomide. After standard radiochemotherapy, patients in the experimental arm received 6–12 cycles of 75–100 mg temozolomide per square meter body surface for 21 consecutive days in a 28-day-cycle. The control arm patients received standard maintenance therapy with temozolomide. No difference was found in median OS and median PFS between both groups (median OS control arm 16.6 vs experimental arm 14.9 months, HR 1.03, P = .63).16
Kong et al.17 investigated cytokine-induced killer (CIK) cells in a phase III trial. The authors randomized 180 patients aged from 19 to 69 years with newly diagnosed glioblastoma after several studies showed a benefit of CIK in other tumor types due to their ability of secreting cytotoxic molecules that regulate immune responses and provide a favorable microenvironment for anti-tumor activity. Glioblastoma patients in the experimental arm received standard radiochemotherapy combined with autologous CIK cells in 14 infusions at different time points. No statistical significant benefit in OS was found (OS control arm 16.9 months vs experimental arm 22.5 months, HR 0.693, P = .5237). Regarding PFS, however, was prolonged in the experimental group as compared to the control group receiving only standard radiochemotherapy with temozolomide (PFS 5.4 vs 8.1 months, HR 0.745, P = .0401).17,18
The CENTRIC trial aimed to investigate the efficacy of cilengitide, an inhibitor of αvβ3 and αvβ5 integrin and angiogenesis, when used in conjunction with standard radiochemotherapy as an experimental treatment strategy. Earlier Phase II trials had demonstrated potential benefits of cilengitide for patients newly diagnosed or experiencing recurrent glioblastoma, particularly those with MGMT promoter methylation. Consequently, the CENTRIC trial specifically recruited such patients, aged between 22 and 81 years, with newly diagnosed glioblastoma. A total of 545 patients participated in the study. Those assigned to the experimental arm received an additional dose of 2000 mg of cilengitide administered intravenously twice weekly, in conjunction with standard chemotherapy involving temozolomide. However, the addition of cilengitide did not demonstrate any benefit across the predefined subpopulations when compared to the control group, which received standard radiochemotherapy with temozolomide alone. This conclusion was based on the median OS rate that remained identical in both groups at 26.3 months (HR 1.02, P = .86), indicating the lack of statistical significance in the experimental treatment’s effectiveness.19–21
The AVAglio trial enrolled 921 patients, aged between 18 and 84 years, all diagnosed with glioblastoma. In the experimental arm, patients underwent radiotherapy (60 Gy, delivered in 2 Gy fractions) and temozolomide treatment (75 mg/m2 of body surface per day), supplemented with bevacizumab (10 mg/kg of body weight every 2 weeks). After a 4-week intermission, the treatment resumed with maintenance doses of bevacizumab and temozolomide (150–200 mg/m2 of body surface for 5 days within a 28-day cycle, over six total cycles). Once the temozolomide treatment was completed, bevacizumab infusions were continued at a dose of 15 mg/kg of body weight every 3 weeks. Patients in the control group were administered a placebo in lieu of bevacizumab. Bevacizumab acts as an anti-vascular endothelial growth factor A (VEGF-A) agent. VEGF holds a critical role in tumor angiogenesis, and glioblastoma typically exhibits overexpression of VEGF-A. The AVAglio trial found a significant improvement in PFS in the experimental arm (10.6 vs 6.2 months in the control group, HR 0.64, P < .001). However, there was no significant difference in the median OS between the two groups, with the control group at 16.7 months and the experimental group at 16.8 months (HR 0.88, P = .10).22
Another phase III trial, RTOG 0825/NCT00884741, also examined the use of bevacizumab in newly diagnosed glioblastoma patients. This trial followed a similar therapeutic strategy to the AVAglio trial, but with a notable difference: bevacizumab (10 mg/kg of body weight) was only initiated from the 4th week of combined radiochemotherapy and continued through up to 12 cycles of maintenance chemotherapy with temozolomide (150–200 mg/m2 of body surface for 5 days in a 28-day cycle, for a total of 6–12 cycles). Despite the varied approaches, the OS did not significantly differ between the two groups, with the control group at 16.1 months and the experimental group at 15.7 months (HR 1.13, P = .21). However, there was a significant difference in the PFS between the control group (7.3 months) and the experimental group (10.7 months, HR 0.79, P = .007), favoring the latter.23
The ACT IV study investigated the potential impact of Rindopepimut, a vaccine developed to target the EGFR mutation EGFRvIII. The study enrolled 745 patients, aged between 51 and 64 years. Amplification in the EGFR gene is a common occurrence in glioblastoma and has been recognized as one of three genetic markers in the updated WHO CNS 2021 classification that defines molecular glioblastoma. In this study, participants in the experimental arm received 500 µg of Rindopepimut and 150 μg of GM-CS monthly, in addition to standard chemotherapy with temozolomide (150–200 mg/m² for 5 of 28 days for 6–12 cycles or longer) following the completion of standard radiochemotherapy without progression. The control arm, on the other hand, received standard radiochemotherapy and a placebo (keyhole limpet hemocyanin). The study was concluded early after an interim analysis. The median OS did not show a significant difference between the two groups: 20.0 months (EGFRvIII+ glioblastoma and minimal residual disease, MRD) and 17.4 months (ITT) for the control group vs 20.1 months (MRD) and 17.4 months (ITT) for the experimental group. Statistical comparison showed a HR of 1.01 (P = .93) for the MRD and 0.89 (P = .22) for the ITT, indicating no significant benefit from the experimental treatment.24,25
The NCT00753246 trial was another study examining the impact of EGFR mutations in newly diagnosed glioblastoma patients. The study focused on Nimotuzumab, a monoclonal antibody against EGFR, and enrolled 149 patients ranging in age from 25 to 71 years. In the experimental arm of the trial, patients received Nimotuzumab as an additional treatment to standard radiochemotherapy with temozolomide. The regimen involved administering Nimotuzumab 400 mg weekly for the first 12 weeks, followed by 400 mg biweekly thereafter. However, the study found no significant difference in median OS between the control group, which received only standard radiochemotherapy, and the experimental group. The median OS was 19.6 months in the control group and 22.3 months in the experimental group, with a HR of 0.86 (P = .49), indicating no statistically significant benefit from the additional treatment with Nimotuzumab.26
Comparison of Study Design/Comparison of Eligibility and Outcome Criteria
Of the 11 identified studies in the final data set allowing younger adults, eight were designed as open-label randomized trials (73%), while only three employed treatment blinding and a placebo control (27%).22–24 The median number of enrolled patients was 573 (range 141–921), with 573 among the three positive trials (range 141–695) and 591 among the negative ones (range 149–921).
Comparison of Eligibility and Outcome Criteria
All but four of the identified investigations allowing younger adults applied similar eligibility criteria, including broad patient characteristics. The remaining four trials focused on specific patient subgroups: CeTeG1 and CENTRIC19 enrolled exclusively patients with methylated MGMT promoter; ASPECT enrolled only patients with completely resectable tumors15; and ACT IV investigated patients with maximally resected and epidermal growth factor receptor vIII (EGFRvIII) positive tumors.24
Due to the study-specific differences in eligibility criteria, the baseline characteristics were partially different, but overall, there was no statistical difference between the positive and negative trials regarding the weighted mean values for gender, age, extent of resection, or MGMT methylation status.
In the positive trials allowing younger patients, the weighted median proportion of male participants was 68% vs 58% in the negative trials, and the mean was 65.1% vs 59.7% males, respectively. With regard to age, median values in positive and negative trials were also similar: the weighted median age in the positive trials was 56 vs 57 years in negative studies (mean: 56.5 vs 57.1 years). However, the two trials published by Gilbert et al.16,23 indicated this variable as the proportion of patients <50 vs ≥50 years and were therefore not included in the analysis. MGMT status also did not differ significantly between positive and negative trials, with a weighted median proportion of 43% MGMT promoter methylated study participants vs 29% without MGMT methylation, and a corresponding mean of 46.6% vs 38.7%, respectively. Notably, one of the three identified positive trials (Herrlinger et al. 2019) and one of the eight negative trials (Westphal et al. 2013) enrolled exclusively patients with methylated MGMT promoters.1,15
Overall, the extent of resection was complete in a median of 50% of patients in both positive and negative trials, with corresponding means of 53.1% and 51.7%, respectively. However, the trial published by Westphal et al. (2013) only enrolled patients with completely resectable tumors, while the trial published by Weller et al. (2017) only enrolled patients with maximally resected and EGFRvIII positive tumors.15,24
It was not possible to directly compare the performance status of patients across trials because they used different scoring systems (Karnofsky or Eastern Cooperative Oncology Group [ECOG]). KPS and ECOG data are listed in Table 2.
Table 2.
Overview of ECOG and Karnofsky performance score distribution in the trials in standard and experimental arm
| Study | Required ECOG PS/KPS | ECOG PS/KPS distribution experimental group (patients involved) | Mean ECOG PS/KPS prior inclusion experimental arm (patients involved) | ECOG PS/KPS distribution control group (patients involved) | Mean ECOG PS/KPS prior inclusion control arm (patients involved) |
|---|---|---|---|---|---|
| Westphal et al. (2013) (“ASPECT”) | KPS ≥ 70 | 70 (n = 18, 15%) 80 (n = 22, 18%) 90 (n = 49, 41%) 100 (n = 30, 25%) |
87.6 (n = 119) | 70 (n = 11, 9%) 80 (n = 23, 20%) 90 (n = 47, 40%) 100 (n = 36, 31%) |
89.2 (n = 117) |
| Gilbert et al. (2013) (“Dose dense TMZ”) | KPS ≥ 60 | 60–80 (n = 146, 35%) 80–100 (n = 276, 65%) |
n.a. | 60–80 (n = 138, 34%) 90–100 (n = 273, 66%) |
n.a. |
| Kong et al. (2017) | KPS ≥ 60 | n.a. | 84.4 (n = 91) | n.a. | 85.7 (n = 89) |
| Stupp et al. (2014) (“CENTRIC”) | ECOG PS 0–1 | 0 (n = 156, 57%) ≥1 (n = 116, 43%) |
n.a | 0 (n = 151, 55%) ≥1 (n = 121, 44%) Missing 1 (n = 1, <1%) |
n.a. |
| Chinot et al. (2014) | KPS ≥ 50 | 50–80 (n = 149, 32.6%) 90–100 (n = 308, 67.4%) |
n.a. | 50–80 (n = 140, 30.3%) 90–100 (n = 322, 69.7%) |
n.a. |
| Gilbert et al. (2014) | KPS ≥ 70 | 60–80 (n = 124, 40%) 90–100 (n = 188, 60%) |
n.a. | 60–80 (n = 119, 39%) 90–100 (n = 190, 61%) |
n.a. |
| Weller et al. (2017) (“ACT IV”) | ECOG PS 0–2 | 0 (n = 165, 45%) 1 (n = 188, 51%) 2 (n = 18, 5%) |
0.6 (n = 371) | 0 (n = 168, 45%) 1 (n = 185, 50%) 2 (n = 21, 6%) |
0.6 (n = 374) |
| Westphal et al. (2015) | KPS ≥ 70 | n.a. | 90.4 (n = 70) | n.a. | 88.9 (n = 70) |
| Herrlinger et al. (2019) | KPS ≥ 70 | 70–80 (n = 9, 13.6%) 90–100 (n = 57, 86.4%) |
n.a. | 70–80% (n = 14, 22.2%) 90–100% (n = 49, 77.8%) |
n.a. |
| Stupp et al. (2005) | ECOG PS 0–2 | 0 (n = 113, 39%) 1 (n = 136, 47%) 2 (n = 38, 13%) |
0.74 (n = 287) | 0 (n = 110, 38%) 1 (n = 141, 49%) 2 (n = 35, 12%) |
0.74 (n = 286) |
| Stupp et al. (2017) | KPS ≥ 70 | ≤80 (n = 154, 33%) 90–100 (n = 308, 66%) Missing (n = 4, 1%) |
n.a. | ≤80 (n = 84, 32%) 90–100 (n = 149, 65%) Missing (n = 6, 3%) |
n.a. |
| Wick et al. (2012) | KPS ≥ 60 | 20–100 (n = 195, 100%, Temozolomide arm) | 70 (n = 195) | 50–100 (n = 178, 100%, radiotherapy arm) | 80 (n = 178) |
| Malmström et al. (2012) | ECOG PS 0–3 | 0–1 (n = 73, 78%, temozolomide arm) 2–3 (n = 20, 22%) 0–1 (n = 78, 80%, hypofractionated radiotherapy arm) 2–3 (n = 20, 20%) |
n.a. | 0–1 (n = 72, 72%, standard radiotherapy arm) 2–3 (n = 28, 28%) |
n.a. |
| Perry et al. (2017) | ECOG PS 0–2 | 0 (n = 74, 26.3%, radiotherapy + temozolomide arm) 1 (n = 141, 50.2%) 2 (n = 66, 23.5%) |
n.a. | 0 (n = 57, 20.3%, radiotherapy alone arm) 1 (n = 160, 56.9%) 2 (n = 64, 22.8%) |
n.a. |
KPS, Karnofsky Performance Score; ECOG PS, Eastern Cooperative Oncology Group Performance Score; n, number.
According to the latest World Health Organization (WHO) classification of Central Nervous System (CNS) tumors (CNS5), glioblastomas are classified differently based on their IDH status. IDH wildtype tumors are still classified as glioblastomas, while IDH-mutated glioblastomas are now classified as grade 4 astrocytomas. These changes present challenges for current treatment in clinical practice and clinical trials. Only the EF-14 and CeTeG trials analyzed the IDH status of their patients, with both showing similar proportions of patients with IDH mutations (5%–8%).1,27
Three other studies showed improved PFS but failed to improve OS: Kong et al. (2017) used CIK cell immunotherapy with radiochemotherapy, Chinot et al. (2014) administered bevacizumab with TMZ and radiotherapy, and Gilbert et al. (2014) also gave bevacizumab in addition to adjuvant TMZ.17,22,23
Quality of Life Data
In the EORTC/NCIC trial, 86% of patients had available health-related quality of life (HRQOL) baseline questionnaires. At the first follow-up, social functioning was better for patients who received radiotherapy alone compared to those who received additional TMZ (difference of 11.6 points [95% CI 3.5–19.7], P = .0052). However, subsequent HRQOL assessments did not show a difference between the groups. In the EF-14 study, 92% of patients completed the baseline HRQOL questionnaire and results showed that TTFields and TMZ did not negatively impact HRQOL over the course of a year, except for causing itchy skin. In the CeTeG trial, the combination of CCNU and TMZ did not reduce HRQOL, with a good proportion of surviving patients providing HRQOL data (over 60% for 2 years). Overall, the three trials that showed improved OS also demonstrated reasonable HRQOL.1,4,6 In all three trials, the NOA-08 trial, the ISRCTN81470623-trial and the NCT00482677 trial, HRQOL was assessed using the EORTC QLQ-C30 and BN20, which are general and brain cancer-specific quality-of-life questionnaires respectively. In the NOA-08 trial, data from 82% of enrolled patients were available. Over time, there were no significant differences observed between the two arms, except for an increase in communication deficits in the radiotherapy group for patients who passed away between 6 and 12 months. On the other hand, in the NCT00482677 trial, nausea and vomiting were found to be more severe in the group that received both temozolomide and radiotherapy, as compared to the group that received radiotherapy alone. Apart from this, the scores for the remaining parameters were similar in both groups. In the ISRCTN81470623 trial, HRQOL data were available for 83% of the 342 patients at the baseline. Of those patients who were alive at follow up, data were available for 59% 6 weeks post-treatment initiation, and for 44% 3 months into therapy. The authors advised that data from 3 months after therapy initiation be interpreted cautiously due to the small number of completed questionnaires. The scarcity of collected questionnaires 6 months after starting therapy precluded their reporting in the study. Interestingly, patients in the temozolomide group reported a higher quality of life (QOL) than those in the radiotherapy groups. However, when evaluated for the global health rating, results were found to be comparable across all groups.10–12
In most of the negative trials, QOL was measured during the trial’s implementation. Gilbert et al.16 reported that a subset of patients completed the EORTC QLQ-C30 and BN20 QOL questionnaires. In this trial, QOL, as measured by the EORTC QLQ-C30/BN20 scales, declined from baseline to cycle 4 in the dose-dense arm.28 In the trial conducted by Kong et al.17, all patients were monitored using global health status scores, and it was noted that the EORTC QLQ-C30 significantly worsened in both treatment arms, with no significant difference between the groups (P = .6409). Chinot et al. reported in their trial that QOL data were collected using the EORTC QLQ-C30 and BN20. They found that baseline HRQOL and performance status were maintained longer in the experimental arm, where bevacizumab was used. During the period of PFS, QOL was described as consistently stable across all domains. Additionally, the deterioration-free survival was significantly longer in the bevacizumab group (P < .05).22 Interestingly, contrary QOL results were found in the bevacizumab group in another trial by Gilbert et al. The authors reported that patients in the bevacizumab arm experienced increased symptom severity and a decline in HRQOL. These patients also had higher deterioration in neurocognitive function, as tested by a neurocognitive-function test battery, compared with the placebo group.23 For QOL assessment, Weller et al.24 used the Anderson Symptom Inventory Brain Tumor (MDASI-BT), EORTC QLQ-C30, and BN20. They found no statistically significant differences between the experimental arm and the control arm. In the trial by Westphal et al., which investigated nimotuzumab, QOL was a secondary endpoint. HRQOL was assessed by EORTC QLQ-C30 and QLQ-BN20. Data was collected four times during the first 8 months, then every 12 weeks thereafter. Differences in functional scales including physical, role, emotional, cognitive, and social functioning did not reach significant levels, even though patients in the experimental arm showed more favorable results than those in the control group. The conclusion from this trial was that nimotuzumab did not negatively impact HRQOL (Table 2).26
Discussion
This systematic review of glioblastoma multiforme clinical trials published since 2005, which included adult patients of all ages, identified three positive studies in young adults: the landmark trial by Stupp et al. (2005) which established temozolomide (TMZ) as the standard of care for subsequent trials; the EF-14 study (Stupp et al. 2017) which added TTFields to adjuvant TMZ chemotherapy; and the CeTeG trial (Herrlinger et al. 2019) which combined TMZ with CCNU.1,4,6 All three of these studies demonstrated similar HRs, indicating a risk reduction of approximately 40%. A 2018 analysis of the glioblastoma clinical study landscape by Vanderbeek and colleagues29 reached a similar conclusion, but did not include the CeTeG trial, which had not been published at the time. It is worth noting that a phase III trial by Perry et al.11 that applied hypofractionated radiotherapy to elderly patients with newly diagnosed glioblastoma (aged 65 years and older) also reported significantly improved survival in this population. The NOA-08 trial showed a non-inferiority of temozolomide therapy compared to radiotherapy alone in older patients with glioblastoma and anaplastic astrocytoma.12 In the ISRCTN81470623-trial standard radiotherapy was associated with poor outcomes, especially in patients older than 70 years of age.10 In this review we tried to identify which trials since 2005 showed survival benefit in younger and older patients. Unfortunately, not all studies (eg, EF-14 trial) distinguish between these two groups which leads to missing data especially in older patients. When prognostic factors such as sex, age, MGMT promoter methylation state, and extent of resection were controlled for, the baseline characteristics of the positive and negative trials allowing younger adults were comparable, and no bias in patient selection that could impact trial results was identified. Additionally, when controlling for prognostic factors such as MGMT promoter methylation state and time to randomization, the median OS of the control groups in these clinical trials was similar. This supports the finding that there was no significant bias in patient selection that could have affected the outcomes of the positive trials compared to the negative ones.
We also considered trials with few participants randomized also if they maybe lack of enough statistical power. But it is worth noting that all trials with less than 100 participants violated other exclusion criteria like “no stupp scheme in control arm” or “no superiority analysis.” One study was terminated due to unacceptable toxicity. Finally, all these studies would have been also excluded if they had a higher number of participants randomized.
The majority of the trials analyzed were open-label and did not incorporate a placebo control, including the three trials with positive outcomes in younger adults. Despite this, there were numerous open-label trials with negative results, such as the CENTRIC trial,19 which utilized conventional medical interventions without a placebo control and failed to demonstrate a survival advantage. Nevertheless, some critics have raised concerns that the results of the EF-14 trial may be confounded by increased medical attention received by patients in the experimental arm. This argument is based on the findings of Temel et al.30, where early palliative care was associated with significant improvements in OS in comparison to the standard of care in patients with metastatic non-small cell lung cancer. Conversely, a meta-analysis by the Cochrane Institute determined that early palliative care did not have a significant impact on OS in oncology patients.31 The authors warned that the results by Temel et al. may not be generalizable to other cancer types and advised clinicians against making blanket claims regarding improved survival outcomes. It is important to underscore that our results should not be interpreted as negating the potential survival benefits of early palliative care integration. Consistent with existing literature, our study maintains that cancer patients, including those diagnosed with glioblastoma, can substantially benefit from early palliative care interventions. These services are essential in managing patients’ physical and psychological needs, which in turn might contribute to improved survival rates. The present study does not counter this established understanding. A post-hoc analysis of the EF-14 trial refutes the suggestion that the positive results were merely due to increased medical attention in the experimental arm by demonstrating a positive correlation between TTFields intensity, dose density applied to the tumor bed, and OS, PFS, and QOL.32,33 These findings suggest that the efficacy observed in the positive trials cannot be solely attributed to a placebo effect. It has been established that while placebo effects may influence patient-reported outcomes such as pain, objective clinical outcomes such as OS and PFS (assessed by a blinded review panel) are insensitive to placebo effects in both cancer therapy and other clinical contexts.34,35 Although the utilization of placebo controls in future neuro-oncology trials is recommended, the absence of placebo controls in the three positive trials does not appear to detract from their validity. The use of TTFields and CCNU/TMZ has been shown to be effective in the treatment of newly diagnosed glioblastoma, but many ongoing clinical trials do not allow for the use of these therapies in the control group. This systematic analysis suggests that both TTFields and CCNU/TMZ, which have demonstrated a survival benefit in phase III trials for newly diagnosed glioblastoma patients, should be considered for inclusion in future clinical studies. This is consistent with the findings of Vanderbeek et al.29, who found that a majority of clinical trial participants are enrolled in negative trials, highlighting the importance of providing the best-available treatment options, particularly in control groups. A retrospective analysis of 16 patients receiving a combination of TTFields and CCNU/TMZ showed that this approach was feasible and safe.36 In a more recent retrospective analysis with a larger sample size (n = 70), treatment with TTFields + CCNU/TMZ was shown to improve OS significantly as opposed to CCNU/TMZ alone, suggesting the need for further investigation of this combination therapy.37 On the contrary, it should be noted that incorporating TMZ, CCNU, and TTFields into control arms for future clinical trials could significantly elevate the costs involved. Rominiyi et al.38 discuss various studies that investigate the cost-effectiveness of TTFields. Using interim data from the EF-14 trial, Bernard-Arnoux et al.39 calculated the cost of achieving 0.34 additional life-years through the use of TTFields at €185 476 from the perspective of French National Health Insurance. Connock et al., using final results from the same trial, estimated that gaining an additional 0.604 life-years would cost €453 848. The authors concluded that TTFields may not be cost-effective, suggesting that price regulation by health authorities could improve accessibility for GBM patients.40 It’s worth emphasizing that in the EF-14 trial, as opposed to the CeTeG and EORTC/NCIC trials, randomization was performed post-radiochemotherapy, and only patients without disease progression were included. This is because the trial evaluated a medical product used in first-line maintenance therapy, excluding patients with a poor disease trajectory. Future and current study participants, however, have a right to the best-available care in control arms, which presents a dilemma. Also, some patients may refuse TTFields treatment due to personal reasons or individual treatment choices, and these patients should not be automatically excluded from participating in clinical trials if TTFields becomes more prevalent in control arms. The combination of CCNU and TMZ can lead to higher toxicity than standard TMZ monotherapy, making it unsuitable for some patients. These complexities could result in more intricate study designs involving multiple treatment arms and higher recruitment numbers for significant study results, thereby increasing trial costs and implementation duration. In conclusion, determining the optimal treatment approach for future GBM trials is challenging. Despite the numerous pitfalls, given the preponderance of unsuccessful trials in recent years, researchers must be acutely aware of their responsibility to patients when designing new trials. Perhaps it would be prudent to invest more effort into trials involving alkylating agents, as they are the only agents that have demonstrated systemic therapy benefits over the past 17 years, while numerous novel, costly antibodies have failed to do so.
The prevalence of negative results in recent phase III studies may stem from subpar preclinical studies, especially those preceding clinical trials. Notably, many glioblastoma preclinical studies lack the standard control arm RT + TMZ when testing new drugs, possibly misrepresenting clinical efficacy. Another issue is the often subtherapeutic exposure of drugs to tumors, though awareness is growing. Microdosing trials, as discussed by Calvert et al.41, are vital for ensuring drug efficacy. Meanwhile, phase II trials display varied designs and endpoints—some focus on PFS while others use composite scores. This inconsistency complicates cross-study comparisons. Standardized phase II trial designs and sample size calculations are imperative for clarity and consistency. Finally, issues with reproducibility underscore the necessity for robust study designs. Addressing these highlighted areas will likely enhance the validity and success rates of clinical trials.
Also further investigation of TTFields for glioblastoma patients may also be promising. Glas et al. re-analyzed EF-14 trial data, focusing on progression patterns associated with TTFields use. They found that TTFields could control local tumors effectively, while the experimental group using TTFields developed different progression patterns directly correlated with TTFields dose distribution. Since TTFields can treat larger volumes with a low toxicity profile and no known effects on healthy brain tissue, this could fill a therapeutic gap.33 Dono et al. identified that molecular markers could predict TTFields response in progressive disease. In a retrospective analysis of 149 patients, 29 were found to be using TTFields. In this cohort, post-progression survival increased when PTEN was mutated (22.2 vs 11.6 months, P = .017). Further research with larger cohorts is needed to understand the use of TTFields based on molecular markers.42 Ongoing investigations into TTFields include the EF-32 phase III trial, which tests TTFields use during radiochemotherapy in newly diagnosed glioblastoma patients (NCT04471844), and the non-interventional TIGER PRO-Active study, investigating changes in daily activity, sleep, and neurocognitive function while using TTFields (NCT04717739).
This report acknowledges several limitations that must be considered. Firstly, the methodology and search criteria used may have resulted in the exclusion of certain trials, and the prolonged time frame and varied standards made it challenging to compare certain patient characteristics. The definitions of “complete resection” and the performance status assessment scales varied across trials. Furthermore, several recently completed trials in newly diagnosed glioblastoma have yet to be published in a peer-reviewed format and have reported negative outcomes with regards to OS in press releases and at congresses, including the INTELLANCE-1 and MIRAGE trials. Checkmate-498 and Checkmate-548 have been published shortly after data cutoff (2022/19/09) but were already known to be negative before.43,44 The recent World Health Organization (WHO) classification of CNS tumors presents advancements in molecular pathology, yet presents challenges in its implementation in clinical practice and future trials. The treatment options for grade 4 astrocytoma, in particular, have not been thoroughly investigated and it is crucial to take into account the new classification in the design of future trials to address this patient population.
Conclusion
This systematic analysis of phase III clinical trials of glioblastoma revealed that only the Stupp et al. trial of 2005 and two other studies, the EF-14 and CeTeG trials, demonstrated positive survival outcomes in newly diagnosed non-elderly glioblastoma patients.
In conclusion, given the findings presented and the numerous rigorous studies with negative results, the integration of these best-available therapies into clinical practice and future clinical trials warrants careful discussion. This aligns with current treatment recommendations from reputable sources such as Hofer et al. (2021), Jiang et al. (2021), Nabors et al. (2020), Stupp et al. (2005), Wen et al. (2020), and Wick (2021).3–5,45–47 However, important counterarguments such as cost considerations, potential for increased toxicity, and the necessity of larger study population sizes should also be given due consideration in this approach.
Contributor Information
Christoph Oster, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Teresa Schmidt, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Sarina Agkatsev, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Lazaros Lazaridis, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Christoph Kleinschnitz, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Ulrich Sure, German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; Department of Neurosurgery and Spine Surgery, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Björn Scheffler, German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Sied Kebir, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Martin Glas, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Funding
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Conflict of interest statement
Christoph Oster received honoraria from Horizon. He also received travel support from Novocure. Lazaros Lazaridis, and Teresa Schmidt received honoraria and travel support from Novocure. Martin Glas has received research grant from Novocure. He has received honoraria from Roche, Seagan, Servier, Novartis, UCB, Abbvie, Daiichi Sankyo, Bayer, Janssen-Cilag, Kyowa Kirin, Medac, Merck and Novocure. He has received travel support from Novocure and Medac. Sied Kebir has Consulting/advisory role to Novocure. He has received travel support from Merck and Novocure. He got honoraria from Biogen, Merck and Novocure. Björn Scheffler is supported by the German Cancer Consortium (DKTK) and the DFG-CRU337. Christoph Kleinschnitz, Sarina Agkatsev and Ulrich Sure declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors contributed to the study conception and design. PRISMA analysis: C.O., S.K. and M.G. Written the manuscript: all authors. Correction of manuscript: M.G., S.K., U.S., C.K. and B.S. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1. Herrlinger U, Tzaridis T, Mack F, et al. ; Neurooncology Working Group of the German Cancer Society. Lomustine–temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Patil N, Cioffi G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nabors LB, Portnow J, Ahluwalia M, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18(11):1537–1570. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:18046. https://www.nature.com/articles/srep18046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–3295. [DOI] [PubMed] [Google Scholar]
- 9. Wong ET, Lok E, Swanson KD.. Alternating Electric fields therapy for malignant gliomas: from bench observation to clinical reality. Prog Neurol Surg. 2018;32:180–195. https://karger.com/books/book/260/chapter-abstract/5179328/Alternating-Electric-Fields-Therapy-for-Malignant?redirectedFrom=fulltext [DOI] [PubMed] [Google Scholar]
- 10. Malmstrom A, Gronberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 11. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 12. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 14. Arakawa Y, Sasaki K, Mineharu Y, et al. A randomized phase III study of short-course radiotherapy combined with Temozolomide in elderly patients with newly diagnosed glioblastoma; Japan clinical oncology group study JCOG1910 (AgedGlio-PIII). BMC Cancer. 2021; 21(1):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westphal M, Yla-Herttuala S, Martin J, et al. ; ASPECT Study Group. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. [DOI] [PubMed] [Google Scholar]
- 16. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong DS, Nam DH, Kang SH, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8(4):7003–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–91.e6. [DOI] [PubMed] [Google Scholar]
- 19. Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC). Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 20. Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–5617. [DOI] [PubMed] [Google Scholar]
- 21. Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(16):2712–2718. [DOI] [PubMed] [Google Scholar]
- 22. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 23. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 25. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westphal M, Heese O, Steinbach JP, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522–532. [DOI] [PubMed] [Google Scholar]
- 27. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanderbeek AM, Rahman R, Fell G, et al. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro Oncol. 2018;20(8):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 31. Haun MW, Estel S, Rucker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6(6):CD011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ballo MT, Urman N, Lavy-Shahaf G, et al. Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: a large-scale numerical simulation-based analysis of data from the phase 3 EF-14 randomized trial. Int J Radiat Oncol Biol Phys. 2019;104(5):1106–1113. [DOI] [PubMed] [Google Scholar]
- 33. Glas M, Ballo MT, Bomzon Z, et al. The impact of tumor treating fields on glioblastoma progression patterns. Int J Radiat Oncol Biol Phys. 2022;112(5):1269–1278. [DOI] [PubMed] [Google Scholar]
- 34. Chvetzoff G, Tannock IF.. Placebo effects in oncology. J Natl Cancer Inst. 2003;95(1):19–29. [DOI] [PubMed] [Google Scholar]
- 35. Hrobjartsson A, Gotzsche PC.. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;2010(1):CD003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lazaridis L, Schafer N, Teuber-Hanselmann S, et al. Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J Cancer Res Clin Oncol. 2020;146(3):787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lazaridis L, Bumes E, Cacilia Spille D, et al. First multicentric real-life experience with the combination of CCNU and temozolomide in newly diagnosed MGMT promoter methylated IDH wildtype glioblastoma. Neurooncol Adv. 2022;4(1):vdac137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rominiyi O, Vanderlinden A, Clenton SJ, et al. Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer. 2021;124(4):697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernard-Arnoux F, Lamure M, Ducray F, et al. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Connock M, Auguste P, Dussart C, Guyotat J, Armoiry X.. Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: an updated evaluation using a partitioned survival model. J Neurooncol. 2019;143(3):605–611. [DOI] [PubMed] [Google Scholar]
- 41. Calvert AH, Plummer R.. The development of phase I cancer trial methodologies: the use of pharmacokinetic and pharmacodynamic end points sets the scene for phase 0 cancer clinical trials. Clin Cancer Res. 2008;14(12):3664–3669. [DOI] [PubMed] [Google Scholar]
- 42. Dono A, Mitra S, Shah M, et al. PTEN mutations predict benefit from tumor treating fields (TTFields) therapy in patients with recurrent glioblastoma. J Neurooncol. 2021;153(1):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Omuro A, Brandes AA, Carpentier AF, et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 2023;25(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim M, Weller M, Idbaih A, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24(11):1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hofer S, Bullinger L, Dierlamm J, et al. Gliome im Erwachsenenalter. 2021. https://www.onkopedia.com/de/onkopedia/guidelines/gliome-im-erwachsenenalter/. Accessed February 12, 2023.
- 46. Wick W, et al. Gliome, S2k-Leitlinie. 2021. https://dgn.org/leitlinien/ll-030-099-gliome-2021/. Accessed February 12, 2023.
- 47. Jiang T, Nam DH, Ram Z, et al. ; Chinese Glioma Cooperative Group (CGCG). Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021;499:60–72. [DOI] [PubMed] [Google Scholar]