Abstract
A human cytomegalovirus mutant that was isolated for resistance (10-fold) to the antisense oligonucleotide fomivirsen (ISIS 2922) exhibited cross-resistance to a modified derivative of fomivirsen with an identical base sequence but little or no resistance to an oligonucleotide with an unrelated sequence. No changes in the mutant’s DNA corresponding to the fomivirsen target sequence were found.
Oligonucleotides, including those that target viral mRNAs via antisense mechanisms, form an approach to antiviral therapy that is proceeding from the laboratory bench to the bedside. One such oligonucleotide, fomivirsen (ISIS 2922), is presently in phase III clinical trials for human cytomegalovirus (CMV) retinitis. Fomivirsen (Table 1) is a 21-base oligonucleotide with phosphorothioate linkages that is complementary to human CMV immediate-early 2 (IE2) mRNA. This compound exhibited more potent anti-CMV activity in cell culture than did a variety of other phosphorothioate oligonucleotides screened. It inhibited IE gene expression in CMV-infected cells and in cells transfected with IE genes, in agreement with an antisense mechanism (1, 2). However, studies of effects on virus adsorption and structure-activity relationships with phosphorothioate oligonucleotides with various sequences indicated that nonantisense mechanisms may contribute to antiviral activity.
TABLE 1.
Sequences of phosphorothioate oligonucleotidesa
| Phosphorothioate oligonucleotide | 5′-3′ Sequence | Target site or characteristic |
|---|---|---|
| Fomivirsen | -GCGTTTGCTCTTCTTCTTGCG- | IE2 mRNA |
| ISIS 13312 | -GCGTTTGCTCTTCTTCTTGCG- | IE2 mRNA |
| ISIS 3383 | -TGGGCACGTGCCTGACACGGC- | Not complementary to any HCMV sequence |
The base sequences of the phosphorothioate oligonucleotides used in this study, synthesized as previously described (1, 2), are shown. Residues in ISIS 13312 that contain 2′-O-methoxyethyl substituents are underlined; C residues, which contain 5-methyl substituents, are indicated by boldface italics.
Studies of drug-resistant viruses have provided insights into the mechanisms of action of antiviral drugs, into the potential for drug resistance in the clinic, into structure-function relationships of drug targets, and into mechanisms of basic viral processes ranging from uncoating through gene expression to pathogenesis (11). In terms of mechanisms of action, the isolation of virus mutants resistant to a drug strongly implies that the drug acts at least in part via a virus-specific process rather than by rendering the host cell incapable of supporting virus replication. Thus, the isolation of a drug-resistant mutant is perhaps the best evidence that an antiviral drug is truly selective (5, 7).
Isolation of CMV resistant to fomivirsen.
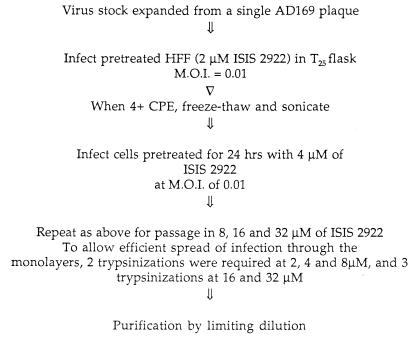
To begin investigations of these issues with antiviral phosphorothioate oligonucleotides, we passaged CMV in increasing concentrations of fomivirsen (Fig. 1). The stock of CMV used was derived from a single plaque of wild-type laboratory strain AD169. Briefly, at each passage, human foreskin fibroblast cells were pretreated overnight with each concentration of drug in serum-free fibroblast growth medium (FGM; Clonetics Corp., Palo Alto, Calif.). After pretreatment, the cells were rinsed with FGM and infected at a multiplicity of infection of 0.01 with virus diluted in FGM. Virus was allowed to adsorb for 1 h at 37°C. Then, the infected cells were overlaid with fresh medium containing the concentration of drug used to pretreat the cells. Virus replication was allowed to continue at the same drug concentration until complete cytopathic effect (4+) was achieved. At the indicated passages (Fig. 1), infected cells were trypsinized and replated to permit efficient spread of the infection. Evidence for a resistant virus subpopulation was obtained following passage at a concentration of 16 μM fomivirsen (8). Following passage at a concentration of 32 μM, virus was purified by limiting dilution, once in the presence of 32 μM fomivirsen and twice in the absence of the drug. The resulting purified virus was designated 2922rA-32-1. This mutant formed plaques similar in size to those of AD169 and replicated to titers similar to those of AD169 during preparation of virus stocks.
FIG. 1.
Procedures used to isolate a mutant resistant to fomivirsen. HFF, human foreskin fibroblasts; M.O.I., multiplicity of infection.
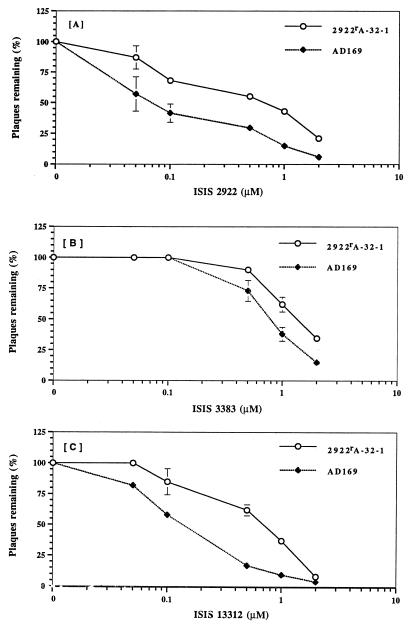
We compared 2922rA-32-1 with parental strain AD169 for susceptibility to fomivirsen by a plaque reduction assay (Fig. 2A) performed as previously described (13), except that cells were pretreated with the indicated concentrations of drug overnight in serum-free FGM and then rinsed with medium. Following adsorption, the cells were overlaid with FGM containing 0.1% fetal bovine serum, 1% methylcellulose, and the indicated concentrations of drug. For AD169, the dose that reduced plaque formation by 50% (ED50) was 70 nM, in agreement with previous results (2). For 2922rA-32-1, the ED50 was 700 nM, which was 10-fold higher than that for AD169. By a two-sample (two-tailed) t test applied to the data plotted in Fig. 2A and those from an additional assay, the ED50 for the mutant was significantly different from that for AD169 (P < 0.02). The ED50 for the mutant was similar to the ED50 observed for the entire virus population following passage at 32 μM (10). Thus, 2922rA-32-1 was resistant to fomivirsen.
FIG. 2.
Susceptibilities to phosphorothioate oligonucleotides. Plaque reduction assays were performed as described in the text. Results presented are the averages of two separate experiments, each performed in duplicate. Similar results were obtained in a third experiment, in which different drug concentrations were used. Error bars represent standard errors of the means. Error bars are not presented for standard errors of the means that were too small to be visible on the plots.
Sequence-dependent resistance.
There is considerable evidence that phosphorothioate oligonucleotides can inhibit viral replication by both sequence-dependent and sequence-independent mechanisms (14). Therefore, we asked whether 2922rA-32-1 had developed resistance to sequence-dependent or sequence-independent inhibition of CMV replication. To investigate this question, we compared the mutant and AD169 for susceptibilities to a phosphorothioate oligonucleotide with a sequence unrelated to that of fomivirsen and to one with a highly related sequence. For an oligonucleotide of unrelated sequence, we tested ISIS 3383 (Table 1), which is not complementary to any CMV sequence and, like many other oligonucleotides, is less potent against CMV than fomivirsen (although ISIS 3383 is similar in potency to ganciclovir) (1, 2). For an oligonucleotide with a sequence related to that of fomivirsen, we tested ISIS 13312, which has a base sequence identical to that of fomivirsen but which contains modifications at the 2′ position at 12 of the 21 residues and 5-methyl substituents on each C residue (Table 1).
As shown in Fig. 2B, the ED50 in plaque reduction assays of the non-sequence-specific compound ISIS 3383 for AD169 was 0.8 μM, which was more than 10 times higher than the ED50 of fomivirsen, similar to previously published values (2). Mutant 2922rA-32-1 exhibited, at most, marginal resistance to ISIS 3383. Its ED50 was <2-fold greater than that for AD169; the mutant and AD169 also exhibited <2-fold differences in plating efficiency at any drug concentration. Moreover, although at certain drug concentrations the error bars for the wild type and mutant did not overlap (Fig. 2B), by a two-sample t test, the ED50 for the mutant was not significantly different from that for AD169 (P > 0.05). Thus, by our previously published criteria (3, 6), the mutant was not resistant to this non-sequence-specific oligonucleotide.
ISIS 13312 was more potent against wild-type CMV strain AD169 (ED50 = 0.15 μM) than was ISIS 3383 (compare Fig. 2B and C), in agreement with its acting in a sequence-dependent manner, although it was not as potent as fomivirsen (compare Fig. 2A and C). In contrast to its sensitivity to ISIS 3383, mutant 2922rA-32-1 exhibited resistance to ISIS 13312, and the ED50 for it was 0.7 μM, which was four- to fivefold higher than that for AD169 (Fig. 2C). By a two-sample t test, the ED50 for the mutant was significantly higher than that for AD169 (P < 0.002). Thus, resistance of the mutant was sequence dependent.
The mutant is not altered in the region complementary to fomivirsen.
Assuming an antisense mechanism for fomivirsen and ISIS 13312, one way in which a mutant could become resistant to these drugs would be by base changes that alter the complementarity of IE mRNA for the oligonucleotides. Therefore, we PCR amplified this region of the CMV genome from the three-times plaque-purified mutant 2922rA-32-1 using primers corresponding to nucleotides 170004 to 170024 and 170305 to 170285 of the AD169 sequence (GenBank accession no. X17403; the fomivirsen target sequence corresponds to nucleotides 170120 to 170140). Virion DNA was isolated as described elsewhere (12), and PCR was performed by using the conditions described elsewhere with 3.5 mM MgCl2 added (4). The relevant PCR product was purified with the Advantage PCR-pure kit (Clontech) and sequenced with the primers employed for PCR by the Biopolymers Facility of the Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, with an ABI sequencer. No differences from AD169 in the region sequenced were observed. Thus, resistance of the mutant was not due to loss of encoded complementarity with the oligonucleotide.
Potential implications.
To our knowledge, there have been no published reports describing viral mutants resistant to an oligonucleotide-based drug. We are aware of studies of human immunodeficiency virus mutants resistant to certain oligonucleotides; however, such mutants have not exhibited sequence-dependent resistance (9). Our ability to isolate a mutant resistant to fomivirsen implies that this compound acts at least in part via a virus-specific process (i.e., selectively against CMV), in agreement with previous studies of its mechanism (1, 2). The mutant’s cross-resistance to ISIS 13312 implies that this derivative of fomivirsen is also a selective anti-CMV compound. The isolation of this mutant in cell culture raises the possibility that resistant mutants could arise during administration of fomivirsen or its derivatives to patients. However, we do not know if the degree of resistance that we observed would be clinically significant. Nor do we know whether such mutants would retain sufficient pathogenicity to cause CMV disease.
We can envision two kinds of mutations that would confer the sequence-dependent resistance of 2922rA-32-1. The first assumes an antisense mechanism of action of fomivirsen. In this case, even though we did not detect any alteration in the region of the mutant genome that is complementary to the oligonucleotide, resistance could be conferred by various mechanisms, including mutations that increased the expression of IE mRNA or the function of IE proteins. The second kind of mutation assumes a novel target for fomivirsen other than IE mRNA. In this case, the mutation could be one that alters this still-unknown target. Although it is time-consuming, mapping the resistance mutation(s) in 2922rA-32-1 should distinguish between these two mechanisms of resistance and shed light on the mechanism of action of fomivirsen.
Acknowledgments
We thank the ISIS oligonucleotide synthesis group for providing phosphorothioate oligonucleotides for selection and testing of virus and C. Mulder for communicating results prior to publication.
This work was supported by a grant from the NIH (AI 26077), with additional support to the Coen laboratory from Isis.
REFERENCES
- 1.Anderson K P, Fox M C, Brown-Driver V, Martin M J, Azad R F. Inhibition of human cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob Agents Chemother. 1996;40:2004–2011. doi: 10.1128/aac.40.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad R F, Driver V B, Tanaka K, Crooke R M, Anderson K P. Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob Agents Chemother. 1993;37:1945–1954. doi: 10.1128/aac.37.9.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou H C, Weller S K, Coen D M. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology. 1985;145:213–226. doi: 10.1016/0042-6822(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 4.Coen D M. Enzymatic amplification of DNA by PCR: standard procedures and optimization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1991. [Google Scholar]
- 5.Coen D M. The implications of resistance to antiviral agents for herpesvirus drug targets and drug therapy. Antivir Res. 1991;15:287–300. doi: 10.1016/0166-3542(91)90010-o. [DOI] [PubMed] [Google Scholar]
- 6.Coen D M, Fleming H E, Jr, Leslie L K, Retondo M J. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J Virol. 1985;53:477–488. doi: 10.1128/jvi.53.2.477-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrmann E C, Jr, Herrmann J A. A working hypothesis—virus resistance development as an indicator of specific antiviral activity. Ann N Y Acad Sci. 1977;284:632–637. doi: 10.1111/j.1749-6632.1977.tb21997.x. [DOI] [PubMed] [Google Scholar]
- 8.Hu, A., and D. M. Coen. 1995. Unpublished results.
- 9.Monroe, J. E., R. Kilkuskie, A. K. Field, and C. Mulder. Personal communication.
- 10.Mulamba, G. B., A. Hu, and D. M. Coen. 1996. Unpublished results.
- 11.Richman D D, editor. Antiviral drug resistance. Chichester, United Kingdom: John Wiley & Sons; 1996. [Google Scholar]
- 12.Sullivan V, Biron K K, Talarico C, Stanat S C, Davis M, Pozzi L M, Coen D M. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob Agents Chemother. 1993;37:19–25. doi: 10.1128/aac.37.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan V, Coen D M. Isolation of foscarnet-resistant human cytomegalovirus: patterns of resistance and sensitivity to other antiviral drugs. J Infect Dis. 1991;164:781–784. doi: 10.1093/infdis/164.4.781. [DOI] [PubMed] [Google Scholar]
- 14.Tonkinson J L, Stein C A. Antisense nucleic acids—prospects for antiviral intervention. Antiviral Chem Chemother. 1993;4:193–200. [Google Scholar]