Abstract
Postherpetic neuralgia (PHN) is a prevalent, intricate, and intractable form of neuropathic pain. The available evidence indicates that alterations in the gut microbiota are significant environmental determinants in the development of chronic neuropathic pain. Nevertheless, the correlation between the gut microbiota and PHN remains elusive. A cross-sectional study was performed on a cohort of 27 patients diagnosed with PHN and 27 matched healthy controls. Fecal samples were collected and subjected to microbiota analysis using 16S ribosomal RNA gene sequencing. Comparable levels of bacterial richness and diversity were observed in the gut microbiota of PHN patients and healthy controls. A significant difference was observed in 37 genera between the two groups. Furthermore, the LEfSe method revealed that the abundance levels of Escherichia-Shigella, Streptococcus, Ligilactobacillus, and Clostridia_UCG-014_unclassified were elevated in PHN patients, while Eubacterium_hallii_group, Butyricicoccus, Tyzzerella, Dorea, Parasutterella, Romboutsia, Megamonas, and Agathobacter genera were reduced in comparison to healthy controls. Significantly, the discriminant model utilizing the predominant microbiota exhibited efficacy in distinguishing PHN patients from healthy controls, with an area under the curve value of 0.824. Moreover, Spearman correlation analysis demonstrated noteworthy correlations between various gut microbiota and clinical symptoms, including disease course, anxiety state, sleep quality, heat pain, pain intensity, and itching intensity. Gut microbiota dysbiosis exists in PHN patients, microbiome differences could be used to distinguish PHN patients from normal healthy individuals with high sensitivity and specificity, and altered gut microbiota are related to clinical manifestations, suggesting potentially novel prevention and therapeutic directions of PHN.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-023-01614-y.
Keywords: Postherpetic neuralgia, Neuropathic pain, Gut microbiota, 16S rRNA sequencing, Clinical symptoms
Introduction
Postherpetic neuralgia (PHN) is a neuropathic pain that is commonly observed, and it endures for a duration of one month or more after the herpes zoster rash has resolved. PHN is the most frequent sequela of herpes zoster (Johnson and Rice 2014). It is clinically characterized by persistent pain, often accompanied by sensory anomalies, sleep disruption, and emotional comorbidities (Geha et al. 2007; Hunt and Mantyh 2001). The disease course of PHN can range from a few months to a lifetime, with an extremely difficult-to-treat nature and a significant impact on patients' quality of life. Despite extensive research, the pathogenesis of PHN remains incompletely understood. Besides neurological damage, risk factors may also act as disease triggers. Diverse clinical presentations and different severity of this disease indicate that risk factors, such as age, the number of herpes, pain intensity, and perceived mental stress may have vital roles in the pathogenesis of PHN (Jung et al. 2004; Takao et al. 2018; Yang et al. 2019). The gut microbiota has garnered significant attention as a potential environmental factor that may impact the health status of a host. There is mounting evidence linking the gut microbiota to various chronic pain conditions in humans, such as inflammatory pain and neuropathic pain (Alizadeh et al. 2022; Guo et al. 2019). However, it is currently unknown how the gut microbiota plays a role in PHN patients.
It was reported that the gut microbiota is involved in the functioning of pain-related receptors or ion channels and exerts direct control over both peripheral and central sensitization through the release of inflammatory mediators (Guo et al. 2019). Furthermore, mounting evidence supports the notion of a causal relationship between the gut microbiome and neuropathic pain in murine models, with potential mechanisms involving circulating bacterial metabolites and lipopolysaccharide levels, immune responses, and microglia activation (Minerbi and Shen 2022). Additionally, gut dysbiosis is linked to pain-associated behavior, including pain sensitivity and depression (Defaye et al. 2020; Lin et al. 2020). Convincing evidence suggests that intestinal microorganisms may play a crucial role in manifestations of neuropathic pain through gut-brain axes (Zhong et al. 2019). Whereas, no study has addressed the relevance between the gut microbiota and PHN to date, and correlations between the gut microbiota and clinical manifestations of PHN remain undefined.
Therefore, we hypothesized that PHN patients exhibit gut microbial dysbiosis that contributes to the development of PHN. To evaluate this hypothesis, we employed 16S rRNA sequencing to compare the gut microbiota composition of PHN patients and healthy controls, identified the biomarkers of PHN, and analyzed the relationship between microbiota and clinical manifestations of PHN. The present study will enhance the comprehension of gut microbiota in PHN pathogenesis, which may suggest new potential prevention strategies and therapeutic directions.
Materials and methods
Study population
This study was approved by the institutional ethics committee at Tongji Medical College, Huazhong University of Science and Technology (No.: S083), which was registered at the Chinese Clinical Trial Registry (No.: ChiCTR2100049883). In total, 54 participants were recruited. We conducted a cross-sectional study of 27 PHN patients and 27 matched healthy controls (HCs). All participants provided written informed consent. All participants recruited in our study resided in Hubei province for a long time to mitigate the potential impact of diverse lifestyles and regions on gut microbial compositions. Patients with PHN were obtained from the pain clinic of the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology during their initial visit to the clinic.
Dietary data was obtained over a period of two weeks through the administration of questionnaires, as documented in Additional file 2: Table S1. Clinical data pertaining to each patient was obtained through the use of medical history records and interview-based questionnaires (Additional file 3: Table S2). The general characteristics, the psychological state, and sleep quality of all participants were also evaluated using questionnaires, as listed in Additional file 3: Table S2. Subsequent to the interview, fecal samples were collected from each participant between October 2021 and June 2022.
The inclusion criteria of the PHN group were as follows: (1) Over 18 years old; (2) Diagnosed with PHN; (3) Without serious complication. The HC group was recruited from healthy volunteers and was matched with the PHN group according to age, gender, and BMI. The following exclusion criteria were conducted for all groups: probiotics or prebiotics has been taken within one month; Hypertension; diabetes; obesity (body mass index (BMI) ≥ 30 kg/m); dyslipidemia; cancer; metabolic syndrome; a history of disease with an autoimmune component (such as rheumatoid arthritis); intestinal dysfunction (such as irritable bowel syndrome, Crohn’s disease, and inflammatory bowel disease); and abnormal liver and kidney function; medications such as pain medications, anti-inflammatory drugs, antibiotics, or psychotropic medications have been taken in the last 6 months.
Clinical manifestations records and assessments
The disease course and the location of the lesion were recorded. Simultaneously, whether the following symptoms existed was also recorded, including preherpetic pain, spontaneous pain, dynamic mechanical allodynia, and sensory disturbance (itching or numbness).
The assessment of depression and anxiety states was conducted through the utilization of the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7), respectively (Kroenke et al. 2001; Spitzer et al. 2006). The scoring system for the PHQ-9 ranging from 0 to 4 indicate no depression, 5–9 indicate mild depression, 10–14 indicate moderate depression, 15–19 indicate moderately severe depression and ≥ 20 indicate severe depression (Wang et al. 2014). Likewise, scores of ≥ 5, ≥ 10, ≥ 14, and ≥ 19 on the GAD-7 represent mild, moderate, moderately severe, and severe levels of anxiety (Spitzer et al. 2006). The sleep quality was assessed using the Insomnia Severity Index (ISI), with scores ranging from 0 to 28. According to the recommended score interpretation guidelines (Bastien et al. 2001), scores of 0–7 indicate no clinically significant insomnia, 8–14 indicate subthreshold insomnia, 15–21 indicate moderate clinical insomnia, and 22–28 indicate severe clinical insomnia. The cold pain and heat pain was assessed. The assessment of pain intensity was conducted through the utilization of a numerical rating scale (NRS), whereby a score of 0 indicated the absence of pain, while scores of 1–3, 4–6, and 7–10 represented mild, moderate, and severe pain, respectively. In a similar manner, the evaluation of itching intensity was performed using an NRS, with scores ranging from 0 to 10, where higher scores indicated a greater severity of itching intensity.
Sample collection and sequencing
Following collection, fecal samples were immediately frozen at a temperature of −80 °C. Subsequently, fecal genomic DNA was extracted utilizing the CTAB method. The quality of the DNA extraction was assessed through agarose gel electrophoresis, and quantification was performed using an ultraviolet spectrophotometer. PCR amplification was carried out using primers 341F (5'-CCTACGGGNGGCWGCAG-3') and 805R(5'-GACTACHVGGGTATCTAATCC-3') which were specific to the V3–4 hypervariable regions of the 16S rRNA gene. The PCR products were purified using AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA), quantified by Qubit (Invitrogen, USA), and recovery was facilitated using the AMPure XT beads recovery kit.
The purified PCR products were assessed using the Agilent 2100 Bioanalyzer (Agilent, USA) and Illumina’s library quantification kit (Kapa Biosciences, Woburn, MA, USA). A library concentration exceeding 2 nM was deemed acceptable. The qualified computer sequencing libraries, which featured non-repeatable index sequences, were subjected to gradient dilution, mixed in accordance with the required sequencing volume, and denatured into a single strand via NaOH for computer sequencing. The NovaSeq 6000 sequencer was employed for 2 × 250 bp double-ended sequencing, utilizing the NovaSeq 6000 SP Reagent Kit (500 cycles).
16S rRNA gene sequencing analysis
For the double-ended data obtained by sequencing, data separation of the sample was performed according to barcode information, and the connector and barcode sequence were removed. Primer sequence and balance base sequence of RawData were were removed according to the Cutadapt (V1.9.1). FLASH (v1.2.8, http://ccb.jhu.edu/software/FLASH/) was employed to concatenate each pair of paired-end reads into a longer tag based on the overlap area. Subsequently, Fqtrim (v0.94, http://ccb.jhu.edu/software/fqtrim/) was utilized to perform window quality scanning on the sequencing reads, with a default scanning window of 100 bp. If the average quality value in the window was lower than 20, the reading part from the beginning of the window to the end of 3’was truncated. Sequences whose length was less than 100 bp, or sequences with content of N (uncertain fuzzy bases) over 5% after truncation were removed. Additionally, the chimera sequence was eliminated through the utilization of Vsearch (v2.3.4, https://github.com/torognes/vsearch). Then, the high-quality Clean Data was finally obtained.
Divisive Amplicon Denoising Algorithm (DADA2) was invoked with QIIME 2 (Bolyen et al. 2019) denoise-paired for length filtering and denoising. The utilization of Amplicon Sequence Variants (ASVs) was employed for the creation of Operational Taxonomic Units (OTU) (Blaxter et al. 2005), resulting in the acquisition of the final ASV feature list and feature sequence.
Species annotation was conducted using the SILVA (Release 138, https://www.arbsilva.de/documentation/release138/) and NT-16S databases, based on the ASV sequence file, and the abundance statistics of each species in each sample were determined using the ASV abundance table. The confidence threshold for comments was set at 0.7.
Statistical analyses
The statistical analysis was conducted utilizing SPSS 26.0 (SPSS Inc., Armonk, New York, United States). Continuous variables that displayed a normal distribution were expressed as mean ± standard deviation (SD), while non-normally distributed variables were presented as median (interquartile range, IQR). Percentages were used to represent other variables. Statistical significance was determined when p < 0.05.
The present study employed R software (Version 3.4.4) to conduct an analysis of alpha and beta diversity. Specifically, alpha diversity was assessed through the calculation of two indices, namely chao1 and shannon, using R's ggplot2 package. Meanwhile, beta diversity was utilized to examine the dissimilarities in gut microbial communities between PHN and HCs. To achieve this, a Principal Coordinate Analysis (PCoA) was performed, and the resulting multidimensional data were visualized using the ade4 and vegan packages in R software.
An analysis of differential abundance of intestinal flora was conducted at the class, order, family, and genus levels using the doBy package (Version 4.6.13) and ggplot2 package (Version 3.3.6) in R software (Version 4.1.3). Taxa with average abundance levels greater than 1%, P values less than 0.05, and Q values less than 0.05 were visualized (White et al. 2009). the linear discriminant analysis (LDA) effect size (LEfSe) method was utilized for biomarker discovery to identify the key fecal microbiota responsible for discriminating between the PHN group and the HC group. Sequentially, the Kruskal–Wallis rank sum test, Wilcoxon rank sum test, and LDA were executed to identify all distinctive biomarkers. The Receiver operating characteristic (ROC) analysis, a statistical tool utilized to evaluate the predictive accuracy of a model, was conducted utilizing SPSS 26.0, and the area under the curve (AUC) was employed to assess the ROC performance. The corrplot package in R software (Version 3.4.4) was employed to compute Spearman rank correlation between gut microbiota and clinical features.
Results
Basic information of recruited subjects
The general characteristics of all participants are summarized in Table 1. There were no statistically significant differences observed in age, gender, BMI, or education between the PHN and HC groups. However, the PHN group exhibited significantly more severe depressive symptoms (p = 0.002), anxiety symptoms (p = 0.006), and insomnia (p = 0.000) compared to the HC group. Additionally, the detailed clinical manifestations of the PHN group are shown in Table 2.
Table 1.
Demographics assessments for all participants
| PHN (n = 27) | NC (n = 27) | P value | |
|---|---|---|---|
| Age (y, mean ± SD) | 58.93 ± 14.597 | 55.70 ± 15.384 | 0.433 |
| Gender (M/F) | 16/11 | 15/12 | 0.788 |
| BMI (kg/m2, median ± SD) | 21.65 ± 2.85 | 22.29 ± 2.33 | 0.37 |
| Education (y, mean ± SD) | 10.52 ± 3.23 | 10.41 ± 3.41 | 0.903 |
| PHQ-9 (score, median [IQR]) | 8.0(3.0–13.0) | 1.0 (0.0–6.0) | 0.002 |
| GAD-7 (score, median [IQR]) | 5.0(1.0- 7.0) | 0.0 (0.0–4.0) | 0.006 |
| ISI (score, median [IQR]) | 10.0(8.0–13.0) | 1.0 (1.0–7.0) | 0.000 |
SD standard deviation, BMI body mass index, n sample size, PHQ-9 Patient Health Questionnaire-9, GAD-7 Generalized Anxiety Disorder Screener-7, NRS numerical rating scale, ISI Insomnia Severity Index
Table 2.
Clinical manifestations of PHN
| PHN (n = 27) | |
|---|---|
| Disease course (month), n (%) | |
| 1–3 | 13 (48.15%) |
| 4–6 | 4 (14.81%) |
| 7–12 | 6 (22.22%) |
| > 12 | 4 (14.81%) |
| Location of lesion, n (%) | |
| Face (Trigeminal nerve region) | 1 (3.70%) |
| Neck and upper limbs (Cervical nerve region) | 8 (29.63%) |
| Trunk (Thoracic nerve region) | 16 (59.26%) |
| Buttocks and lower limbs (Lumbar and Sacral nerves region) | 2 (7.41%) |
| Preherpetic pain, n (%) | 17 (62.96%) |
| Spontaneous pain, n (%) | 25 (92.59%) |
| Dynamic mechanical allodynia, n (%) | 16 (59.26%) |
| Sensory disturbance, n (%) | |
| Itching | 15 (55.56%) |
| Numbness | 13 (48.15%) |
| PHQ-9 (score), n (%) | |
| No depression (0–4) | 10 (37.04%) |
| mild (5–9) | 7 (25.93%) |
| moderate (10–14) | 4 (14.81%) |
| moderate-severe (15–19) | 4 (14.81%) |
| Severe (20–27) | 2 (7.41%) |
| GAD-7 (score), n (%) | |
| No anxiety (0–4) | 13 (48.15%) |
| Mild (5–9) | 12 (44.44%) |
| Moderate (10–13) | 0 (0%) |
| Moderate-severe (14–18) | 0 (0%) |
| Severe (19–21) | 2 (7.41%) |
| ISI (score), n (%) | |
| Insomnia without clinical significance (0–7) | 6 (22.22%) |
| Subclinical insomnia (8–14) | 16 (59.26%) |
| Clinical insomnia (moderate) (15–21) | 4 (14.81%) |
| Clinical insomnia (severe) (22–28) | 1 (3.70%) |
| Cold pain, n (%) | |
| Normal | 10 (37.04%) |
| Hypalgesia | 14 (51.85%) |
| Hyperpathia | 3 (11.11%) |
| Heat pain, n (%) | |
| Normal | 15 (55.56%) |
| Hypalgesia | 8 (29.63%) |
| Hyperpathia | 4 (14.81%) |
| Pain intensity (NRS 0–10), n (%) | |
| No pain (0) | 1 (3.70%) |
| Mild (1–3) | 12 (44.44%) |
| Moderate (4–6) | 6 (22.22%) |
| Severe (7–10) | 8 (29.63%) |
| Itching intensity (NRS 0–10), n (%) | |
| No itching (0) | 9 (33.33%) |
| Mild (1–3) | 12 (44.44%) |
| Moderate (4–6) | 5 (18.52%) |
| Severe (7–10) | 1 (3.70%) |
PHQ-9 Patient Health Questionnaire-9, GAD-7 Generalized Anxiety Disorder Screener-7, NRS numerical rating scale, ISI Insomnia Severity Index
Differences in gut microbiota composition between postherpetic neuralgia patients and healthy controls
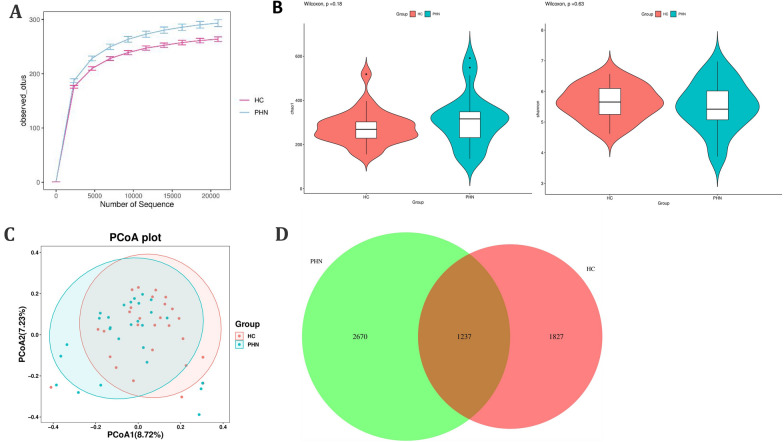
The examination of rarefaction curves at the species level indicates that the sequencing sample size employed in this study was of sufficient magnitude and reliability. Furthermore, the gut microbiota in PHN patients demonstrated a tendency towards greater species richness (observed OTU number) in comparison to that of the healthy controls (Fig. 1A). The analysis of α-diversity involved the consideration of both species richness and evenness, as measured by the Chao1 index and the Shannon diversity index, respectively. However, no statistically significant differences were observed in the comparison of α-diversity between the two groups (p > 0.05) (Fig. 1B). The PCoA plot, as determined by the β-diversity analysis, indicated no statistically significant differences in the degree of similarity among microbial communities between the two groups (Fig. 1C). Additionally, the Venn diagram analysis revealed that 1237 ASVs were common to both the PHN and HC groups, while 2670 and 1827 ASVs were unique to PHN patients and HCs, respectively (Fig. 1D).
Fig. 1.
Rarefaction curves and comparison of diversity indexes between PHN patients and HCs. A Rarefaction curves of patients with PHN and HCs. B α-diversity indexes in PHN patients and HCs (chao1, Shannon). C PcoA for β-diversity analysis. D Venn diagram
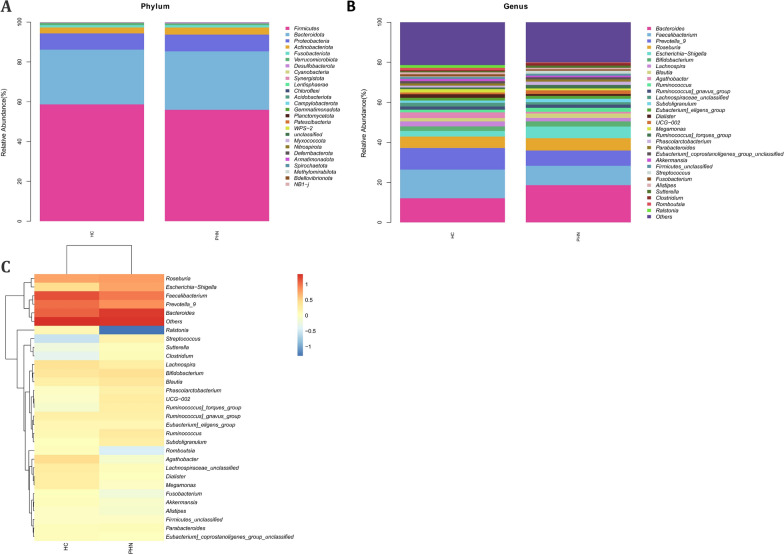
The analysis of Community Profiling revealed no statistically significant differences between the PHN group and HCs from phylum to species levels (Additional file 1: Figure S1 and Fig. 2). The bacterial phyla Firmicutes, Bacteroidota, and Proteobacteria were found to be predominant (Fig. 2A). In comparison to HCs, PHN patients exhibited lower levels of Firmicutes, but higher levels of Bacteroidota and Proteobacteria. At the class level, Bacteroidota and Gammaproteobacteria were found to be more abundant in PHN patients, while Clostridia and Negativicutes were more prevalent in HCs. At the level of order, it was observed that PHN patients exhibited an increase in Bacteroidales and Enterobacterales, but a decrease in Oscillospirales and Lachnospirales, in comparison to HCs. The family level analysis revealed that Bacteroidaceae and Enterobacteriaceae were more abundant in the PHN group, while Lachnospiraceae, Ruminococcaceae, and Prevotellaceae were more prevalent in the healthy group. At the genus level, Bacteroides and Faecalibacterium were the most dominant in both groups, with Bacteroides being more abundant in the PHN group, and Faecalibacterium and Prevotella_9 being more prevalent in the HCs group (Fig. 2B and C). At the species level, the abundance of Roseburia_unclassified and Escherichia-Shigella_unclassified was observed to be higher in the PHN group, whereas Faecalibacterium_unclassified and Prevotella_9_unclassified were more prevalent in the HCs.
Fig. 2.
Community Profiling analysis showing differential relative abundances of fecal microbiota in PHN patients and HCs. A Microbiome composition of the two groups at the phylum level. B Microbiome composition of the two groups at the genus level. C Relative abundance of the top 30 genus in each sample
These findings suggest that the gut microbiota of PHN patients and healthy controls exhibit comparable levels of bacterial richness and diversity, but the overall composition of the gut microbiota differs significantly between the two groups.
Alteration in gut microbiota between postherpetic neuralgia patients and healthy controls
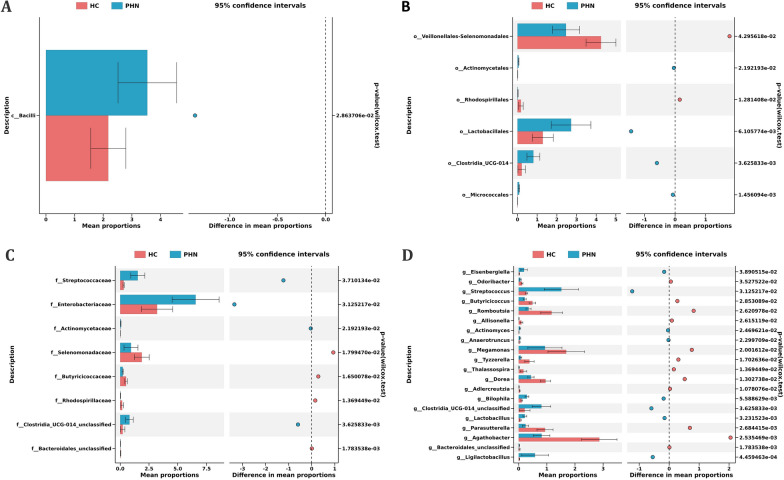
A significant difference was observed between PHN patients and HCs in 3 classes, 13 orders, 16 families, 37 genera, and 46 species of gut microbiota (Additional file 4: Table S3). Taxa with average abundance levels exceeding 1% were graphically represented. Specifically, at the class level, the proportion of Bacilli was found to be higher in the PHN group compared to the HCs (Fig. 3A). At the order level, Actinomycetales, Lactobacillales, Clostridia_UCG-014, and Micrococcales were more abundant in the PHN group, while Veillonellales-Selenomonadales, and Rhodospirillales were more prevalent in the HCs (Fig. 3B). At the family level, proportions of Streptococcaceae, Enterobacteriaceae, Actinomycetaceae, and Clostridia_UCG-014 unclassified were higher in the PHN group than that in the HCs, while Selenomonadaceae, Butyricicoccaceae, Rhodospirillaceae, and Bacteroidales_unclassified were lower (Fig. 3C). At the genus level, 20 genera displayed substantial variation between the PHN group and HCs. Specifically, the proportions of Odoribacter, Butyricicoccus, Romboutsia, Allisonella, Megamonas, Tyzzerella, Thalassospira, Dorea, Adlercreutzia, Parasutterella, Agathobacter, and Bacteroidales_unclassified genera were decreased, whereas the proportions of Eisenbergiella, Streptococcus, Actinomyces, Anaerotruncus, Bilophila, Clostridia_UCG-014_unclassified, Lactobacillus, and Ligilactobacillus genera were increased in PHN patients (Fig. 3D).
Fig. 3.
Gut microbiota differences between PHN patients and controls. Gut microbiota is compared between PHN patients and healthy control subjects at class (A), order (B), family (C), and genus (D) levels. Only the taxa with average abundance levels exceeding 1% are plotted. The bars on the left side of each figure show the relative abundance. On the right side of each figure, the center of circles represents the difference between the means of the two groups. The error bars represent the 95% confidence interval. P values of unpaired t-test are listed on the right
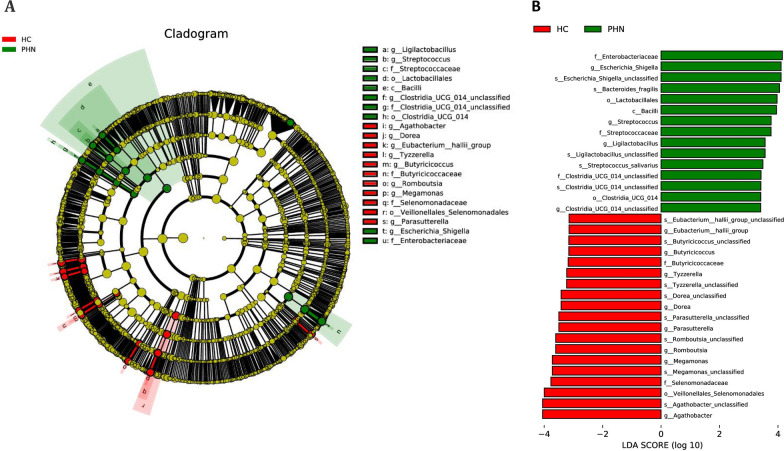
To further explore the specific bacterial taxa related to PHN, the LEfSe algorithm was employed to assess the abundance of the fecal microbiota (Fig. 4). A cladogram was generated to facilitate the comparison of the phylogenetic distribution between healthy controls and PHN patients. The results revealed significant differences at each taxonomic level analyzed (LDA > 3, p < 0.05), with 34 differential ASVs being identified. Concretely, one class, two orders, three families, four genera, and five species were found to be enriched in PHN patients, while one order, two families, eight genera, and eight species were more abundant in HCs (p < 0.05). In comparison to NCs, patients with PHN exhibited ten enriched ASVs primarily belonging to the class Bacilli. Notably, the families Streptococcaceae, Clostridia_UCG-014_unclassified, and Enterobacteriaceae were more abundant in PHN patients, while the Butyricicoccaceae and Selenomonadaceae families were more prevalent in NCs. At the genus level, Escherichia-Shigella, Streptococcus, Ligilactobacillus, and Clostridia_UCG-014_unclassified were enriched in PHN patients, whereas Eubacterium_hallii_group, Butyricicoccus, Tyzzerella, Dorea, Parasutterella, Romboutsia, Megamonas, and Agathobacter were enriched in NCs. The findings indicate notable variations in the intestinal microbiota between PHN and HC groups.
Fig. 4.
LEfSe identified the taxa with the greatest differences in abundance between PHN patients and HCs. A Cladogram showing differential bacterial abundance in the PHN and control groups. B Microbiome biomarkers were identified. The green color represents the PHN group, and the red color represents the HCs. LDA score for discriminative features > 3.0
ROC curve analysis
Consequently, our objective was to ascertain potential biomarkers that could differentiate between the two groups. We identified the five most prominent genera (Escherichia_Shigella, Agathobacter, Streptococcus, Megamonas, and Romboutsia) based on their LDA value, and subsequently employed these microbiota to construct the ROC curve (Fig. 5). The AUC of the ROC curve was determined to be 0.824, indicating that the model possessed the ability to differentiate between the two groups. Additionally, the model exhibited a specificity and sensitivity of 92.6% and 63.0%, respectively, signifying a notable diagnostic efficacy. These findings suggest that the gut microbiota may serve as a robust predictor of PHN.
Fig. 5.
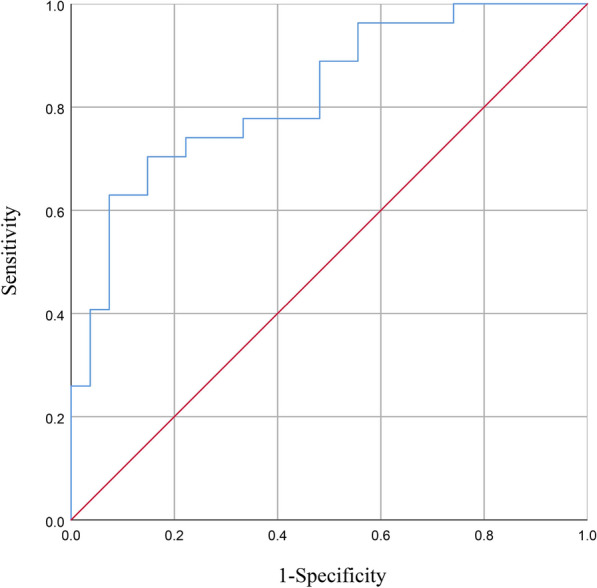
ROC curves of the gut genus bacterium relative abundance for the prediction of PHN. Vertical coordinate indicated the sensitivity of prediction, horizontal coordinate indicated the 1-specificity of prediction, AUC > 0.5 indicated a predictive efficiency of the gut bacterium
Clinical manifestations correlated with the gut microbiota
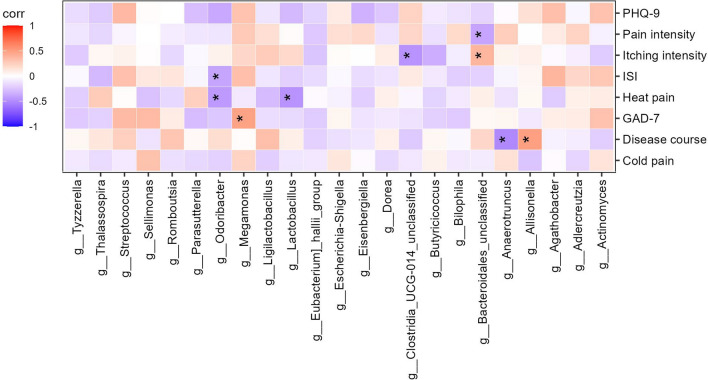
The associations between the gut microbiota and clinical manifestations were investigated (Fig. 6). The results revealed a negative correlation between Anaerotruncus and disease course, while Allisonella exhibited a positive correlation with disease course. Additionally, a positive trend was observed between Megamonas abundance and GAD-7, and Odoribacter demonstrated a direct negative association with ISI. Moreover, Lactobacillus and Odoribacter were negatively correlated with heat pain. Bacteroidales_unclassified was negatively associated with pain intensity, but positively correlated with itching intensity. Additionally, Clostridia_UCG-014_unclassified was negatively associated with itching intensity.
Fig. 6.
Spearman correlation analysis of PHN clinical symptoms. The intensity of the color indicates the r value (correlation). The red color represents a positive score, and the purple color represents a negative one. *p < 0.05
Discussion
In recent years, accumulating evidence has demonstrated that the disorder of gut microbiota plays a significant role in modulating the development of neuropathic pain (Lin et al. 2020). This study employed 16S rRNA sequencing analysis to characterize the gut microbiota of PHN patients for the first time. The results revealed that PHN patients displayed an altered gut microbiota composition. Furthermore, a prediction model was developed based on the results of LEfSe and achieved high values of AUC. Moreover, the Spearman correlation analysis revealed robust associations between differentiated gut microbiota and diverse clinical presentations. These results indicate that dysbiosis of gut microbiota is a crucial factor in the pathogenesis of PHN.
In our study, we observed a lower abundance of Firmicutes at the phylum level in PHN patients, while Bacteroidota exhibited a higher abundance. Several previous studies also observed marked decreases in Firmicutes and increase in Bacteroidota in the intestines of patients with neuralgia (Lin et al. 2020; Zhang et al. 2019). It is noteworthy that phylum Firmicutes is known to play a crucial role in regulating inflammatory responses and human metabolic functions (Bhat and Kapila 2017). The depletion of the Firmicutes phylum may result in the production of pro-inflammatory cytokines and toxic metabolites, while simultaneously reducing the presence of beneficial substances such as short-chain fatty acids (SCFAs), ultimately leading to damage to the gut epithelial barrier. The Bacteroidota phylum is characterized by its major outer membrane component, lipopolysaccharide (LPS). LPS is known to play a critical role in the initiation of systemic inflammation and the release of pro-inflammatory cytokines. Thus, the missing phylum Firmicutes and enriched phylum Bacteroidota in PHN patients may exacerbate neuroinflammation in humans, thereby augmenting the likelihood and advancement of PHN.
At the genus level, a reduction in the abundance levels of many microbiotas was observed in PHN patients. According to the LEfSe results, Eubacterium_hallii_group, Butyricicoccus, Tyzzerella, Dorea, Parasutterella, Romboutsia, Megamonas, and Agathobacter were decreased in PHN patients. Previous research has established the significant roles played by these species in preserving human health. Eubacterium_hallii, Butyricicoccus, and Agathobacter were shown to produce butyrate (Duncan et al. 2004; Geirnaert et al. 2014; Iversen et al. 2022). According to Hamer et al., butyrate serves as the primary energy source for colonocytes, thereby promoting the maintenance of gastrointestinal health by enhancing epithelial barrier integrity and suppressing inflammation (Hamer et al. 2008). Furthermore, recent studies have shown that butyrate possesses the ability to ameliorate neuropathic pain (Bonomo et al. 2020; Kukkar et al. 2014). The genera Tyzzerella, Dorea, Parasutterella, Romboutsia, and Megamonas have been shown to produce SCFAs, which are important for maintaining the health of the gut lining and supporting immune function (Huang et al. 2022; Jiao et al. 2018; Xiao et al. 2020; Xu et al. 2021; Zhang et al. 2022). Therefore, we hypothesized that a decrease in these genera of bacteria might be detrimental to PHN by affecting the abundance of SCFAs. Furthermore, Dorea has been demonstrated negatively linked to inflammatory diseases (Bajaj et al. 2012). It was shown that Romboutsia and Megamonas may be beneficial for individuals with inflammatory bowel disease or other inflammatory conditions because of their anti-inflammatory effects (Qiu et al. 2020; Yu et al. 2021). Consequently, the reduced levels of these species in PHN patients may contribute to the destruction of the intestinal mucosal barrier, thereby triggering inflammation and immune responses and ultimately exacerbating the pathology of PHN.
While, the abundance levels of several genera were found to be increased in PHN patients according to the LEfSe results. Specifically, Escherichia_Shigella, Streptococcus, Ligilactobacillus, and Clostridia_UCG-014_unclassified were observed to be elevated in PHN patients. Previous studies have linked certain species with increased abundance levels to inflammatory diseases or neuropathic pain. Notably, Escherichia_Shigella has been associated with a pro-inflammatory status, and chronic and persistent peripheral inflammation has been observed in individuals with persistent infection with this species (Qiu et al. 2020). Similar to our findings, previous studies also revealed remarkably increased Streptococcus in rats with CCI-induced neuropathic pain (Chen et al. 2021). Additionally, Streptococcus is associated with inflammatory pain (Chakravarthy et al. 2014; Guo et al. 2019). Thus, we hypothesized that an increase in these genera of bacteria might contribute to PHN development by exacerbating inflammation. Interestingly, it was reported that Ligilactobacillus and Clostridia_UCG-014_unclassified may be involved in maintaining a healthy gut environment (Guerrero Sanchez et al. 2022; Liu et al. 2021). It is noteworthy that in our study, there was a negative correlation between Clostridia_UCG-014_unclassified and itching intensity in patients with PHN. Further investigation is necessary to determine the potential impact of elevated levels of these particular species on PHN in humans.
We used LEfSe analysis to select five species based on their LDA values for use as biomarkers for predicting disease status. A ROC Curve was constructed using the abundance of the top five genera, selected from a pool of 12 genera, resulting in an AUC value of 0.824. Therefore, it was confirmed that the discriminant model could effectively distinguish PHN patients from healthy controls, which suggests that the gut microbiota could be used to forecast PHN. Additionally, a heat map was employed to depict the associations between species and clinical phenotype, revealing that certain gut microbiota exhibited significant correlations with clinical manifestations related to PHN, including disease course, anxiety states, sleep quality, heat pain, pain intensity, and itching intensity. These outcomes establish a basis for investigating the interplay between the gut microbiota and the host in relation to the onset of PHN.
One of the primary strengths of our study is the utilization of the 16S rRNA gene sequencing method to profile the gut microbiota of patients with PHN. Additionally, we have developed a pioneering predictive model and conducted Spearman correlation analysis to explore associations between differentiated gut microbiota and clinical presentations in PHN. Theoretically, the variability of the microbiome may offer a degree of plausible explanations for longstanding clinical enigmas, such as the development of chronic pain in certain patients following the resolution of herpes zoster rash and the variability of clinical symptoms among patients. However, it is imperative to acknowledge that this study is in its preliminary stages and is subject to several limitations that require further investigation. Firstly, our study is a single-center, cross-sectional study with a limited sample size. Secondly, despite age-, gender-, BMI-, and diet matching of PHN patients in the analysis, other confounding factors such as stress may have influenced our results. Thirdly, it was suggested that SCFAs may be implicated in the development of PHN in our study, while the association of fecal or plasma levels of SCFAs with gut microbiota in PHN patients was not further explored. Additionally, the impact of certain treatments for PHN, including pharmacological, nonpharmacological, and interventional, on the gut microbiota remains unclear. Lastly, our research lacks animal experiments to investigate the underlying mechanisms linking gut microbiota dysbiosis to the development of PHN, as well as animal experiments exploring the potential of fecal microbiota transplantation as a treatment for PHN. Consequently, further research is imperative, incorporating larger sample sizes, multicenter designs, follow-up studies after treatment, animal experimentation, and innovative research techniques such as metagenomics and metabonomics. This will facilitate the exploration of potential causal mechanisms between gut microbiota and PHN, thereby providing guidance for future scientific investigations and interventions aimed at the prevention and treatment of PHN.
In summary, our study revealed a significant correlation between gut microbiomes and PHN, suggesting that disruptions in gut microbiota may play a role in the development of PHN. Our results provide evidence to support the potential use of gut microbiota as a predictive tool for PHN and targeting gut microbiota as a novel therapeutic approach for PHN.
Supplementary Information
Additional file 1: Figure S1. The bacterial community in both groups at diferent taxonomic levels. Bar graphs indicated the relative abundance of class-level, order-level, family-level taxa, and species-level.
Additional file 2: Table S1. Dietary data of all participants.
Additional file 3: Table S2. Clinical information for all samples.
Additional file 4: Table S3. The difference of gut microbiota abundance between PHN patients and HCs.
Acknowledgements
No application.
Author contributions
Study conception and design were performed by BJ, MZ and XZ. Material preparation, public data collection and analysis were performed by XC, CZ, WZ and SY. Patients and stool samples were collected by BJ, XC, and CZ. The first draft of the manuscript was written by BJ. Supervision and revision were done by MZ and XZ. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The data that support the findings of this study are openly available in BioProject at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA975052, reference number PRJNA975052.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee at Tongji Medical College, Huazhong University of Science and Technology (No.: S083), which was registered at the Chinese Clinical Trial Registry (No.: ChiCTR2100049883). All subjects were informed consent.
Consent for publication
All authors agree to be published.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mi Zhang, Email: misscat0311@163.com.
Xianwei Zhang, Email: ourpain@163.com.
References
- Alizadeh N, Naderi G, Kahrizi MS, Haghgouei T, Mobed A, Shah-Abadi ME. Microbiota-pain association; recent discoveries and research progress. Curr Microbiol. 2022;80(1):29. doi: 10.1007/s00284-022-03124-9. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G675–685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bhat MI, Kapila R. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev. 2017;75(5):374–389. doi: 10.1093/nutrit/nux001. [DOI] [PubMed] [Google Scholar]
- Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc Lond B Biol Sci. 2005;360(1462):1935–1943. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vazquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Author correction: reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(9):1091. doi: 10.1038/s41587-019-0252-6. [DOI] [PubMed] [Google Scholar]
- Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, Zima AV, Brown IA, Dugas LR, Zakharian E, Aubert G, Alonzo F, 3rd, Calcutt NA, Mansuy-Aubert V. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci U S A. 2020;117(42):26482–26493. doi: 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy K, Faltus R, Robinson G, Sevilla R, Shin J, Zielstorff M, Byford A, Leccese E, Caniga MJ, Hseih S, Zhang S, Chiu CS, Zhang-Hoover J, Moy LY, McLeod RL, Stoffregen D, Zhang W, Murtaza A, Cicmil M. Etanercept ameliorates inflammation and pain in a novel mono-arthritic multi-flare model of streptococcal cell wall induced arthritis. BMC Musculoskelet Disord. 2014;15:409. doi: 10.1186/1471-2474-15-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Wang C, Ren YN, Ye ZJ, Jiang C, Wu ZB. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol Brain. 2021;14(1):50. doi: 10.1186/s13041-021-00765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defaye M, Gervason S, Altier C, Berthon JY, Ardid D, Filaire E, Carvalho FA. Microbiota: a novel regulator of pain. J Neural Transm (vienna) 2020;127(4):445–465. doi: 10.1007/s00702-019-02083-z. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128(1–2):88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirnaert A, Steyaert A, Eeckhaut V, Debruyne B, Arends JB, Van Immerseel F, Boon N, Van de Wiele T. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe. 2014;30:70–74. doi: 10.1016/j.anaerobe.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Guerrero Sanchez M, Passot S, Campoy S, Olivares M, Fonseca F. Ligilactobacillus salivarius functionalities, applications, and manufacturing challenges. Appl Microbiol Biotechnol. 2022;106(1):57–80. doi: 10.1007/s00253-021-11694-0. [DOI] [PubMed] [Google Scholar]
- Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth. 2019;123(5):637–654. doi: 10.1016/j.bja.2019.07.026. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Huang WC, Tung CL, Yang YSH, Lin IH, Ng XE, Tung YT. Endurance exercise ameliorates western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci Rep. 2022;12(1):3612. doi: 10.1038/s41598-022-07317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Iversen KN, Dicksved J, Zoki C, Fristedt R, Pelve EA, Langton M, Landberg R. The effects of high fiber rye, compared to refined wheat, on gut microbiota composition, plasma short chain fatty acids, and implications for weight loss and metabolic risk factors (the RyeWeight Study) Nutrients. 2022 doi: 10.3390/nu14081669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao N, Baker SS, Nugent CA, Tsompana M, Cai L, Wang Y, Buck MJ, Genco RJ, Baker RD, Zhu R, Zhu L. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol Genomics. 2018;50(4):244–254. doi: 10.1152/physiolgenomics.00114.2017. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Rice AS. Clinical practice. Postherpetic Neuralgia N Engl J Med. 2014;371(16):1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62(9):1545–1551. doi: 10.1212/01.wnl.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkar A, Singh N, Jaggi AS. Attenuation of neuropathic pain by sodium butyrate in an experimental model of chronic constriction injury in rats. J Formos Med Assoc. 2014;113(12):921–928. doi: 10.1016/j.jfma.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain. 2020;21(1):103. doi: 10.1186/s10194-020-01170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhou M, Yang M, Jin C, Song Y, Chen J, Gao M, Ai Z, Su D. Pulsatilla chinensis Saponins Ameliorate Inflammation and DSS-induced ulcerative colitis in rats by regulating the composition and diversity of intestinal flora. Front Cell Infect Microbiol. 2021;11:728929. doi: 10.3389/fcimb.2021.728929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minerbi A, Shen S. Gut microbiome in anesthesiology and pain medicine. Anesthesiology. 2022;137(1):93–108. doi: 10.1097/ALN.0000000000004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Zhao X, Cui X, Mao X, Tang N, Jiao C, Wang D, Zhang Y, Ye Z, Zhang H. Characterization of fungal and bacterial dysbiosis in young adult Chinese patients with Crohn's disease. Therap Adv Gastroenterol. 2020;13:1756284820971202. doi: 10.1177/1756284820971202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Takao Y, Okuno Y, Mori Y, Asada H, Yamanishi K, Iso H. Associations of perceived mental stress, sense of purpose in life, and negative life events with the risk of incident herpes zoster and postherpetic neuralgia: the SHEZ study. Am J Epidemiol. 2018;187(2):251–259. doi: 10.1093/aje/kwx249. [DOI] [PubMed] [Google Scholar]
- Wang W, Bian Q, Zhao Y, Li X, Wang W, Du J, Zhang G, Zhou Q, Zhao M. Reliability and validity of the Chinese version of the patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. 2014;36(5):539–544. doi: 10.1016/j.genhosppsych.2014.05.021. [DOI] [PubMed] [Google Scholar]
- White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4):e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Liu C, Chen M, Zou J, Zhang Z, Cui X, Jiang S, Shang E, Qian D, Duan J. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104(1):303–317. doi: 10.1007/s00253-019-10174-w. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang Y, Li H, Dai Y, Chen D, Wang M, Jiang X, Huang Z, Yu H, Huang J, Xiong Z. Altered fecal microbiota composition in older adults with frailty. Front Cell Infect Microbiol. 2021;11:696186. doi: 10.3389/fcimb.2021.696186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Yu S, Fan B, Liu Y, Chen YX, Kudel I, Concialdi K, DiBonaventura M, Hopps M, Hlavacek P, Cappelleri JC, Sadosky A, Parsons B, Udall M. The epidemiology of herpes zoster and postherpetic neuralgia in China: results from a cross-sectional study. Pain Ther. 2019;8(2):249–259. doi: 10.1007/s40122-019-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Du J, Pu X, Zheng L, Chen S, Wang N, Li J, Chen S, Pan S, Shen B. The gut microbiome and metabolites are altered and interrelated in patients with rheumatoid arthritis. Front Cell Infect Microbiol. 2021;11:763507. doi: 10.3389/fcimb.2021.763507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Jing Y, Zhang W, Zhang J, Yang M, Du L, Jia Y, Chen L, Gong H, Li J, Gao F, Liu H, Qin C, Liu C, Wang Y, Shi W, Zhou H, Liu Z, Yang D, Li J. Dysbiosis of gut microbiota is associated with serum lipid profiles in male patients with chronic traumatic cervical spinal cord injury. Am J Transl Res. 2019;11(8):4817–4834. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang B, Peng B, Wang J, Hu Y, Wang R, Wang S. Different dose of sucrose consumption divergently influences gut microbiota and PPAR-gamma/MAPK/NF-kappaB pathway in DSS-induced colitis mice. Nutrients. 2022 doi: 10.3390/nu14132765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhou Z, Liang Y, Cheng X, Li Y, Teng W, Zhao M, Liu C, Guan M, Zhao C. Targeting strategies for chemotherapy-induced peripheral neuropathy: does gut microbiota play a role? Crit Rev Microbiol. 2019;45(4):369–393. doi: 10.1080/1040841X.2019.1608905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The bacterial community in both groups at diferent taxonomic levels. Bar graphs indicated the relative abundance of class-level, order-level, family-level taxa, and species-level.
Additional file 2: Table S1. Dietary data of all participants.
Additional file 3: Table S2. Clinical information for all samples.
Additional file 4: Table S3. The difference of gut microbiota abundance between PHN patients and HCs.
Data Availability Statement
The data that support the findings of this study are openly available in BioProject at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA975052, reference number PRJNA975052.