Abstract
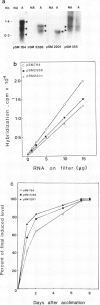
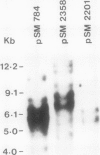
Cold-acclimation-specific (CAS) gene expression has been examined by screening a cDNA library prepared from poly(A)+ RNA of cold-acclimated seedlings of a freezing-tolerant variety of alfalfa (Medicago falcata cv Anik). Three CAS cDNA clones, pSM784, pSM2201, and pSM2358, representing different sequence species, have been used to investigate the relative abundance and time-course of accumulation of corresponding transcripts. Results obtained show that the expression of these CAS genes is regulated in a coordinated manner most likely at the level of transcription. The expression of genes, as measured by mRNA abundance corresponding to the three CAS cDNA clones, is not stimulated or induced by heat shock, water stress, abscisic acid, or wounding. A positive correlation is observed between the expression of these cloned sequences and the degree of freezing-tolerance in four alfalfa cultivars.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cloutier Y. Changes in the Electrophoretic Patterns of the Soluble Proteins of Winter Wheat and Rye following Cold Acclimation and Desiccation Stress. Plant Physiol. 1983 Feb;71(2):400–403. doi: 10.1104/pp.71.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Campbell W. F. Response of Tomato Plants to Stressful Temperatures : INCREASE IN ABSCISIC ACID CONCENTRATIONS. Plant Physiol. 1981 Jan;67(1):26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faw W. F., Jung G. A. Electrophoretic protein patterns in relation to low temperature tolerance and growth regulation of alfalfa. Cryobiology. 1972 Dec;9(6):548–555. doi: 10.1016/0011-2240(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guy C. L., Haskell D. Induction of freezing tolerance in spinach is associated with the synthesis of cold acclimation induced proteins. Plant Physiol. 1987 Jul;84(3):872–878. doi: 10.1104/pp.84.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. L., Niemi K. J., Brambl R. Altered gene expression during cold acclimation of spinach. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3673–3677. doi: 10.1073/pnas.82.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., McLeester R. C., McCown B. H., Beck G. E. Enzyme changes during deacclimation of willow stem. Cryobiology. 1970 Sep-Oct;7(2):130–135. doi: 10.1016/0011-2240(70)90009-x. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnuk M., Jung G. A., Witham F. H. Electrophoretic studies of several dehydrogenases in relation to the cold tolerance of alfalfa. Cryobiology. 1976 Jun;13(3):375–393. doi: 10.1016/0011-2240(76)90121-8. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Noyes B. E., Agarwal K. L. Detection of gastrin-specific mRNA using oligodeoxynucleotide probes of defined sequence. J Biol Chem. 1979 Aug 25;254(16):7472–7475. [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Abscisic Acid-regulated gene expression in relation to freezing tolerance in alfalfa. Plant Physiol. 1988 Jun;87(2):468–473. doi: 10.1104/pp.87.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Changes in Protein Patterns and Translatable Messenger RNA Populations during Cold Acclimation of Alfalfa. Plant Physiol. 1987 Aug;84(4):1172–1176. doi: 10.1104/pp.84.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsanyi-Breiner A., Gusella J. F., Keys C., Housman D. E., Sullivan D., Brisson N., Verma D. P. The organization of a nuclear DNA sequence from a higher plant: molecular cloning and characterization of soybean ribosomal DNA. Gene. 1979 Nov;7(3-4):317–334. doi: 10.1016/0378-1119(79)90051-9. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]