Abstract
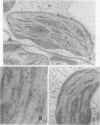
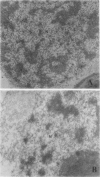
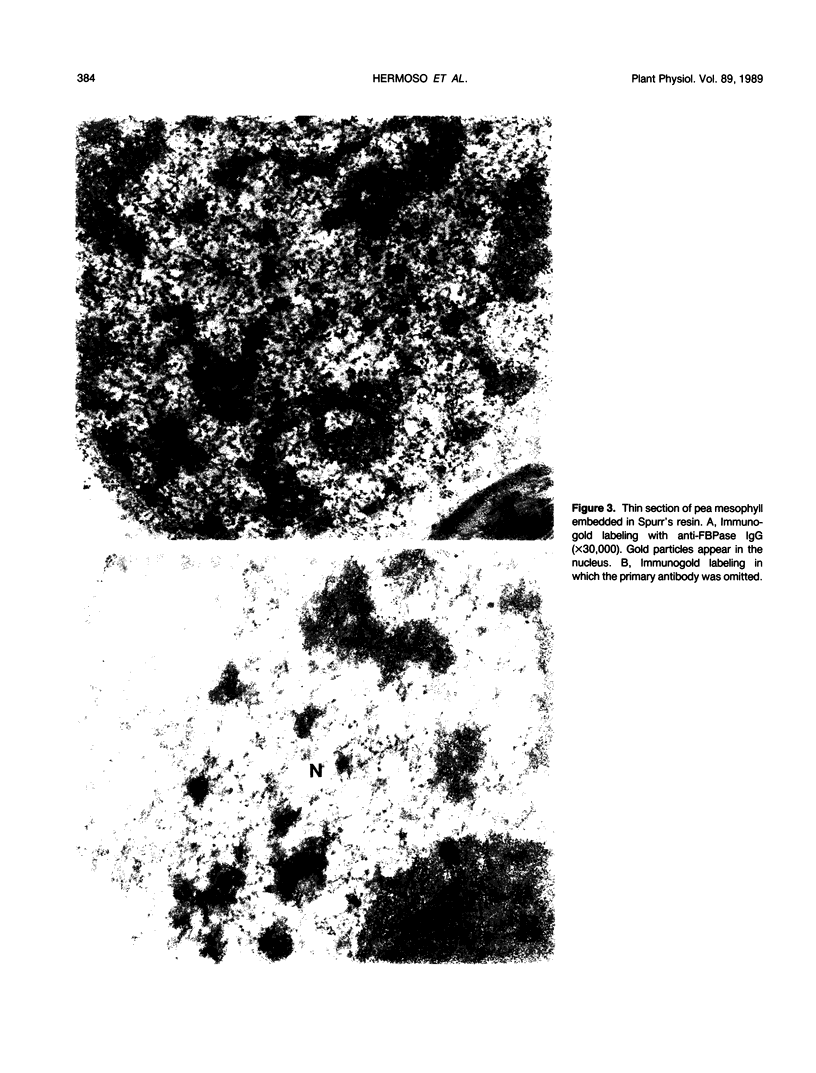
An enriched IgG serum fraction obtained from rabbits immunized against pea chloroplast fructose-1,6-bisphosphatase (FBPase) was used, coupled to colloidal gold (15 nanometer particles) goat anti-rabbit IgG, to analyze by electron microscopy the location of photosynthetic FBPase in pea (Pisum sativum L.) leaf ultrathin sections. In accordance with earlier biochemical studies on distribution of FBPase activity, the enzyme was visualized both in the stromal space and bound to the chloroplast membranes. Some gold particles also appear in the cytoplasm, which can be related to the presence in the cytosol of a high molecular weight precursor of this nuclear coded enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alscher-Herman R. Chloroplast alkaline fructose 1,6-bisphosphatase exists in a membrane-bound form. Plant Physiol. 1982 Sep;70(3):728–734. doi: 10.1104/pp.70.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Brennan T., Anderson L. E. Thioredoxin-like Activity of Thylakoid Membranes: THIOREDOXIN CATALYZING THE REDUCTIVE INACTIVATION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE OCCURS IN BOTH SOLUBLE AND MEMBRANE-BOUND FORM. Plant Physiol. 1980 Oct;66(4):605–608. doi: 10.1104/pp.66.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Bassat D., Anderson L. E. Light-Induced Release of Bound Glucose-6-phosphate Dehydrogenase to the Stroma in Pea Chloroplasts. Plant Physiol. 1981 Aug;68(2):279–283. doi: 10.1104/pp.68.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueca A., Lázaro J. J., Gorgé J. L. Light-Induced Nuclear Synthesis of Spinach Chloroplast Fructose-1,6-bisphosphatase. Plant Physiol. 1984 Jul;75(3):539–541. doi: 10.1104/pp.75.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K. H., Latzko E. Chloroplast ribulose-5-phosphate kinase: light-mediated activation, and detection of both soluble and membrane-associated activity. Biochem Biophys Res Commun. 1979 Jul 12;89(1):300–306. doi: 10.1016/0006-291x(79)90978-1. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Harrsch P. B., Kim Y., Fox J. L., Marcus F. Amino acid sequence similarity between spinach chloroplast and mammalian gluconeogenic fructose-1,6-bisphosphatase. Biochem Biophys Res Commun. 1985 Dec 17;133(2):520–526. doi: 10.1016/0006-291x(85)90937-4. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Gibbs M. Characterization of a Photosynthesizing Reconstituted Spinach Chloroplast Preparation : REGULATION BY PRIMER, ADENYLATES, FERREDOXIN, AND PYRIDINE NUCLEOTIDES. Plant Physiol. 1982 Jan;69(1):179–186. doi: 10.1104/pp.69.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pla A., Lopez-Gorge J. Thioredoxin/fructose-1,6-bisphosphatase affinity in the enzyme activation by the ferredoxin-thioredoxin system. Biochim Biophys Acta. 1981 Jun 12;636(1):113–118. doi: 10.1016/0005-2728(81)90082-7. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn K. C. Two immunological approaches to the detection of ribulose-1,5-bisphosphate carboxylase in guard cell chloroplasts. Plant Physiol. 1987 May;84(1):188–196. doi: 10.1104/pp.84.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivo A., Felipe M. R. Cytochemical location and biochemical analysis of the starch phosphorylase enzyme in pea seeds (Pisum sativum L. cv. Rivalin). Cell Mol Biol. 1986;32(4):471–476. [PubMed] [Google Scholar]