Abstract
The current global shortage of organ resources, the imbalance in donor-recipient demand and the increasing number of high-risk donors make organ preservation a necessity to consider appropriate storage options. The current method of use often has risks such as blood group mismatch, short shelf life, and susceptibility. HBOCs have positive effects such as anti-apoptotic, anti-inflammatory, antioxidant and anti-proliferative, which have significant advantages in organ storage. Therefore, it is the common pursuit of researchers to design and synthesize HBOCs with safety, ideal oxygen-carrying capacity, easy storage, etc. that are widely applicable and optimal for different organs. There has been a recent advancement in understanding HBOCs mechanisms, which is discussed in this review.
Keywords: Hemoglobin-based oxygen carriers, Chemical modification, Red blood cells, Organs storage
Graphical abstract
Abbreviations
- BHb
Bovine hemoglobin
- ECD
Expanded criteria donor
- DHSG
1,5-O-dihexadecyl-N-succinyl-l-glutamate
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine
- EDTA
Ethylene diamine tetraacetic acid
- Hb
Hemoglobin
- HBOCs
Hemoglobin-based oxygen carriers
- Hb-V
Hemoglobin vesicles
- HHb
Human hemoglobin
- HSA
Human Serum Albumin
- IRI
Ischemia reperfusion injury
- GA
Glutaraldehyde
- LBL
Layer by layer
- MQ
Milli-Q
- nCV
Normalized cell viability
- PDA
Polydopamine
- PEG
Polyethylene glycol
- PEG-DSPE
1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-poly (ethylene glycol)
- RBC
Red blood cells
- SOT
Solid organ transplantation
1. Information
The increased success of solid organ transplantation (SOT), the treatment of choice for end-stage organ disease, has been hampered by ischemia-reperfusion injury (IRI) [1]. According to the data from the OPTN/SRTR 2021 Annual Data Report about Deceased Organ Donation, there were 13 862 deceased donors, a 10.1% increase from 12 588 in 2020, and an increase from 11 870 in 2019; this number has been increasing since 2010 [2]. IRI unavoidably occurs during organ resection and transplantation, may compromise the short-term and long-term after transplantation, and remains a critical organ transplantation challenge. With the increase in expanded criteria donor (ECD), the selection of better preservation methods to improve the preservation time of isolated organs, tissue oxygenation, etc., and to further reduce organ IRI remains an urgent issue [3].
The methods commonly used today to preserve organs are: under room temperature conditions using crystalloid and RBCs or under cryogenic conditions at 4 °C using clinically standard preservation solutions [4,5]. However, the above methods suffer from storage fluid cross-matching, scarcity, and infection-induced oxidative metabolism of the organ, which in turn exacerbates oxidative stress damage to the organ [6]. In addition, the limitation of the preservation fluid makes it necessary to use the organs in a short period of time, otherwise thousands of organs will be abandoned. Therefore, we have a critical need for better organ isolation preservation fluids to provide near physiological conditions for the duration of organ preservation, thereby improving the quality of transplanted organs to address the global crisis of organ shortage due to organ storage.
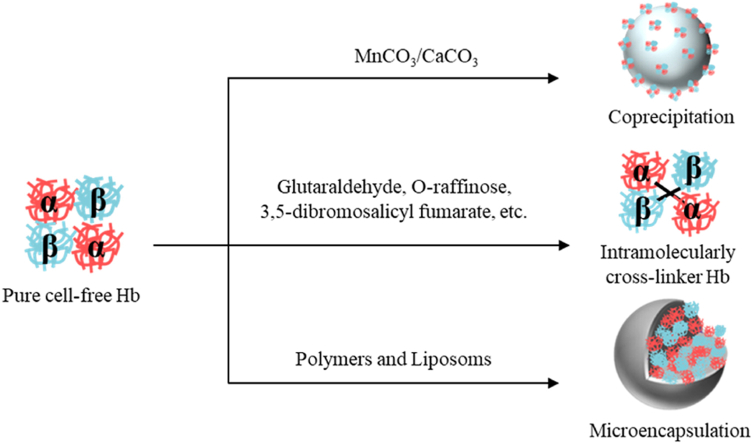
HBOCs (hemoglobin-based oxygen carriers) have expanded from their initial use as a blood substitute to ischemia and hypoxia therapy as a near physiologically conditioned oxygen carrier. Among the many properties that make hemoglobin (Hb) the ideal oxygen carrier within red blood cells are its oxygen affinity, long-term stability, stability of tetramers, and cooperativity [7,8]. During the past few decades, diverse nanoscale carriers have been developed for physical encapsulation or chemical conjugation of Hb, which were known as HBOCs [9]. To obtain desirable HBOCs for organ preservation, HBOCs of different particle sizes were synthesized in different buffers by chemical modification means, such as co-precipitation, desolvation, cross-linking, microencapsulation, selected from the above-mentioned methods (Table 1).
Table 1.
Different HBOCs sources, solvents and particle size.
| Polyhemoglobin | Source | various of buffers | Mean diameter | Ref. |
|---|---|---|---|---|
| Hb-MPs | BHb | EDTA solution (0.2 M, pH 7.4, 20 mL) | 3.30 ± 0.80 μm | [10] |
| ZnPc-loaded HbMs | BHb | Phosphate buffer (10 mM, pH = 7.4) | 66.01 ± 0.95 nm | [11] |
| PLGAHb/M-NCs | BHb | Tris(hydroXymethyl) aminomethane (TRIS) | ~95 nm | [[12], [13]] |
| PDA-Hb | BHb | Tris-HCl buffer (10 mM, pH 8.5) | 3.32 ± 0.49 μm | [14,15] |
| Hb@lipo | HHb | pH 7.4 PBS | ~120 nm | [16] |
| BP QDs-Hb-encapsulated biocompatible | HHb | Deionized water | NA | [17] |
| GelMA hydrogel | ||||
| Hb-PDA | BHb | Tris-HCl buffer (10 mM, pH 8.5) | 6–8 nm | [18] |
| Hb-conjugated biotins | HHb | Phosphate buffer (50 mM, pH 6.5) | NA | [19] |
| SA@Hb@CQDs | HHb | Deionized water | NA | [20] |
| ZIF-8P-Hb | BHb | Deionized water | 106.0 ± 9.7 nm | [21] |
| SFHbNP | HHb | Phosphate buffer (10 mM, pH 7.4) | ~90 nm | [22] |
| HbAvHb | HHb | Phosphate buffer (50 mM, pH 6.5) | NA | [23] |
| SPolyHb | Guinea pig hemoglobin | Phosphate buffer (50 mM, pH 7.4) | >500 kDa | [24] |
| Hb@lipo | HHb | phosphate-buffered (pH 7.4) | ~120 nm | [25] |
| HEP | BHb | phosphate-buffered (pH 7.4) | ~200 nm | [26] |
| Hb-PEG | HHb | Phosphate buffered (10 mM Na2HPO4, pH 7.4) | 10.6 ± 3.6 nm | [27] |
| PolyhHbs | BHb | Phosphate buffered (10 mM, pH 7.4) | ~80 nm | [28] |
| Hb-V | HHb | Phosphate buffered (10 mM, pH 7.4) | 250–280 nm | [29] |
| Fe3O4-PEI-PA-Yb3+ | BHb | Tris-HCl (pH 6) | ~105 nm | [30] |
In this review, we summarize the use of purified hemoglobin that was structurally modified for organ storage. Most examples presented here have been published within the past 5 years, and greater emphasis has been given to recent examples that are illustrative principles.
2. Materials of HBOCs-Hb
The function of Hb includes maintaining the pH of the blood, carrying oxygen to peripheral tissues, as well as transporting carbon dioxide. Structurally, hemoglobin chains retain the classic globin fold, which is shared by several proteins. Each hemoglobin chain harbors a heme group in a hydrophobic pocket. A ferrous ion (Fe2+) of the heme group associated with each hemoglobin chain acts as a cofactor for this tetrameric protein. An Hb molecule is also categorized as a supramolecular assembly that consists of two α and two β subunits (α2β2). They are assembled using a combination of non-covalent interactions as hydrogen bonds, hydrophobic forces, van der Waals forces, and electrostatic effects. Its tetrameric structure(α2β2) is fundamentally stable under physiological conditions, but dissociates reversibly into dimers, thereby exchanging dimers intermolecularly(αβ) [31]. Specifically, the amino acids that comprise each of the three coding sequences of Hb are responsible for the following functions: i) heme contacts essential for oxygenation, ii) contacts α1 and β1 are essential for a cooperative dimer, iii) α1-β1 contacts essential for cooperative tetramer, iv) the Bohr effect that modulates oxygen loading and unloading from the lung to tissues, and v) oxygen affinity regulation requires 2,3-diphosphoglycerate binding [32,33].
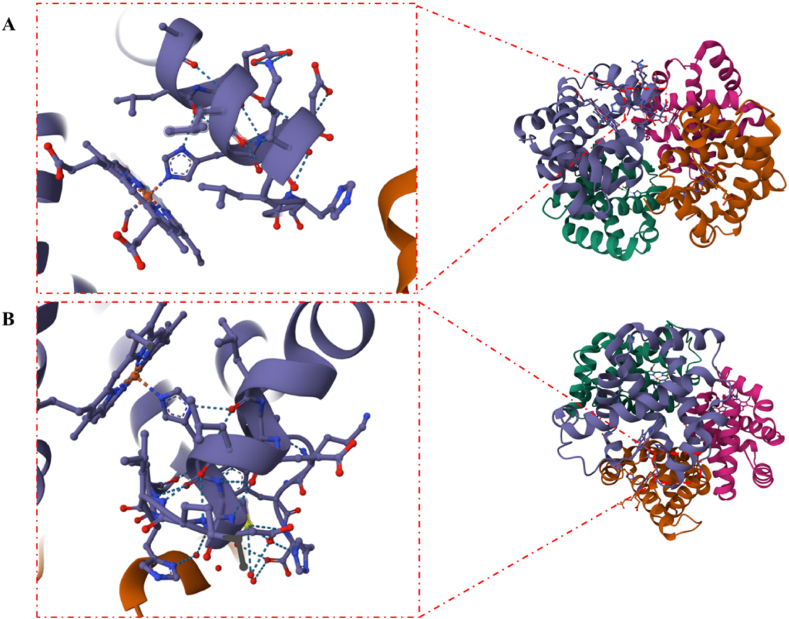
Due to its genetic similarity to human hemoglobin (HHb) at 90% of amino acid sequence and its better oxygen-carrying capacity, bovine hemoglobin (BHb) is commonly used in hemoglobin synthesis [34,35] (Fig. 1). Both BHb and HHb consists of two α-chains with 141 amino acid residues each and two β-chains with 146 amino acid residues each that form a tetrameric protein molecule [36]. In fact, Hes_63, His_92, and Phe_42 in beads proteins act as markers to maintain the position and orientation of the heme group, while Cys_93 in this protein uses NO as a site-modifying enzyme in Hb [37]. Although mammalian Hb contains six cysteines, all of which are capable of binding NO, β-Cys_93 has two unique properties (one in each α-globin and two in each β-globin): it is not only the most active Cys residue in Hb, but also its activity is related to the binding and release of oxygen at the heme site (“thermodynamic link”) [38,39].
Fig. 1.
Comparison of the spatial structure of BHb and HHb. A. BHb and β-Cys_93; B. HHb and β-Cys_93 (PDB: 6IHX and 1A3N).
3. Chemical modification
3.1. Coprecipitation
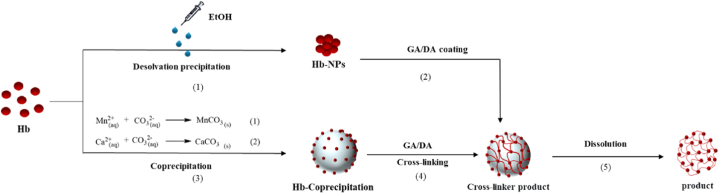
Since the affinity of neutral salt to water molecules is greater than that of Hb, it causes the hydration layer around protein molecules to weaken or even disappear. As the ionic strength changes with the addition of Hb to the neutral salt, the charge on the Hb surface is heavily neutralized, leading to a decrease in solubility and allowing co-precipitation by aggregation between molecules [38](Fig. 2). When a precipitant is added, homogeneous precipitation can be obtained. By adopting this method, precipitated particles with uniform chemical composition, small particle size, and uniform steps can be obtained. Carbonate ions (CO32−) and nanoparticles are used as precipitants for co-precipitation. a. MnCO3: A combination of MnSO4 and NH4HCO3 was used to synthesize MnCO3 particles from Chunmei yu for its excellent absorption capacity [40]. As a result of their high oxygen affinity, PDA-Hb microcapsules can bind and reversibly release oxygen. Metal carbonates of MnCO3 are formed when MnCl2 reacts with Na2CO3, encapsulating Hb in M. Emily [41]. CaCO3: Using a co-precipitation process between Hb and CaCO3, followed by covalent sphere assembly between Hb and GA, Li and co-workers have recently developed highly loaded Hb spheres [14,15]. They used CaCO3 particles as templates for fabricating Hb spheres that contain a high loading content in order to exploit their special properties, such as porous channel-like structures and high surface areas. CaCl2 and Na2CO3 were co-precipitated to produce CaCO3 particles. For every CaCO3 particle, there were 1.36 g/cm3 of Hb.
Fig. 2.
Synthesis of HBOCs based on co-precipitation method.
b. Desolvation precipitation: Desolvation results in the effective supersaturation of a protein solution by replacing water with an antisolvent, which is insoluble in water [42]. Precipitates are commonly dissolved with polar solvents. By applying classical nucleation theory, precipitates are formed as protein-protein interactions take precedence over protein-solvent interactions as the antisolvent is added [43]. Chen and colleagues used a magnetic stirring apparatus to dissolve Hb with different concentrations of ultrapure water (MQ), using EtOH as an excipient for protein dissolution [44]. EtOH was then added dropwise to Hb solutions under continuous stirring at different volumes based on the water phase. In order to remove the turbid suspensions, a benchtop centrifuge was used and MQ washes were used to spin the suspensions down. However, sonication and resuspension of Hb-NPs were both successful. The method is simple to operate, does not introduce toxic solvents, has uniform particle size, high encapsulation efficiency, and good re-dispersibility in water, and is a relatively common method. The synthesis of HBOCs by coprecipitation usually controls the amount of Hb in the spheres by regulating the initial concentration of hemoglobin, which in turn improves the oxygen-carrying capacity. Nevertheless, the Hb loading efficiency in the spheres can also be adversely affected by high concentrations. In addition, the stability of HBOCs obtained by this method is relatively poor. Therefore, on this basis, other chemical modification methods were introduced for structural modifications.
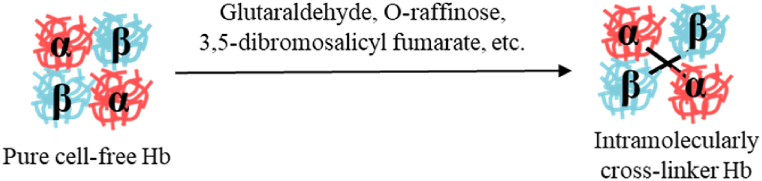
3.2. Cross-linking
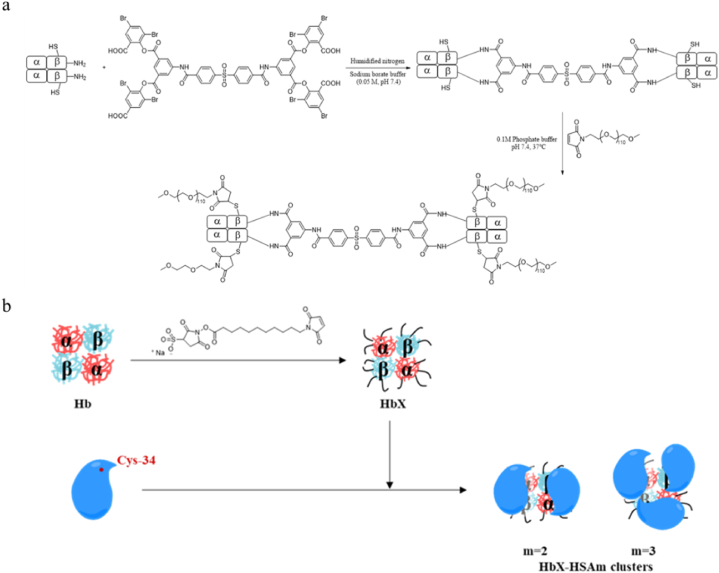
Cross-linking selects functionalities of the components to form polymers of a certain size by cross-linking with exposed amino acid residues –SH or –NH2 of Hb [45](Fig. 3). By increasing the particle size of Hb, cross-linking improves a certain oxygen-carrying capacity, increases the half-life of HBOC, and reduces the degradation of metabolic enzymes. However, to a certain extent, it causes side effects such as vasoconstriction. HBOCs are commonly used as a polymer of glutaraldehyde, polydopamine and glucan oxide and so on [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]]. The purified hemoglobin was dissolved in the anti-solvent ice ethanol, and oxidized dextran (2 wt%) was added for cross-linking reaction when the solution became turbidity. The reaction was completed by adding NaBH3CN to quench the reaction. Subsequently, the template is usually removed with EDTA or Na2EDTA after the cross-linked Hb is completed. It has been noted that hemoglobin's nitrite reductase activity is a potential source of biologically active NO during hypoxia and ischemia. Activating variable structure-controlled nitrite reductase with hemoglobin's heme fraction allows nitrite in the blood to be converted to NO [47,48]. Protein modifications that alter the binding affinity of the ligand to heme or its redox potential are considered to contribute to the enhancement of nitrite reductase activity. Ronald Kluger produced the PEGylated bis-tetramers with cross-linking that makes hemoglobin (BT-HB-PEG5K4)(Fig. 4a). In order to develop functional oxygen therapeutics that are effective, these polymers must have high oxygen affinity and nitrite reductase activity [49]. Their research has led to the production of PEGylated bis-tetramers of hemoglobin (BT-Hb-PEG5K4), enhancement of oxygen affinity, and improvement of nitrite reductase activity, all of which are critical for the development of functional oxygen therapies. Daiki Tomita used α-succinimidyl-ε-maleimide crosslinker constructs to covalently wrap Hb with HSAs to generate new core-shell protein clusters [50](Fig. 4b). O2-carrying HbX-HSAm clusters with negative surface net charges, high O2 affinity, and lower P50 value, does not cause vasoconstriction and NADH-dependent reductase unit shells can provide O2 therapeutic reagent in various clinical situations.
Fig. 3.
Synthesis of HBOCs based on cross-linking method.
Fig. 4.
Hemoglobin oxygen carriers was synthesized by cross-linking method. a) Synthesis of hemoglobin oxygen carrier BT-HB-PEG5K4 based on the cross-linking method; b) Schematic illustrations of the synthetic route of the HbX-HSAm cluster.
4. Microencapsulation
In order to further improve the oxygen capacity of the Hb vector and increase the function of the carrier, the researchers used the polymer and liposomes to encapsulate the hemoglobin, which formed the scale of the micro sodium level and made up a more stable and multivariate oxygen delivery system. These methods not only increase the quality of the hemoglobin package, but also improve the compatibility of the objects, and adapt the more complex biological environment to the prospect of a broader application of the hemoglobin vector.
4.1. Polymer
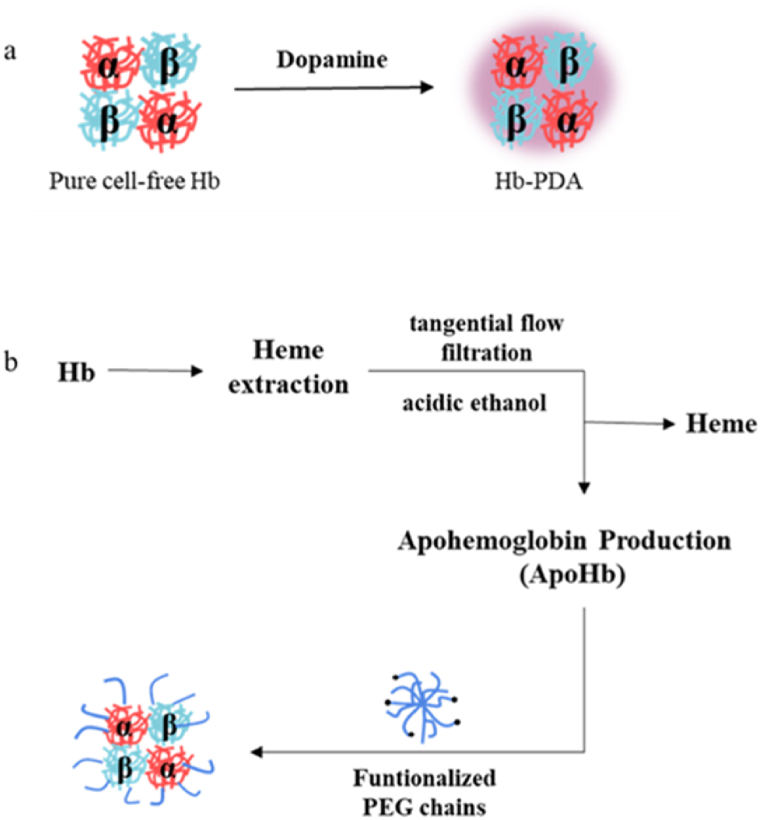
Polymersomes are of interest as nanocarriers due to their physical and chemical robustness, which arises from the macromolecular nature of their block copolymer components. The polymer of the polymerized hemoglobin: dextran, dopamine, PEG, etc. The polydopamine (PDA) coating material is highly adherent to all surface types of substrates and is simple and easy to apply. The PDA not only has little effect on the survival and proliferation of many kinds of mammal cells but also as an antioxidant agent, removing free radicals from distinct hydroquinone moiety. Platelet adhesion and fibrinogen conformation transition could be effectively inhibited by surfaces modified with PDA [[51], [52], [53]]. By encapsulating Hb in one step using a simple PDA coating, Wang and colleagues focused on the coating's desirable properties [54](Fig. 5a). An incubation mixture containing dopamine hydrochloride and Hb was incubated at room temperature for 3.5 h with slight stirring using a Tris-HCl buffer. The total volume of the reaction system was 2 mL, and it was dialyzed in PBS solution to remove excess dopamine hydrochloride. In addition, DA was oxidatively polymerized in TRIS 1 for coating Hb with PDA by Jansman [55]. Briefly, 8 mg/mL solution of Hb and 1.6 mg/mL solution of DA were mixed at 1:1 vol ratio in TRIS 1 and rotators for 3 h were used. The resulting HbPDA was washed in TRIS 2 using a bench-top centrifuge and amicon centrifugal filters. By conjugating polyethylene glycol (PEG) to the protein surface, protein therapeutics can increase their circulatory half-life in vivo. PEG is used as a standard for nanoparticle and protein coupling surfaces due to its excellent biocompatibility and increased hydration radius after coupling. Furthermore, PEG chains possess bristle-like structures on their molecular surfaces that prevent T-cell recognition and reduce clearance of PEG-coupled therapeutic molecules [56,57]. As a result of PEGylated apoHb (PEG-apoHb) coupling of PEG to apoproteins via thiol-maleimide, Ivans S and colleagues improved lipoprotein stability, circulating half-lives, and the prevention of PEG-apoHb extravasation [58](Fig. 5b).
Fig. 5.
Synthesis of hemoglobin oxygen carrier in polymeric form. a) Synthetic pathway of Hb-PDA; b) Synthetic pathway of PEG-apoHb.
4.2. Liposome
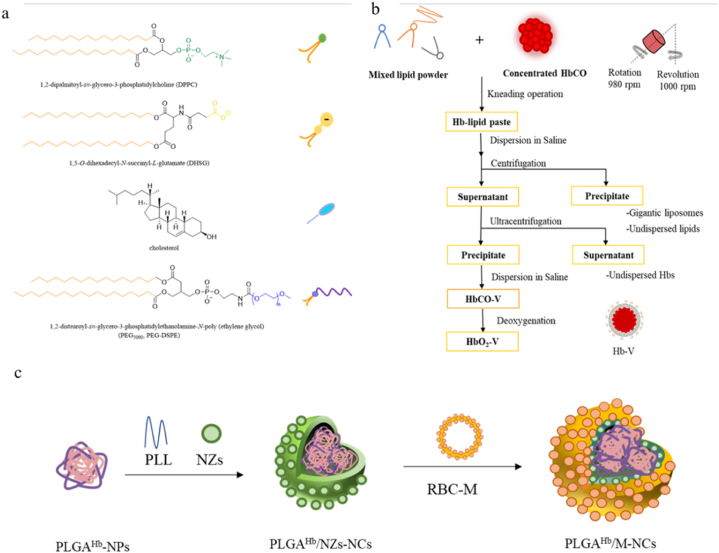
Hb encapsulated in lipid membranes without antigen, and thereby reducing renal excretion, would not only increase circulation time, but eliminate the need for matching blood groups [22,59,60]. Despite PEG being the standard for delivering stealth properties to intravenously administered carriers, the production of anti-PEG antibodies is increasingly recognized as a concern. By creating PEG antibodies, the liver and spleen recognize and eliminate PEGylated drugs and NPs by creating mononuclear phagocytes (MPS). In order to avoid this drawback, alternative PEGylation techniques are highly sought after [[61], [62], [63]]. As the most common substitute for biological membranes, amphiphilic molecular phospholipid vesicles or liposomes self-assemble in water to form bilayers (Fig. 6a) [64]. The interaction with plasma proteins poses difficulties in controlling particle size and inhibiting aggregation [65]. Using the extrusion method, particles of phospholipids are dispersed in an aqueous phase and then extruded through filters with different pore sizes [66]. Viscosity increases further when lipids are added to hemoglobin vesicles (Hb-V) to enhance oxygen carrying capacity. Extrusion methods clog filters due to the mixture's high viscosity. The mixture of lipids was therefore limited [67]. It has been proposed that freeze-dried liposomes can be mixed to resolve this difficulty. However, Hb remains limited in how much lipid can be mixed with it. Tomoko Kure and Hiromi Sakai developed “dual (asymmetric) centrifugation (DAC or DC)" after carefully understanding the background and difficulties presented above [68]. Using planetary motion, raw materials are sealed inside a cylindrical container (vessel) that rotates around a central axis and a second axis simultaneously. To prepare the mixed lipids, specific molar ratios of DPPC, DHSG, and PEG-DSPE were dissolved in 2-methyl-2-propanol by stirring in a 500 mL flask at 60 °C(Fig. 6a and b). Then, the lipid mixture solution was freeze-dried for 1 day to obtain a powdered lipid mixture. Liposomal preparation can be performed in fewer steps with this mixer without contamination, rapidly and aseptically. In addition, Michelle Maria Theresia Jansman and his colleagues chose human blood cells as phospholipid membranes to wrap the Hb(Fig. 6c) [59]. Nanocarriers with membrane coatings were shown to be novel oxygen carriers that combine antioxidant and stealth properties.
Fig. 6.
Synthesis of hemoglobin oxygen carrier in microcapsule form. a) Chemical structure of DPPC, DHSG cholesterol, and PEG-DSPE; b) Synthetic pathway of HbO2-V; c) Synthetic pathway of PLGAHb/M-NCs.
5. Biocompatibility and hemocompatibility
1Good biocompatibility is a central aspect when developing HBOCs to preserve the normal physiological functions of the body [44]. According to the literature, HUVEC, RAW 264.7, HEK 293T, HepG 2 cell lines were usually selected for HBOCs for biocompatibility determination by CCK-8 and other methods. HbPDA was not significantly cytotoxic after 24 h incubation in different concentration ranges [18]. The biocompatibility of the Hb/PDA-NPs was evaluated by assessing the in vitro cell viability of HUVEC and RAW 264.7 cells [44]. It was shown to inhibit cells by approximately 15% at only higher concentrations of 30 000 NPs/μL. The PDA-Hb microcapsules designed by Yu C did not produce significant cytotoxic activity on HEK 293T cells compared to the control [40]. The IL/PDA nanocapsules studied by Tan et al. still showed 80% cell viability at 200 μg/mL. Therefore, it was shown to have no significant cellular activity [53][69]. RAW264.7 cells in PLGAHb/M − NCs showed a significant decrease in cellular activity with increasing concentrations of NCs, whereas this did not occur in HUVEC. Leticia Hosta-Rigau suggested that the decrease in RAW264.7 cellular activity was the result of phagocytosis of NCs by cells, independent of cell membrane concentration [55]. In addition, the study by Leticia Hosta-Rigau's group found no significant differences based on different coating methods to cover Hb.
Hemocompatibility as an important essential property of intravenous carriers has also been used to examine the safety of HBOCs. According to the literature the threshold value of hemolysis rate of HBOCs is below 5%, which indicates that the microcapsules do not interact with other components of the blood and therefore do not have adverse effects on the blood after introduction [40,44,51,59]. The Hb-NPs and Hb/PDA-NPs did not hemolysis to occur, even at higher concentrations [40,44]. The hemolysis rates of PLGA/HbPDA/(CeO2-NPs)-NCs and PLGA/HbPDA/(CeO2-NPs)-NCs were both well below 5% under the wrapping of cell membranes [51,55].
6. Conclusion
HOBCs have an important role as a blood substitute used clinically to maintain normal physiological functions of the body. With the continuous development and optimization of synthesis technology, it is now common to combine one or more of the above methods to synthesize the required HBOCs with the advantages of higher oxygen-carrying capacity, higher stability, improved half-life, and lower toxicity. However, there are some unavoidable drawbacks (Table 2). For example, although the coprecipitation method for the synthesis of HBOCs is simple in operation, without the introduction of toxic substances and with greater solubility in various buffers, its stability is poor compared to several other methods, so the cross-linking agent and the formation of microcapsules are introduced on this basis. However, the introduction of cross-linking agents largely causes vasoconstriction and has a potential risk of causing hypertension. In addition, the formation of hemoglobin into polymers leaves relatively small molecules or unreacted Hb, and when liposomes are encapsulated, the formation of membranes requires the addition of other substances to fill the shell.
Table 2.
Merits and disadvantages of different synthesis methods.
| Method of synthesis | Merits | Disadvantages |
|---|---|---|
| coprecipitation | simple, no toxic substances introduced, high encapsulation efficiency and solubility in a polar solvent | lower stability |
| cross-linking | increased half-life, improved stability, and oxygen-carrying capacity, reduced protein immunogenicity | blood pressure rises, vasoconstriction |
| polymer | increased half-life, smaller side effects, and larger particle size | residual unreacted Hb |
| liposome | uniform particle size, good stability | cumbersome steps |
HBOCs were initially developed and used as blood substitutes as an ideal class of oxygen carriers for early applications in hemorrhagic shock. However, the ethical problems associated with the use of modified HBOCs in emergency care, the significant cardiovascular dysfunction and the apparent increased mortality caused by them led to the extended use of most HBOCs in organ preservation fluids. In organ storage, HBOCs have positive effects such as anti-apoptotic, anti-inflammatory and anti-proliferative. Not only that, HBOCs improve oxygen supply and preserve optimal metabolic activity, reduce oxidation-mediated tissue damage and enhance liver capacity storage, but also have positive effects in preserving and improving marginal organs. Mechanistic studies related to vascular resistance, methemoglobin and oxidative damage in transplants and recipients should also be closely investigated in future studies. In the future, according to the characteristics of storing different types of organs, we will screen the synthesis method that is most suitable for storing HBOCs of that type of organ, so as to construct the optimized storage solution.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
1) conceived and designed the experiments.
2) performed the experiments.
3) analyzed and interpreted the data.
4) contributed reagents, materials, analysis tools or data.
5) wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the financial support provided by Key Research Project of Application Foundation of Logistics Support Department of Central Military Commission of China [BWS21J002].
References
- 1.McFarlane L., Nelson P., Dugbartey G G., Sener A. Pre-Treatment of transplant donors with hydrogen sulfide to protect against warm and cold ischemia-reperfusion injury in kidney and other transplantable solid organs. Int. J. Mol. Sci. 2023;24(April):3518. doi: 10.3390/ijms24043518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israni A., Zaun D., Gauntt K. OPTN/SRTR 2021 annual data Report: deceased organ donation. OPTN/SRTR 2021 annual data Report: preface. Am. J. Transplant. 2023;21:444–545. doi: 10.1016/j.ajt.2023.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Cao M., et al. Hemoglobin-based oxygen carriers: potential applications in solid organ preservation. Front. Pharmacol. 2021;11(December) doi: 10.3389/fphar.2021.760215. 760215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosgood S., Brown R., Nicholson M., Michael N. Advances in kidney preservation techniques and their application in clinical practice. Transplantation. 2021;105:202–214. doi: 10.1097/TP.0000000000003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahboub P., et al. The efficacy of HBOC-201 in ex situ gradual rewarming kidney perfusion in rat model. Artif. Organs. 2020;44(January):81–90. doi: 10.1111/aor.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv Y., et al. There is no dose-response relationship between allogeneic blood transfusion and healthcare-associated infection: a retrospective cohort study. Antimicrob. Resist. Infect. Control. 2020;(October):62. doi: 10.1186/s13756-021-00928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faggiano S., et al. From hemoglobin allostery to hemoglobin-based oxygen carriers. Mol. Aspect. Med. 2022;4(August) doi: 10.1016/j.mam.2021.101050. [DOI] [PubMed] [Google Scholar]
- 8.Wang A., Kluger R. Increasing efficiency in protein-protein coupling: subunit-directed acetylation and phase-directed cuaac (“click coupling”) in the formation of hemoglobin bis-tetramers. Biochemistry. 2014;53(April):6793–6801. doi: 10.1021/bi501054b. [DOI] [PubMed] [Google Scholar]
- 9.Charbe N., et al. A new era in oxygen therapeutics? From perfluorocarbon systems to haemoglobin-based oxygen carriers. Blood Rev. 2022;54(July) doi: 10.1016/j.blre.2022.100927. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y., et al. Hemoglobin-based oxygen carrier microparticles: synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules. 2021;13:3292–3300. doi: 10.1021/bm301085x. [DOI] [PubMed] [Google Scholar]
- 11.Wang S., et al. Synthesis of hemoglobin conjugated polymeric micelle: a znpc carrier with oxygen self-compensating ability for photodynamic therapy. Biomacromolecules. 2015;16(September):2693–2700. doi: 10.1021/acs.biomac.5b00571. [DOI] [PubMed] [Google Scholar]
- 12.Coll C, Michelle M, Peter W, Leticia H. Optimization of Hemoglobin Encapsulation within PLGA Nanoparticles and Their Investigation as Potential Oxygen Carriers. Pharmaceutics. November 2021;13:1958. doi: 10.3390/pharmaceutics13111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kure T., Sakai H. Preparation of artificial red blood cells (hemoglobin vesicles) using the rotation-revolution mixer for high encapsulation efficiency. ACS Biomater. Sci. Eng. 2021;7(June):2835–2844. doi: 10.1021/acsbiomaterials.1c00424. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y., Duan L., Li J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv. Mater. 2016;28(June):1312–1320. doi: 10.1002/adma.201502581. [DOI] [PubMed] [Google Scholar]
- 15.Duan L., Yan X., Wang A., Jia Y., Li J. Highly loaded hemoglobin spheres as promising artificial oxygen carriers. ACS Nano. 2012;6(August):6897–6904. doi: 10.1021/nn301735u. [DOI] [PubMed] [Google Scholar]
- 16.Jiang M., et al. Inhibiting hypoxia and chemotherapy-induced cancer cell metastasis under a valid therapeutic effect by an assistance of biomimetic oxygen delivery. Mol. Pharm. 2019;16(November):6897–6904. doi: 10.1021/acs.molpharmaceut.9b00663. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Chen G., Liu Y., Sun L., Sun L., Zhao Y. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing. ACS Nano. 2020;14(May):5901–5908. doi: 10.1021/acsnano.0c01059. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q., et al. Bioinspired polydopamine-coated hemoglobin as potential oxygen carrier with antioxidant properties. Biomacromolecules. 2017;18(April):1333–1341. doi: 10.1021/acs.biomac.7b00077. [DOI] [PubMed] [Google Scholar]
- 19.Singh S., luger R. Self-assembly of a functional triple protein: hemoglobin-avidin hemoglobin via biotin-avidin interactions. Biochemistry. 2016;55(May):2875–2882. doi: 10.1021/acs.biochem.6b00215. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., et al. Injectable in situ self-cross-linking hydrogels based on hemoglobin, carbon quantum dots, and sodium alginate for real-time detection of wound bacterial infection and efficient postoperative prevention of tumor recurrence. Langmuir. 2020;36(11):13263–13273. doi: 10.1021/acs.langmuir.0c02219. [DOI] [PubMed] [Google Scholar]
- 21.Gu X., Palmer A. ZIF-8 metal-organic framework nanoparticles loaded with hemoglobin as a potential red blood cell substitute. ACS Appl. Nano Mater. 2022;5(April):8832–8844. doi: 10.1021/acsanm.2c00608. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto W., et al. Core-shell structured hemoglobin nanoparticles as artificial O2 carriers. ACS Appl. Bio Mater. 2022;5(December):5844–5853. doi: 10.1021/acsabm.2c00813. [DOI] [PubMed] [Google Scholar]
- 23.Singh S., Kluger R. Self-assembly of a functional triple protein: hemoglobin-avidinhemoglobin via biotin-avidin interactions. Biochemistry. 2016;55(May):2875–2882. doi: 10.1021/acs.biochem.6b00215. [DOI] [PubMed] [Google Scholar]
- 24.Williams A., et al. Polymerized hemoglobin with increased molecular size reduces toxicity in healthy Guinea pigs. ACS Appl. Bio Mater. 2020;15(May):2976–2985. doi: 10.1021/acsabm.0c00039. [DOI] [PubMed] [Google Scholar]
- 25.Jiang M., et al. Inhibiting hypoxia and chemotherapy-induced cancer cell metastasis under a valid therapeutic effect by an assistance of biomimetic oxygen delivery. Mol. Pharm. 2019;16(November):4530–4541. doi: 10.1021/acs.molpharmaceut.9b00663. [DOI] [PubMed] [Google Scholar]
- 26.Kim J., Jeong S., Korneev R., Shin K., Kim K. Cross-linked polymersomes with reversible deformability and oxygen transportability. Biomacromolecules. 2019;20(June):2430–2439. doi: 10.1021/acs.biomac.9b00485. [DOI] [PubMed] [Google Scholar]
- 27.Matsuhira T., Yamamoto K., Sakai H. Ring-opening polymerization of hemoglobin. Biomacromolecules. 2019;20(April):1592–1602. doi: 10.1021/acs.biomac.8b01789. [DOI] [PubMed] [Google Scholar]
- 28.Donald A., Clayton T., Evan L., Pires I., Palmer A. Controlled polymerization and ultrafiltration increase the consistency of polymerized hemoglobin for use as an oxygen carrier. Bioconjugate Chem. 2020;31(March):605–621. doi: 10.1021/acs.bioconjchem.9b00766. [DOI] [PubMed] [Google Scholar]
- 29.Kure K., Sakai H. Transmembrane difference in colloid osmotic pressure affects the lipid membrane fluidity of liposomes encapsulating a concentrated protein solution. Langmuir. 2017;33(June):1533–1540. doi: 10.1021/acs.langmuir.6b04643. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Guan H., Han Q., Tian S., Liang Q., Ding M. Fabrication of Yb3+-immobilized hydrophilic phytic-acid-coated magnetic nanocomposites for the selective separation of bovine hemoglobin from bovine serum. ACS Biomater. Sci. Eng. 2019;5(June):2740–2749. doi: 10.1021/acsbiomaterials.9b00074. [DOI] [PubMed] [Google Scholar]
- 31.Matsuhira T., Sakai H. Entropy-driven supramolecular ring-opening polymerization of a cyclic hemoglobin monomer for constructing a hemoglobin-PEG alternating polymer with structural regularity. Biomacromolecules. 2021;22(May):1944–1954. doi: 10.1021/acs.biomac.1c00061. [DOI] [PubMed] [Google Scholar]
- 32.Morita Y., Yamada T., Kureishi M., Kihira K., Komatsu T. Quaternary structure analysis of a hemoglobin core in hemoglobin-albumin cluster. J. Phys. Chem. B. 2018;122(January):12031–12039. doi: 10.1021/acs.jpcb.8b10077. [DOI] [PubMed] [Google Scholar]
- 33.Laganowsky A., et al. Origin of complexity in haemoglobin evolution. Nature. 2020;583(June):480–485. doi: 10.1038/s41586-020-2292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amanat A., Soman S., Vijayan R. Dynamics of camel and human hemoglobin revealed by molecular simulations. Sci. Rep. 2022;12(January):122. doi: 10.1038/s41598-021-04112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Zhang H., Zhou Q. Studies on the interaction of caffeine with bovine hemoglobin. Eur. J. Med. Chem. 2009;44(May):2100–2105. doi: 10.1016/j.ejmech.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Liu B., Yan X., Cao S., Chong B., Yang C., Lu K. Interaction of salicylic acid with bovine hemoglobin and effect of the coexistent metal ion on the reaction. Spectrosc. Lett. 2013;46(March):165–174. doi: 10.1080/00387010.2012.718725. [DOI] [Google Scholar]
- 37.Premont R., Reynolds J., Zhang R., Stamler J. Red blood cell-mediated S-nitrosohemoglobin-dependent vasodilation: lessons learned from a β-globin cys93 knock-in mouse. Antioxidants Redox Signal. 2021;34(June):936–961. doi: 10.1089/ars.2020.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isbell T., et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat. Med. 2008;14(June) doi: 10.1038/nm1771. 155-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R., et al. Hemoglobin beta-Cys93 is essential for cardiovascular function and integrated response to hypoxia. Proc. Natl. Acad. Sci. U.S.A. 2015;112:6425–6430. doi: 10.1073/pnas.1502285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu C., et al. Fabrication and evaluation of hemoglobin-based polydopamine microcapsules as oxygen carriers. Chem. Commun. 2018;54(April):4136–4139. doi: 10.1039/c8cc00095f. [DOI] [PubMed] [Google Scholar]
- 41.Emily M., Hickey R., Palmer A. Sonication effectively reduces nanoparticle size in hemoglobin-based oxygen carriers (HBOCs) produced through coprecipitation: implications for red blood cell substitutes. ACS Appl. Nano Mater. 2020;3(December):11736–11742. doi: 10.1021/acsanm.0c02176. [DOI] [Google Scholar]
- 42.Marty J., Oppenheim R., Speiser P. Nanoparticles a new colloidal drug delivery system. Pharm. Acta Helv. 1978;53(January):17–23. [PubMed] [Google Scholar]
- 43.Kalikmano V. Vol. 12. Springer; 2013. (Nucleation Theory). March. [Google Scholar]
- 44.Chen J., Michelle M., Liu X., Hosta-Rigau L. Synthesis of nanoparticles fully made of hemoglobin with antioxidant properties: a step toward the creation of successful oxygen carriers. Langmuir. 2021;37(September):11561–11572. doi: 10.1021/acs.langmuir.1c01855. [DOI] [PubMed] [Google Scholar]
- 45.Matsuhira T., Sakai H. Artificial oxygen carriers, from nanometer to micrometer-sized particles, made of hemoglobin composites substituting for red blood cells. Particuology. 2022;64(December):43–55. doi: 10.1016/j.partic.2021.08.010. [DOI] [Google Scholar]
- 46.Hickey R., Plamer A. Synthesis of hemoglobin-based oxygen carrier nanoparticles by desolvation precipitation. Langmuir. 2020;36(December):14166–14172. doi: 10.1021/acs.langmuir.0c01698. [DOI] [PubMed] [Google Scholar]
- 47.Yu B., Bloch K., Zapol W. Hemoglobin-based red blood cell substitutes and nitric oxide. Trends Cardiovasc. Med. 2009;19(March):103–110. doi: 10.1016/j.tcm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kehmeier E., et al. Serial measurements of whole blood nitrite in an intensive care setting. Free Radical Biol. Med. 2008;44(December):1945–1950. doi: 10.1016/j.freeradbiomed. [DOI] [PubMed] [Google Scholar]
- 49.Lui F., Kluger R. Enhancing nitrite reductase activity of modified hemoglobin: bis-tetramers and their pegylated derivatives. Biochemistry. 2009;48(December):11912–11921. doi: 10.1021/bi9014105. [DOI] [PubMed] [Google Scholar]
- 50.Tomita D., et al. Covalent core−shell architecture of hemoglobin and human serum albumin as an artificial O2 carrier. Biomacromolecules. 2013;14(June):1816–1825. doi: 10.1021/bm400204y. [DOI] [PubMed] [Google Scholar]
- 51.Lu M., et al. Preparation, characterization and in vivo investigation of blood-compatible hemoglobin-loaded nanoparticles as oxygen carriers. Colloids Surf., B. 2016;139(December):171–180. doi: 10.1016/j.colsurfb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y., Zheng Y., Zhao C., You J., Qu F. Hollow PDA-Au nanoparticles-enabled signal amplification for sensitive nonenzymatic colorimetric immunodetection of carbohydrate antigen. Biosens. Bioelectron. 2015;71(December):200–206. doi: 10.1016/j.bios.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Cheng W., et al. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano. 2019;13(August):8537–8565. doi: 10.1021/acsnano.9b04436. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q., et al. Bioinspired polydopamine-coated hemoglobin as potential oxygen carrier with antioxidant properties. Biomacromolecules. 2017;18(April):1333–1341. doi: 10.1021/acs.biomac.7b00077. [DOI] [PubMed] [Google Scholar]
- 55.Michelle M., et al. Hemoglobin-based oxygen carriers incorporating nanozymes for the depletion of reactive oxygen species. ACS Appl. Mater. Interfaces. 2020;45(December):50275–50286. doi: 10.1021/acsami.0c14822. [DOI] [PubMed] [Google Scholar]
- 56.Awwad S., Ginn C., Brocchini S. Elsevier; 2018. The Case for Protein Pegylation Engineering of Biomaterials for Drug Delivery Systems: beyond Polyethylene Glycol; pp. 27–49. [DOI] [Google Scholar]
- 57.Savla C., Palmer A. Structural stability and biophysical properties of the mega-protein erythrocruorin are regulated by polyethylene glycol surface coverage. Biomacromolecules. 2021;22(May):2081–2093. doi: 10.1021/acs.biomac.1c00196. [DOI] [PubMed] [Google Scholar]
- 58.Pires I.S., Savla C., Palmer A. Poly (ethylene glycol) surface-conjugated apohemoglobin as a synthetic heme scavenger. Biomacromolecules. 2020;21(June):2155–2164. doi: 10.1021/acs.biomac.0c00141. [DOI] [PubMed] [Google Scholar]
- 59.Jansman M., et al. Hemoglobin-based oxygen carriers camouflaged with membranes extracted from red blood cells: optimization and assessment of functionality. Biomater. Adv. 2022;134(June) doi: 10.1016/j.msec.2022.112691. [DOI] [PubMed] [Google Scholar]
- 60.Thi T., et al. The importance of poly (ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers. 2020;12(February):298. doi: 10.3390/polym12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.d'Avanzo N., et al. Immunogenicity of polyethylene glycol-based nanomedicines: mechanisms, clinical implications and systematic approach. Adv. Ther. 2020;3(March) doi: 10.1002/adtp.201900170. [DOI] [Google Scholar]
- 62.Bangham A., Horne R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;5(April):660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 63.Djordjevich L., Miller I. Lipid encapsulated hemoglobin as a synthetic erythrocyte, Fed. SAVE Proc. 1977;8(May):207–224. doi: 10.3233/bir-1987-24212. [DOI] [Google Scholar]
- 64.Phillips W., et al. Polyethylene glycol-modified liposome-encapsulated hemoglobin: a long circulating red cell substitute. J. Pharmacol. Exp. Therapeut. 1999;288(February):665–670. [PubMed] [Google Scholar]
- 65.Keitaro S., Yoshiyasu N., Taro E., Takeoka S., Tsuchida E. Effective encapsulation of proteins into size-controlled phospholipid vesicles using freeze-thawing and extrusion. Biotechnol. Prog. 2003;19(May):1547–1552. doi: 10.1021/bp0201004. [DOI] [PubMed] [Google Scholar]
- 66.Has C., Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020;30(April):336–365. doi: 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- 67.Massing U., Ingebrigtsen S., Skalko B., Holsæter A. Tech. 2017. Dual centrifugation - a novel “in-vial” liposome processing technique, in Liposomes. [Google Scholar]
- 68.Tan L., et al. Biocompatible hollow polydopamine nanoparticles loaded ionic liquid enhanced tumor microwave thermal ablation in vivo. ACS Appl. Mater. Interfaces. 2016;8(May):11237–11245. doi: 10.1021/acsami.5b12329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.