Abstract
This study aimed to evaluate the protective effect of Spirulina platensis primary against dental fluorosis and secondary against oxidative stress in lambs reared in endemic fluorosis areas. Forty-eight lambs aged 5 months were divided into four equal groups (each one including 6 males and 6 females). Groups I and II served as controls belonging respectively to fluorosis-free (Settat) and endemic fluorosis (El Fokra) areas, while the other two Groups III and IV (belonging to El Fokra) received respectively a fixed daily intake of 250 and 500 mg/kg bodyweight (BW) of Spirulina platensis. The experiment was carried out for 13 months until the adult incisors appeared for all animals. According to the Dean’s Fluorosis Index (DFI), 500 mg/kg BW/day of Spirulina platensis (Group IV) protected against dental fluorosis. Moreover, in both male and female lambs, this dose significantly (p < 0.0001) reduced the plasmatic levels of fluoride, proteins, GSH, and MDA compared to the Group II. Furthermore, enzymatic activities of catalase and SOD increased significantly (p < 0.0001) in male and female lambs of the Group IV as compared to Group II. In conclusion, our findings support the potential use of Spirulina platensis as a valuable solution for addressing fluorosis in sheep, warranting further clinical trials.
Subject terms: Environmental sciences, Natural hazards
Introduction
Fluorine is one of the most reactive elements in nature1,2. It plays an important role in the production and growth of both humans and animals3,4. However, chronic exposure to high fluoride level can lead to several chronic intoxications such as dental and skeletal fluorosis5. Another important aspect of fluoride toxicity is its impact on the antioxidant system6 and generation of free radicals7. Fluorosis is a global health problem and it is endemic in different parts of the world8,9. It has been documented in humans and animals, mainly ruminants10,11. All over the world, many regions are well known for their fluoride contamination. In certain cases, countries such as India, China, Turkey, Morocco, Mexico and Australia, sheep have gained more attention from researchers12,13. Furthermore, chronic ingestion of fluoride from drinking water, or from roughage growing in soils rich in fluoride or contaminated with fluorinated compounds from volcanic or industrial sources, has a negative impact on the growth and production of sheep14,15. This causes serious socio-economic problems for the farmers16.
To prevent sheep from the harmful effects of this poisoning, several strategies have been adopted either by breeders or by researchers. Since fluorosis only manifests after the appearance of the first two adult teeth in sheep, breeders commonly adopt the strategy of selling lambs before their first teeth erupt and buying adult sheep from fluorosis-free areas. This causes a loss of herd benefits due to the low selling prices of young animals and handicaps the genetic programs17. Besides, several technologies have been practiced in different regions of the world for the defluoridation of water18. However, in many cases, infrastructure requirements, technical expertise and high operating costs are major factors limiting their wide use19,20. Moreover, various compounds have been tested to alleviate fluorosis in sheep such as aluminum sulfate21, aluminum chloride22 and aluminum hydroxide23. However, chronic ingestion of these synthetic minerals could also cause a toxic effect in animals24. Recently, a number of reports documented the beneficial effect of natural sources of biomolecules originating from Spirulina platensis (microalgae) and Tamarindus indica L. (medicinal plant), such as bivalent minerals and antioxidants to reduce or prevent fluoride toxicity in rats, mice, rabbits and cattle6,19,25,26. However, all these studies investigated only experimentally induced fluorosis.
Spirulina platensis is known for its richness in various minerals mainly magnesium, manganese, copper, zinc, and selenium27, which could chelate fluoride excess and promote its excretion. In addition, this microalga contains a wide range of antioxidant including phycocyanin, carotenoids, chlorophyll, acidic polyphenols, linoleic acid and vitamin C, which could correct the oxidative stress generated by this halogen. Therefore, the present study aimed to evaluate the potential protective effect of Spirulina platensis at two doses (250 and 500 mg/kg BW/day) primary against dental fluorosis and secondary against oxidative stress in naturally exposed lambs in the commune of El Fokra, belonging to the province of Khouribga, Morocco, an endemic fluorosis area. The control group includs lambs reared in the fluorosis-free Settat region, considered healthy. The null hypothesis of this study was that there is no protective effect of Spirulina platensis at two doses (250 and 500 mg/kg BW/day) against dental fluorosis and oxidative stress in lambs.
Results
Fluoride levels in groundwater and soil
Table 1 presents the comparison of fluoride levels in groundwater and soil samples between El Fokra commune and the Settat area. The results revealed that fluoride levels in both water and soil samples were significantly (p < 0.0001) higher in El Fokra commune compared to Settat area.
Table 1.
Comparison of fluoride levels in groundwater and soil between El Fokra commune and Settat area.
| Fluoride levels (ppm) | El Fokra Commune | Settat area |
|---|---|---|
| Groundater | 1.82 ± 0.34A | 0.72 ± 0.09B |
| Soil | 750.30 ± 32.29A | 158.90 ± 7.66B |
Data were expressed as the mean ± SEM.
A and B different superscripts within each column show significant differences at the level of fluoride levels between El Fokra commune and Settat area.
Clinical examination and prevalence of dental fluorosis
Table 2 presents the results of clinical examination of dental fluorosis. According to Fisher’s exact test, the statistical results indicated that there was a significant (p < 0.0001) association between the supplementation of Spirulina platensis and the state of dentition in lambs reared in an endemic fluorosis area. Moreover, it revealed that in all studied groups, male animals had a higher incidence of dental fluorosis compared to females, however, the difference was not statistically significant (p > 0.05). A 100% of the females and 83% of the males reared in fluorosis-free area (Group I) had a normal score, while the rest of males (17%) had a questionable score. Moreover, female, and male animals reared in endemic fluorosis area (Group II) presented dental lesions which intensity varied from very mild to moderate scores. The 250 mg/kg BW/day intake of Spirulina platensis (Group III) improved the protection of the animals’ teeth with 66% of females and 50% of males presenting a normal score, 17% of females and males presenting a questionable score, and 17% of females and 34% of males with a very mild score. Finally, the 500 mg/kg BW/day intake of Spirulina platensis (Group IV) protected the animals’ teeth from fluorosis, with only 17% of females and 34% of males showing a questionable score, while the remaining 83% of females and 66% of males had a normal score. It has to be highlighted that Groups III and IV went only as far as very mild and questionable scores, respectively, while Group II showed mild and moderate scores.
Table 2.
Effect of Spirulina platensis on the state of dentition in lambs reared in endemic fluorosis area.
| Score | Group I* | Group II* | Group III* | Group IV* | ||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Normal, n (%) | 6 (100) | 5 (83) | 0 | 0 | 4 (66) | 3 (50) | 5 (83) | 4 (66) |
| Questionable, n (%) | 0 | 1 (17) | 0 | 0 | 1 (17) | 1 (17) | 1 (17) | 2 (33) |
| Very mild, n (%) | 0 | 0 | 2 (33) | 1 (17) | 1 (17) | 2 (33) | 0 | 0 |
| Mild, n (%) | 0 | 0 | 3 (50) | 2 (33) | 0 | 0 | 0 | 0 |
| Moderate, n (%) | 0 | 0 | 1 (17) | 3 (50) | 0 | 0 | 0 | 0 |
| Severe, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total, n (%) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
*Indicates significant difference in the severity the state of dentition between male and female groups (p < 0.0001); n: number of animals; Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis.
Plasma fluoride level
The results indicated that the plasma fluoride level was significantly higher in female (p < 0.0001) and male (p < 0.0001) lambs reared in endemic fluorosis area (Group II) compared to the those reared in fluorosis-free area (Group I) (Fig. 1). Furthermore, the group supplemented with 250 mg/kg BW/day (Group III) of Spirulina platensis revealed that the plasma fluoride level was significantly higher in females (p < 0.0001) and males (p = 0.0002) compared to the Group I and significantly lower compared to those from Group II (p < 0.0001) (Fig. 1). Concerning the group supplemented by 500 mg/kg BW/day (Group IV) of Spirulina platensis revealed that the plasma fluoride level was significantly higher in females (p < 0.0454) and males (p < 0.0023) compared to the Group I and significantly lower compared to those of Group II (p < 0.0001) (Fig. 1). The results also showed that the dose of 250 mg/kg BW per day resulted in a fewer effective decreases of plasma fluoride with only 51.60% in females and 65.61% in males. In contrast, 500 mg/kg of Spirulina platensis per day resulted in a better decrease of plasma fluoride in both male and female lambs. Specifically, the reduction in plasma fluoride was 73.15% for females and 81.28% for males.
Figure 1.
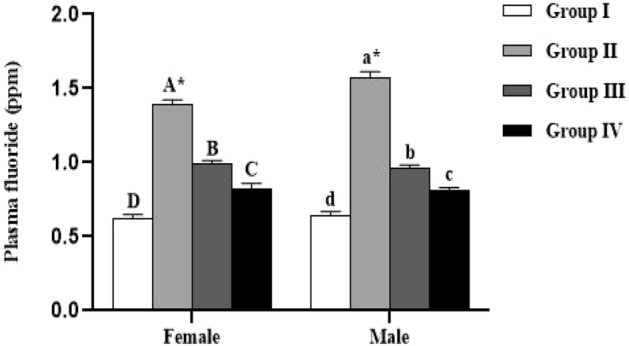
Effect of Spirulina platensis on plasma fluoride level in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A, B, C and D different superscripts within each column show significant differences at the level of plasma fluoride between the females of each group. a, b, c and d different superscripts within each column show significant differences at the level of plasma fluoride between the males of each group. *: indicates the significant difference at the level of plasma fluoride between males and females of each group.
Fecal fluoride level
The Fig. 2 shows the level of fluoride in the feces samples. The results indicate that the parameter under investigation was not significantly affected by either the studied groups or sex (p > 0.05).
Figure 2.
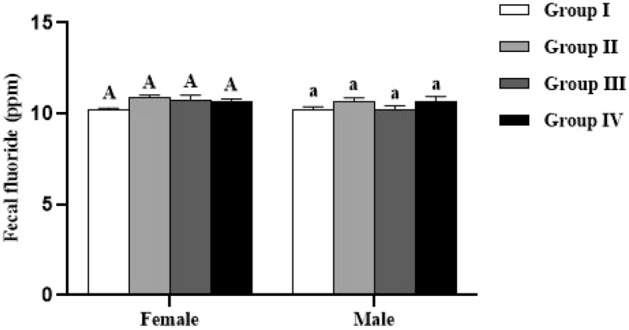
Effect of Spirulina platensis on fecal fluoride levels in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A: indicates that there is no significant difference in fecal fluoride levels between the females of each group. a indicates that there is no significant difference in fecal fluoride levels between the males of each group.
Biochemical parameters
The levels of proteins, reduced glutathione (GSH) and lipid peroxidation (MDA) in plasma of the studied animals are summarized in Figs. 3, 4 and 5. All these parameters were not significantly influenced by the sex (p > 0.05). In both males and females, significant (p < 0.0001) difference in plasma protein, GSH and MDA levels was recorded between all the studied groups.
Figure 3.
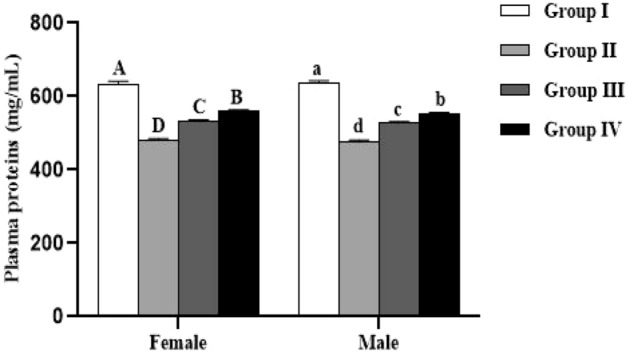
Effect of Spirulina platensis on plasma proteins level in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A, B, C and D different superscripts within each column show significant differences at the level of plasma proteins between the females of each group. a, b, c and d different superscripts within each column show significant differences at the level of plasma proteins between the males of each group.
Figure 4.
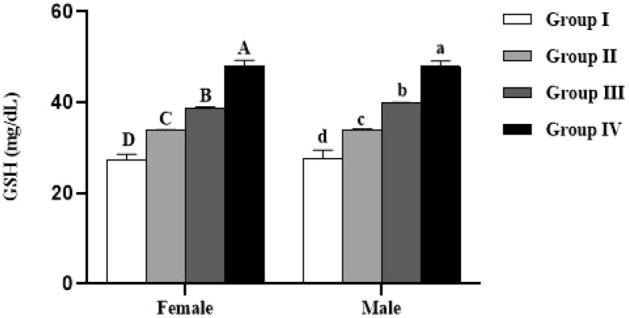
Effect of Spirulina platensis on reduced glutathione (GSH) level in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A, B, C and D different superscripts within each column show significant differences at the level of GSH between the females of each group (p < 0.05). a, b, c and d different superscripts within each column show significant differences at the level of GSH between the males of each group.
Figure 5.
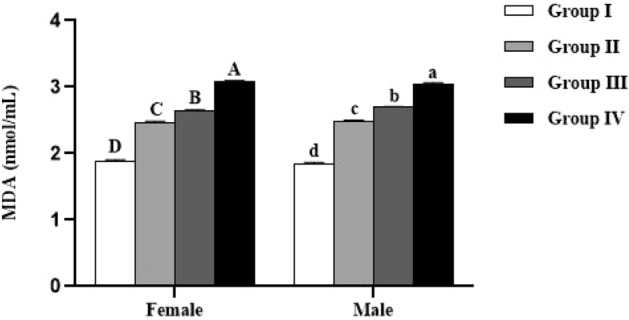
Effect of Spirulina platensis on malondialdehyde (MDA) level in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A, B, C and D different superscripts within each column show significant differences at the level of MDA between the females of each group. a, b, c and d different superscripts within each column show significant differences at the level of MDA between the males of each group.
Concerning enzymatic activity, the results showed that catalase and SOD activities were significantly lower in female (p < 0.0001) and male (p < 0.0001) lambs reared in endemic fluorosis area (Group II) compared to those reared in fluorosis-free area (Group I) (Figs. 6 and 7). Moreover, in both male and female lambs supplemented with 500 mg/kg BW/day of Spirulina platensis (Group IV), it was revealed that catalase (CAT) and superoxide dismutase (SOD) are significantly lower (p < 0.0001) compared to Group I and significantly higher (p < 0.0001) compared to Group II (Figs. 6 and 7). Concerning the group supplemented with 250 mg/kg BW/day of Spirulina platensis (Group III), the results revealed that there was no significant difference (p > 0.05) in catalase activity in both sexes compared to the Group II, whereas SOD activity was significantly lower (p < 0.0001) than Group I and significantly higher than Group II (p < 0.0001).
Figure 6.

Effect of Spirulina platensis on catalase (CAT) activity in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A, B, C and D different superscripts within each column show significant differences at the level of CAT activity between the females of each group. a, b, c and d different superscripts within each column show significant differences at the level of CAT activity between the males of each group.
Figure 7.
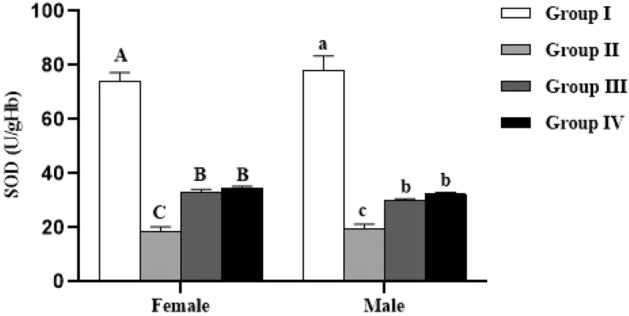
Effect of Spirulina platensis on superoxide dismutase (SOD) activity in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A, B, C and D different superscripts within each column show significant differences at the level of SOD activity between the females of each group. a, b, c and d different superscripts within each column show significant differences at the level of SOD activity between the males of each group.
Coming back to the level of hemoglobin, the results of Fig. 8 indicated that this parameter was not significantly affected by either the studied groups or sex (p > 0.05).
Figure 8.
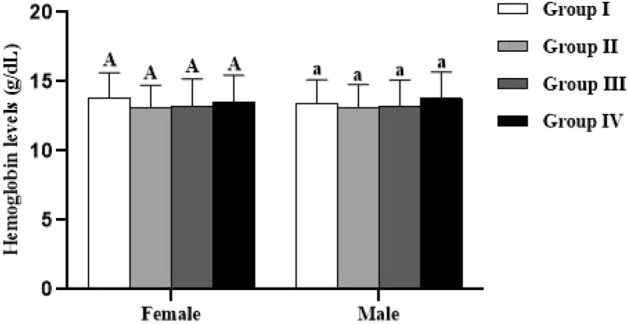
Effect of Spirulina platensis on hemoglobin (Hb) levels in lambs reared in endemic fluorosis area. Data were expressed as the mean ± SEM. Group I: lambs reared in fluorosis-free area, Group II: lambs reared in endemic fluorosis area, Group III: lambs supplemented with 250 mg/kg BW/day of Spirulina platensis, Group IV: lambs supplemented with 500 mg/kg BW/day of Spirulina platensis. A: indicates that there is no significant difference in hemoglobin levels between the females of each group. a indicates that there is no significant difference in hemoglobin levels between the males of each group.
According to the Rho Spearman test (Table 3), the results revealed that plasma fluoride level were positively correlated with GSH (r = 0.18; p < 0.0001) and MDA (r = 0.19; p < 0.0001), and negatively correlated with proteins (r = − 0.14; p < 0.0001), catalase (r = − 0.11; p < 0.0001) and SOD (r = − 0.22; p < 0.0001).
Table 3.
Correlation between fluoride, protein, MDA, GSH levels and catalase and SOD activities.
| Fluoride | Proteins | GSH | MDA | Catalase | SOD | |
|---|---|---|---|---|---|---|
| Fluoride | 1 | − 0.14* | 0.18* | 0.19* | − 0.11* | − 0.22* |
| Proteins | − 0.14* | 1 | − 0.24* | − 0.15* | 0.22* | 0.11* |
| GSH | 0.18* | − 0.24* | 1 | 0.55* | − 0.58* | − 0.38* |
| MDA | 0.19* | − 0.15* | 0.55* | 1 | − 0.39* | − 0.37* |
| Catalase | − 0.11* | 0.22* | − 0.58* | − 0.39* | 1 | 0.36* |
| SOD | − 0.22* | 0.11* | − 0.38* | − 0.37* | 0.36* | 1 |
*Correlation is significant at the 0.0001 level.
Discussion
This study investigated the effects of the natural microalgae called Spirulina platensis supplemented to lambs reared in El fokra commune, belonging to Khouribga-Morocco where fluorosis is endemic. In adherence to the null hypothesis, which postulated that there would be no significant protective effect of Spirulina platensis supplementation at two different doses (250 and 500 mg/kg BW/day) against dental fluorosis and oxidative stress in lambs, the present study was conducted. However, the findings lead us to reject the null hypothesis.
In Morocco, two distinct types of fluorosis are recognized, industrial and hydrotelluric28. However, the predominant form in Morocco is hydrotelluric fluorosis, mainly affecting areas rich in phosphate29, notably the province of Khouribga17. These regions are home to natural deposits of fluoride within phosphate rocks, and weathering of these rocks releases a significant amount of fluoride into groundwater and soil23.
Our study indicates significantly elevated fluoride levels in soils and groundwater in the commune of El Fokra compared to the Settat region, confirming the prevalence of hydrotelluric fluorosis in the former area. Furthermore, another study revealed higher fluoride concentrations in plant species (Chrysanthemum coronarium, Hordeum vulgare, Papaver rhoeas, Reseda alba, Silene vulgaris, Sinapis alba and Fumaria parviflora) collected in the grazing areas of El Fokra, as opposed to Settat30. This suggests that consumption of water or plants grown in fluoride-contaminated soil by lambs reared in El Fokra commune can lead to various forms of fluorosis.
The results obtained showed that the lambs reared in this commune presented dental lesions with a score varying between very mild to moderate according to Dean’s Index (DI). Several studies have shown that fluorosis in sheep, whether endemic or experimentally induced, results in dental lesions of varying severity depending on the concentration and the daily amount of ingested fluoride14,31–33. Daily intake of 250 mg/kg BW/day showed an ameliorative effect by reducing the degree of severity of dental lesions, while 500 mg/kg BW/day of Spirulina platensis protected against dental fluorosis in lambs. Rahim et al.27 reported that Spirulina platensis used in this study contains high levels of certain minerals, including calcium, magnesium, manganese, and zinc. These minerals have a strong affinity to fluoride, which means they can bind to it and reduce its harmful effects34. According to other studies, the high levels of calcium and ascorbic acid naturally present in plants, grass, and forage were proven to be responsible for the lower prevalence of dental and bone lesions in sheep reared in fluorosis endemic areas35,36.
Higher plasma fluoride level in fluorotic lambs was observed in this study compared to healthy control. Several studies reported that supplementing animals with some synthetic minerals reduced plasma fluoride in sheep21,22. This study was the first to evaluate the effect of a natural microalgae (Spirulina platensis) in lambs. Daily intake of 500 mg/kg BW of this microalgae reduced the plasma fluoride level by about 67.51%. On the other hand, the drop of plasma protein level in fluorotic animals in this study was similar to other findings37,38. In this regard, it was reported that fluoride can trigger endoplasmic reticulum stress, which in turn can reduce protein synthesis and secretion39. Moreover, DenBesten and Li40 reported that the inhibition of protein synthesis produced by fluoride toxicity during enamel development was responsible for dental fluorosis in the incisors of the animals. Hence, the beneficial effect of Spirulina platensis in increasing the level of plasma proteins in this work can be explained by its high crude protein content41,42. The term crude proteins refer to the total protein content in Spirulina platensis without further refinement or processing, a standard metric in nutritional analysis encompassing all forms of proteins without distinguishing types or quality. Spirulina platensis, used in this research, contains 53.31 g of proteins per 100 g of the microalgae, including essential amino acids and various protein compounds27. This substantial presence of crude proteins holds significance as it suggests Spirulina platensis may contribute to overall protein intake in animals. This aspect potentially plays a role in mitigating the adverse effects of fluoride toxicity on protein synthesis43,44. Furthermore, the findings of the current study revealed that the fluoride excretion level via feces was the same in all studied groups. This could be explained by the fact that the main route of fluoride excretion is urination (50–70%)20.
Regarding antioxidant system parameters, the results indicated that the erythrocytic enzymatic activities of CAT and SOD were lower while plasma levels of GSH and MDA were higher in fluorotic lambs compared to the healthy control. From the previous studies, it has been shown that fluoride is related to oxidative stress45–47. Moreover, it has been suggested that there are many groups of enzymes that can be affected by fluoride. More precisely, several enzymes require divalent metal ions as cofactors such as magnesium, calcium, zinc and selenium which have a strong affinity with fluoride, and thus can induce oxidative stress resulting from an inhibitory interaction of these enzymes13,48,49. For that, recent studies focused on supplementation of animals with antioxidants such as gallic acid50, quercetin51, vitamins7,46,52 and medicinal plants6,19,26. In this study, Spirulina platensis showed an ameliorative effect against oxidative stress in fluorotic sheep species. The ability of Spirulina platensis to prevent lipid peroxidation through chronic fluoride ingestion could be linked to its arsenal of antioxidants including phenolic compounds, β-carotene, vitamins and chlorophyll53. It also contains several natural phycobiliproteins, mainly phycocyanin which is a hydrophilic and intense blue-colored molecule, having a linear tetrapyrol prosthetic group called bilin. The latter gives it a strong ability to scavenge radical oxygen species and can behave as an antioxidant54. Additionally, phycocyanin originated from Spirulina platensis contains high levels of glutamic acid, glycine, and cysteine which are precursors of glutathione55. The latter is a powerful antioxidant that protect cells from oxidative damage. β-carotene, zeaxanthin, lycopene, lutein and chlorophyll which are present in Spirulina platensis have received considerable attention, due to their ability to strengthen the physiological system against oxidative stress53,56. These molecules can directly scavenge free radicals and inhibit lipid peroxidation, or indirectly improve the enzymatic activity of SOD and catalase57,58. Liu et al.59 observed a significant inverse association of the skeletal fluorosis with dietary intake of β-carotene, lutein/zeaxanthin, lycopene, and total carotenoids. Oxidative stress has been shown to play a potential etiological function through the expression and signalling process of NF-κB ligand (RANKL), which enhances osteoclast genesis and promotes osteoclastic differentiation in bone loss60,61. On the other hand, lycopene may exert dual anti-catabolic and pro-anabolic activities in bone by counteracting the NF-κB signal transduction pathway, inhibiting osteoclast differentiation and promoting osteoblast mineralization62. Moreover, it has been proposed that lycopene has a beneficial impact on bone formation and may imply possible consequences due to its use in the chemoprevention of skeletal fluorosis59.
Coming back to other components in Spirulina platensis that could have a main role in explaining our findings, it has to be highlighted that this microalga is a rich source of natural acidic polyphenols63. The most abundant polyphenols in Spirulina platensis are gallic acid and caffeic acid64, histopathological studies have shown that caffeic acid protects against fluoride-induced liver damage, and decreases serum fluoride levels and regulates the level of enzymatic and non-enzymatic antioxidants. In addition, caffeic acid inhibits fluoride induced apoptosis by altering the expression of the Bax and caspase-3p20 genes65. Another study showed that gallic acid can attenuate nephrotoxicity and oxidative stress induced by fluoride51. It also protected against fluoride-induced hypertension and hepatorenal toxicity by lowering blood pressure, inhibiting lipid peroxidation, protein carbonylation, and enhancing the enzymatic and non-enzymatic antioxidant pathways66. Further, and more importantly, to minimize the cost of Spirulina platensis, several recent studies have been focused on the culture of this microalgae in natural and cheaper mediums instead of the standard Zarrouk67 which is synthetic and expensive68–71. Accordingly, Spirulina platensis used in this study is cultivated using a natural and cheap medium based on well water and wood ash27. This medium allowed the production of a biomass like the Zarrouk medium. Furthermore, it increased the production of antioxidants such as phycocyanin, carotenoids and water-soluble vitamins mainly ascorbic acid27.
This study indeed provides valuable insights into the potential protective effects of Spirulina platensis against dental fluorosis and oxidative stress in naturally exposed lambs, rejecting the null hypothesis. The importance of extrapolating these findings to human contexts is acknowledged. While animal studies like this one serve as essential preliminary investigations, it is crucial to recognize the limitations and the need for cautious interpretation. Firstly, the small sample size in the study is indeed a valid point. However, it is essential to clarify that this animal study was conducted as a Phase I interventional study. Such studies often start with small sample sizes to assess safety, feasibility, and preliminary efficacy trends before larger, more generalizable trials are undertaken. The decision to employ a small sample size was not arbitrary but rather a deliberate choice made to ensure the ethical use of animals and minimize harm while providing a foundation for future research. Regarding statistical analysis, the study was conducted with a focus on achieving a high statistical power, set at 95%. This choice was based on the acceptable level of type II error (false negatives), ensuring a strong likelihood of detecting true differences if existed. The choice of Fisher’s exact test for analyzing categorical data with small sample sizes was motivated by the suitability of this method for such scenarios. To sum up, while this study does have limitations, including the small sample size and specificity to a particular geographic area, it is essential to view these limitations within the context of the study’s purpose and design. This animal study serves as an initial step in exploring the potential benefits of Spirulina platensis in reducing dental fluorosis and oxidative stress. Further research, including larger-scale studies and human trials, will be necessary to validate and generalize the findings to broader populations.
Conclusion
In conclusion, this interventional clinical trial provides strong evidence supporting the efficacy of Spirulina platensis supplementation in reducing dental fluorosis severity and improving oxidative stress markers in lambs naturally exposed to fluoride. These results directly concern the central questions of our study. While the potential for broader applications of Spirulina platensis in addressing fluorosis in other animal populations and potentially in human contexts is an area of interest, it is important to emphasize that our conclusions are firmly based on the evidence obtained in this specific research study. Further investigation, including Phase I/II trials, is warranted to explore the practical implications of Spirulina platensis in managing fluorosis in diverse and varied environments.
Methods
Microalgae material
Spirulina platensis was purchased from an artisanal farm situated at Chichaoua-Morocco (31° 32′ 38″ north, 8° 45′ 58″ west). It was cultivated in natural climatic conditions and wholly characterized by Rahim et al.27.
Study site
This study was conducted in El Fokra commune, located in the western region of Khouribga, Morocco (32° 53′ 9.683″ N, 6° 55′ 15.116″ W). El Fokra commune is a part of the phosphate zone, characterized by geological conditions that contribute to the prevalence of hydrotelluric fluorosis in the area30. Hydrotelluric fluorosis indicates that the two main sources of contamination in this region are water and soil. The region’s semi-arid climate, marked by hot and dry summers, leads to elevated evaporation rates, potentially impacting fluoride concentrations in local water sources, soil, and vegetation, and influencing the health and behavior of the animal subjects. For comparison, lambs from the fluorosis-free Settat region, located nearby (33° 0′ 0″ N, 7° 37′ 0.12″W), were also included in the study.
Collection of groundwater and soil samples
A total of 20 well water samples were collected, with 20 samples originating from the El Fokra commune and 20 samples from the Settat region. These wells had depths ranging between 60 and 80 m, corresponding to the depth at which water is typically accessed from the groundwater table. Each water sample, approximately 100 mL in volume, was carefully collected in clean and sterile polyethylene bottles, and stored at 4 °C until analyzed.
Soil samples were collected from various locations in both the El Fokra and Settat regions, with 20 samples gathered from each location. Each soil sample was carefully placed in clean and sterile polyethylene bottles and transported to the laboratory. Subsequently, the soil samples were subjected to a drying process in an oven at 105 °C for 24 h and then ground into a fine powder using a mortar and pestle. For the digestion process, 1 g of powdered soil from each sample was placed in a crucible, covered with 5 mL of 8 M NaOH solution, and heated on a hot plate for a minimum of 5 min. The crucibles were then placed in a 200 °C oven for 16 h. After cooling, the crucibles were rehydrated with 15 mL of distilled water, gently shaken until homogeneous, and neutralized to pH 7.2–7.5 using 37% hydrochloric acid. Finally, the solutions were diluted with 25 mL of distilled water, and stored until analyzed72.
Animals and experimental design
All animal care protocols were accomplished in agreement with the Hassan II Institute of Agronomy and Veterinary Medicine of Rabat and the Moroccan Ministry of Agriculture recommendations which are in accordance with international ethical standards (European Union Directive 2010/63/EU) legislation and ARRIVE (Animal Research Reporting of In Vivo Experiments) guidelines.
A total number of forty-eight Sardi lambs aged 5 months were chosen for the present experiment. This work was conducted as a Phase I interventional study, which typically starts with small sample sizes to assess safety, feasibility, and preliminary efficacy trends before larger trials. This choice aimed to ensure ethical use of animals and minimize harm while providing a foundation for future research. The decision to employ a small sample size was deliberate and not arbitrary. It was made to address ethical concerns and align with the study’s initial goals. Practical considerations, including the availability of lambs meeting specific criteria and limitations in available resources (time, personnel, budget), influenced the final sample size choice. To reinforce the reliability of our findings, we utilized an online sample size calculator, setting a desired statistical power of 95% and a significance level of 0.05. This approach ensured a 95% chance of detecting true differences if they existed while maintaining a 5% threshold for the risk of type I errors, aligning with common standards in scientific research.
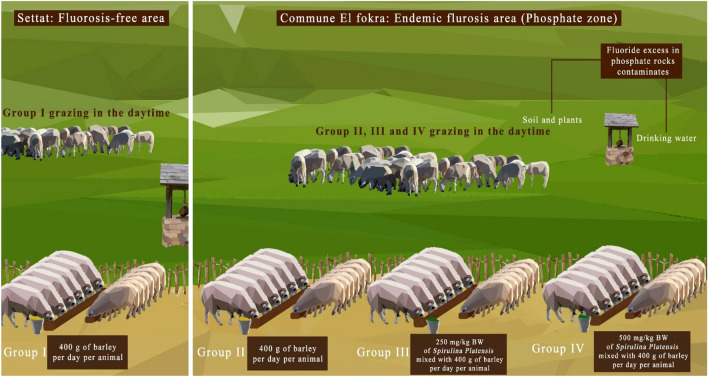
They animals were weighed and then treated against enterotoxaemia and internal and external parasites. The experiment started when the lambs were 5 months old and ended in August 2021 after the eruption of their first two milk teeth and the total appearance of their incisors. Twelve lambs reared at INRA belonging to the Regional Agricultural Research Center station in Settat city, were used as a control of a fluorosis-free area and then used as healthy control (Group I). The Thirty-six animals reared in the endemic fluorosis (El fokra-Khouribga) area were separated into 3 equal groups (II, III and IV). Each group was composed of 6 females and 6 males (Fig. 9).
Group I: healthy control reared in a fluorosis-free area and each animal received every morning 400 g of barley without Spirulina platensis.
Group II: reared in endemic fluorosis area and each animal received every morning 400 g of barley without Spirulina platensis.
Group III: received every morning 250 mg/kg BW of Spirulina platensis mixed with 400 g of barley, for each animal.
Group IV: received every morning 500 mg/kg BW of Spirulina platensis mixed with 400 g of barley, for each animal.
Figure 9.
Diagram presenting the experimental protocol.
All animals were allowed to be taken to pasture and reared extensively. Groups III and IV were weighed every 15 days to readjust the quantity of Spirulina platensis according to BW of the animal.
Dental examination
After the eruption of their two first milk teeth, lambs were clinically examined for lesions on their incisors. Dental lesions were scored according to the Dean’s Fluorosis Index (DFI)73. This index based on six scores attributed to tooth damage from fluorosis23.
Collection of blood samples and feces
The samples of blood were collected every 15 days from the jugular vein in heparinized tubes. Then the samples were centrifuged at 3000 g/10 min at 4 °C, and the supernatant containing plasma was stored at − 20 °C until use. Besides, the pellet containing the erythrocytes was washed by NaCl (9 g/L) three times to eliminate contaminants before being stored at − 20 °C until use.
Concerning feces samples, a thick filter paper was used to catch the feces from each group of animals. Afterward, the obtained feces were dried at 100 °C and grounded with a mortar. Then, the dried and powdered fecal samples underwent digestion using the same method employed for soil samples72, and stored until analyzed.
Determination of fluoride in plasma and feces
To determine the level of plasma fluoride, 1 mL of plasma of each animal was buffered with 1 mL of the commercial total ionic strength adjustment buffer II solution (Orion 940,909; Thermo Fisher Scientific, Waltham, MA, USA) and evaluated using a fluoride electrode (Orion 96-09; Thermo Fisher Scientific, Waltham, MA, USA), coupled to an ion analyzer (Star A214; Thermo Fisher Scientific, Waltham, MA, USA). The electrode was calibrated with standard fluoride solutions (Orion 940,907; Thermo Fisher Scientific, Waltham, MA, USA).
Biochemical parameters
A total number of 1500 blood samples collected during 13 months of the experiment underwent various biochemical analyzes to judge the effect of Spirulina platensis.
Reduced glutathione level
Reduced glutathione (GSH) level in plasma was determined according to Ellman74. Briefly, 200 µL of trichloroacetic acid 5% was mixed with 400 µL of plasma. After centrifugation for 10 min at 12,000 g, 50 µL of supernatant was mixed with 850 µL of phosphate buffer (50 mM, pH 8) and 100 µL of 6 mM 5,5-dithiobis (2-nitrobenzoic acid (DTNB)). The yellow-colored solution was immediately read at 412 nm, using a Shimadzu UV-1601 Spectrophotometer (Shimadzu; Kyoto, Japan). Glutathione reduced was used as a standard.
Malondialdehyde level
Malondialdehyde level was measured according to the standard method75. Briefly, 1.5 mL of TBA (0.8%) was added to 1 mL of the plasma of each animal. Then 0.4 mL of SDS (8.1%) and 1.5 mL of acetic acid were added. The mixture was finally made up to 5 mL with distilled water and placed in hot water bath at 95 °C for 1 H. After cooling 1 mL of distilled water and 5 mL of n-butanol was added. The mixture was vortexed and after centrifugation for 10 min at 4000 g, the absorbance of the organic layer (upper layer) was measured at 532 nm by a Shimadzu UV-1601 Spectrophotometer (Shimadzu; Kyoto, Japan).
Assays of antioxidant enzymes
Catalase activity was determined by the method developed by Ni et al.76. The 200 µL reaction mixture containing 10 µL of the aliquot was mixed with 30 µL of a 7.3 mM H2O2 solution. After 3 min of incubation, the reaction was stopped by adding 20 µL of H2SO4 at 6N and then 140 µL of KmNO4 at 2 mM. The absorbance was read at 480 nm by a Tecan Microplate Reader Infinite 200 Pro (Tecan; Männedorf, Switzerland). The specific activity of CAT was expressed as micromoles per minute per milligram of protein. Superoxide dismutase (SOD) activity was assayed as described by Beyer and Fridovich77. The reaction mixture contained 50 mM phosphate buffer, 0.025% Triton X-100, 0.1 mM EDTA (pH 8), 12 mM L-methionine, 75 mM NBT, aliquot and 2 µM riboflavin. The tubes were shaken and placed 30 cm below a light bank consisting of a 15 W fluorescent lamp for 10 min. The reaction was stopped by switching off the light and the absorbance was read at 560 nm by a Tecan Microplate Reader Infinite 200 Pro (Tecan; Männedorf, Switzerland).
Proteins and hemoglobin levels
Plasma proteins were estimated using the BCA Protein Assay Kit78. Hemoglobin (Hb) concentrations were determined using drabkin’s cyanmethemoglobin method79. All samples were analyzed in triplicates.
Statistical analysis
To determine the appropriate sample size, we utilized the online tool available at ‘https://clincalc.com/stats/samplesize.aspx’. The desired statistical power represents the probability of correctly detecting a true difference if it exists. It was chosen based on the acceptable level of type II error (false negatives) and was set at 95%. The significance level was set at 0.05. To compare the dentition among the four groups, we used the R programming language and specifically, the fisher test function. Fisher’s exact test was chosen due to its suitability for analyzing categorical data when comparing multiple groups with small sample sizes. The null hypothesis assumed no association between the dentition differences and the groups, while the alternative hypothesis suggested a significant association. Additionally, a comparison of means for plasma fluoride, fecal fluoride, and biochemical parameters between groups and sexes was performed using JMP SAS (SAS Institute; Cary, NC, USA). The Wilcoxon test was employed as the statistical method in this analysis.
Acknowledgements
The authors would like to acknowledge the support through the R&D Initiative – Appel à projects APPHOS – sponsored by OCP (OCP Foundation, R&D OCP, Mohammed VI Polytechnic University, National Center of Scientific and Technical Research CNRST, Ministry of Higher Education, Scientific Research and Professional Training of Morocco MESRSFC) under the project SHS-ELM-01/2017.
Author contributions
A.R. Writing - Original Draft, Methodology, Investigation. M.S. Data curation, Resources, Writing- Original draft preparation. A.E. Writing - Review and Editing, Visualization, Supervision. B.E.A. Conceptualization, Visualization, Investigation, Writing - Review and Editing, Supervision.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dinoiu V. Fluorine chemistry: past, present and future. Rev. Roum. Chim. 2006;51:1141–1152. doi: 10.1002/chin.200744226. [DOI] [Google Scholar]
- 2.Perumal E, Paul V, Govindarajan V, Panneerselvam L. A brief review on experimental fluorosis. Toxicol. Lett. 2013;223:236–251. doi: 10.1016/j.toxlet.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Dhar V, Bhatnagar M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009;20:350. doi: 10.4103/0970-9290.57379. [DOI] [PubMed] [Google Scholar]
- 4.Peckham S, Awofeso N. Water fluoridation: A critical review of the physiological effects of ingested fluoride as a public health intervention. Sci. World J. 2014;2014:293019. doi: 10.1155/2014/293019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson BM, Shu Y-Z, Zhuo X, Meanwell NA. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 2020;63:6315–6386. doi: 10.1021/acs.jmedchem.9b01877. [DOI] [PubMed] [Google Scholar]
- 6.Samal P, et al. Effect of Tamarindus indica leaf powder on plasma concentrations of copper, zinc, and iron in fluorotic cows. Vet. World. 2016;9:1121–1124. doi: 10.14202/vetworld.2016.1121-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dede S, Taşpinar M, Yüksek V, Çetin S, Usta A. The effects of vitamin D application on NaF-induced cytotoxicity in osteoblast cells (hFOB 1.19) Biol. Trace. Elem. Res. 2023;201(2):698–705. doi: 10.1007/s12011-022-03177-8. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava S, Flora S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health. Rep. 2020;7:140–146. doi: 10.1007/s40572-020-00270-9. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary V, Kumar M, Sharma M, Yadav BS. Fluoride, boron and nitrate toxicity in ground water of northwest Rajasthan. India. Environ. Monit. Assess. 2010;161:343–348. doi: 10.1007/s10661-009-0750-y. [DOI] [PubMed] [Google Scholar]
- 10.Choubisa SL. Fluoridated ground water and its toxic effects on domesticated animals residing in rural tribal areas of Rajasthan. India. Int J. Environ. Stud. 2007;64:151–159. doi: 10.1080/00207230601173713. [DOI] [Google Scholar]
- 11.Choubisa SL. Some observations on endemic fluorosis in domestic animals in Southern Rajasthan (India) Vet. Res. Commun. 1999;23:457–465. doi: 10.1023/A:1006325710222. [DOI] [PubMed] [Google Scholar]
- 12.Ayoob S, Gupta AK. Fluoride in drinking water: A review on the status and stress effects. Crit. Rev. Environ. Sci. Technol. 2006;36:433–487. doi: 10.4236/oalib.1100546. [DOI] [Google Scholar]
- 13.Rahim A, Aydogmus-Öztürk F, Cakir C, Essamadi A, El Amiri B. Mitigating fluoride, lead, arsenic and cadmium toxicities in laboratory animals and ruminants through natural products. Rec. Agric. Food. Chem. 2022;2(2):1–17. doi: 10.25135/rfac.6.2202.2365. [DOI] [Google Scholar]
- 14.Rahim A, Essamadi A, El Amiri B. Endemic fluorosis in ruminants and its socioeconomic impact in Morocco. Afri. Med. Agri. J. 2023;138:77–95. [Google Scholar]
- 15.Abdel-Rahman GH, El-Hallawany HA, Dohreig RA. Effect of excess fluoride on reproductive potentials in farm animals (ovine) Alex. J. Vet. Sci. 2018;57:41–57. doi: 10.5455/ajvs.296364. [DOI] [Google Scholar]
- 16.Kessabi M, Hamliri A, Braun JP, Rico AG. Experimental acute sodium fluoride poisoning in sheep: Renal, hepatic, and metabolic effects. Fundam. Appl. Toxicol. 1985;5:1025–1033. doi: 10.1016/0272-0590(85)90139-3. [DOI] [PubMed] [Google Scholar]
- 17.El Amiri B, Rahim A, Sibaoueih M. Perception et pratiques d’élevage pour atténuer la fluorose chez les ovins dans trois communes de la province de Khouribga-Maroc. Afri. Med. Agri. J. 2021;133:1–17. [Google Scholar]
- 18.Khairnar MR, Dodamani AS, Jadhav HC, Naik RG, Deshmukh MA. Mitigation of fluorosis - A review. J Clin. Diagn. Res. 2015;9:ZE05–ZE09. doi: 10.7860/JCDR/2015/13261.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey S, et al. Ameliorative effect of Tamarindus indica L. on biochemical parameters of serum and urine in cattle from fluoride endemic area. Vet. Arh. 2013;83:487–496. [Google Scholar]
- 20.Kashyap SJ, Sankannavar R, Madhu GM. Fluoride sources, toxicity and fluorosis management techniques–A brief review. J. H. M. Letters. 2021;2:100033. doi: 10.1016/j.hazl.2021.100033. [DOI] [Google Scholar]
- 21.Kessabi M, Hamliri A, Braun JP. Experimental fluorosis in sheep: Alleviating effects of aluminum. Vet. Hum. Toxicol. 1986;28:300–304. [PubMed] [Google Scholar]
- 22.Said AN, Slagsvold P, Bergh H, Laksesvela B. High fluorine water to wether sheep maintained in pens. Aluminum chloride as a possible alleviator of fluorosis. Nord. Vet. Med. 1977;29:172–180. [PubMed] [Google Scholar]
- 23.Rahim A, Essamadi A, El Amiri B. A comprehensive review on endemic and experimental fluorosis in sheep: Its diverse effects and prevention. Toxicology. 2022;465:153025. doi: 10.1016/j.tox.2021.153025. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Voet GB, Schijns O, de Wolff FA. Fluoride enhances the effect of aluminium chloride on interconnections between aggregates of hippocampal neurons. Arch. Physiol. Biochem. 1999;107:15–21. doi: 10.1076/apab.107.1.15.4356. [DOI] [PubMed] [Google Scholar]
- 25.Perumal E, et al. Therapeutic efficacy of Tamarindus indica (L) to protect against fluoride-induced oxidative stress in the liver of female rats. Fluoride. 2010;43:134. [Google Scholar]
- 26.Gupta AR, Dey S, Saini M, Swarup D. Toxic effect of sodium fluoride on hydroxyproline level and expression of collagen-1 gene in rat bone and its amelioration by Tamrindus indica L. fruit pulp extract. Interdiscip. Toxicol. 2016;9:12–16. doi: 10.1515/intox-2016-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahim A, et al. Chemical characterization and nutritional value of Spirulina platensis cultivated in natural conditions of Chichaoua region (Morocco) S. Afr. J. Bot. 2021;141:235–242. doi: 10.1016/j.sajb.2021.05.006. [DOI] [Google Scholar]
- 28.Kessabi M, Assimi B, Braun JP. The effects of fluoride on animals and plants in the South Safi zone. Sci. Total Environ. 1984;38:63–68. doi: 10.1016/0048-9697(84)90208-0. [DOI] [PubMed] [Google Scholar]
- 29.Abdennebi EH, Fandi R, Lamnaouer D. Human fluorosis in Morocco: Analytical and clinical investigations. Vet Hum Toxicol. 1995;37:465–468. [PubMed] [Google Scholar]
- 30.Sibaoueih M, Rahim A, El Amiri B. Les teneurs en fluorure des plantes influencent-elles leur qualité fourragère pour ruminant? Afric. Med. Agri J. 2022;136:81–96. [Google Scholar]
- 31.Ullah R, Zafar MS, Shahani N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic. Med. Sci. 2017;20(8):841–848. doi: 10.22038/IJBMS.2017.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osuji OO, et al. Risk factors for dental fluorosis in a fluoridated community. J. Dent. Res. 1988;67(12):1488–1492. doi: 10.1177/00220345880670120901. [DOI] [PubMed] [Google Scholar]
- 33.Tabchoury CM, Holt T, Pearson SK, Bowen WH. The effects of fluoride concentration and the level of cariogenic challenge on caries development in desalivated rats. Arch. Oral Biol. 1998;43(12):917–924. doi: 10.1016/S0003-9969(98)00093-4. [DOI] [PubMed] [Google Scholar]
- 34.Rahim A, El Amiri B. Effects of heat stress and chemical pollutants on sheep reproduction and strategies to mitigate them. In: Mabrouki J, Mourade A, Irshad A, Chaudhry SA, editors. Advanced Technology for Smart Environment and Energy. Springer International Publishing; 2023. pp. 173–185. [Google Scholar]
- 35.Choubisa SL. Natural amelioration of fluoride toxicity (fluorosis) in goats and sheep. Curr. Sci. 2010;99:1331–1332. [Google Scholar]
- 36.Choubisa S, et al. Food, fluoride, and fluorosis in domestic ruminants in the Dungarpur district of Rajasthan. India. Res. Rep. 2011;44(2):70–76. [Google Scholar]
- 37.Cenesiz S, Ozcan A, Kaya N, Baysu N, Karabulut A. Chronic effects of fluoride in TUJ sheep on serum levels of total protein, albumin, uric acid, and nitric oxide and activities of lactate dehydrogenase and leucine aminopeptidase. Fluoride. 2005;38:52–56. [Google Scholar]
- 38.Mert H, Comba B, Mert N. Advanced oxidation protein products (AOPP) levels and kidney function in fluorotic sheep. Fluoride. 2016;49(3):366–342. [Google Scholar]
- 39.Yur F, Belge F, Mert N, Yörük I. Changes in erythrocyte parameters of fluorotic sheep. Fluoride. 2003;36(3):152–156. [Google Scholar]
- 40.DenBesten P, Li W. Chronic fluoride toxicity: Dental fluorosis. Monogr. Oral. Sci. 2011;22:81–96. doi: 10.1159/000327028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Hinai M, Al Kalbani A, Al Rubkhi A, Al Kalbani U, Walke S. Protein extraction from Spirulina Platensis. Int. J. Eng. Innov. Technol. 2019;8(12):1524–1530. [Google Scholar]
- 42.Ramírez-Rodrigues MM, Estrada-Beristain C, Metri-Ojeda J, Pérez-Alva A, Baigts-Allende DK. Spirulina platensis protein as sustainable ingredient for nutritional food products development. Sustainability (Switzerland) 2021;13(12):6849. doi: 10.3390/su13126849. [DOI] [Google Scholar]
- 43.Liang Z. Ameliorative effect of protein and calcium on fluoride-induced hepatotoxicity in rabbits. Afr. J. Biotechnol. 2012;11:13801–13808. doi: 10.5897/AJB12.1761. [DOI] [Google Scholar]
- 44.Lohakare J, Pattanaik AK. Effects of addition of fluorine in diets differing in protein content on urinary fluoride excretion, clinical chemistry and thyroid hormones in calves. R. Bras. Zootec. 2013;42:751–758. doi: 10.1590/S1516-35982013001000009. [DOI] [Google Scholar]
- 45.Yasar S, Yur F. Antioxidant vitamin and mineral levels in sheep with fluorosis. Biol. Trace. Elem. Res. 2008;123:139–143. doi: 10.1007/s12011-008-8089-8. [DOI] [PubMed] [Google Scholar]
- 46.Yüksek V, Çetin S, Usta A, Kömüroğlu A, Dede S. Effect of some vitamins on antioxidant/prooxidant parameters in sodium fluoride (NaF)-treated cell line (hFOB 1.19) Turkish J. Vet. Res. 2017;1:1–6. [Google Scholar]
- 47.Çetin S, Yur F, Taspinar M, Yüksek V. The effects of some minerals on apoptosis and DNA damage in sodium fluoride-administered renal and osteoblast cell lines. Fluoride. 2019;52:362–378. [Google Scholar]
- 48.Basha PM, Sujitha NS. Combined influence of intermittent exercise and temperature stress on the modulation of fluoride toxicity. Biol. Trace Elem. Res. 2012;148:69–75. doi: 10.1007/s12011-012-9338-4. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguti PM, et al. Effects of single exposure of sodium fluoride on lipid peroxidation and antioxidant enzymes in salivary glands of rats. Oxid. Med. Cell. Longev. 2013;2013:e674593. doi: 10.1155/2013/674593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nabavi SM, et al. Protective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat’s kidney. Mol. Cell. Biochem. 2013;372:233–239. doi: 10.1007/s11010-012-1464-y. [DOI] [PubMed] [Google Scholar]
- 51.Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012;132:931–935. doi: 10.1039/c2fo10264a. [DOI] [Google Scholar]
- 52.Çetin S, Usta A, Yüksek V. The effect of lycopene on dna damage and repair in fluoride-treated NRK-52E cell line. Biol. Trace. Elem. Res. 2021;199:1979–1985. doi: 10.1007/s12011-020-02288-4. [DOI] [PubMed] [Google Scholar]
- 53.Banji D, Banji O, Pratusha N, Annamalai AR. Investigation on the role of Spirulina platensis in ameliorating behavioural changes, thyroid dysfunction and oxidative stress in offspring of pregnant rats exposed to fluoride. Food Chem. 2013;140:321–331. doi: 10.1016/j.foodchem.2013.02.076. [DOI] [PubMed] [Google Scholar]
- 54.Soni B, Trivedi U, Madamwar D. A novel method of single step hydrophobic interaction chromatography for the purification of phycocyanin from Phormidium fragile and its characterization for antioxidant property. Bioresour. Technol. 2008;99:188–194. doi: 10.1016/j.biortech.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Wu H-L, Wang G-H, Xiang W-Z, Li T, He H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016;19:2349–2362. doi: 10.1080/10942912.2015.1038564. [DOI] [Google Scholar]
- 56.Ghaeni M, Roomiani L. Review for application and medicine effects of spirulina, Spirulina platensis microalgae. J. Adv. Agric. Technol. 2016;3:114–117. doi: 10.18178/joaat.3.2.114-117. [DOI] [Google Scholar]
- 57.Ourida A, Amiali M, Bouzid N, Belkacemi K, Bitam A. Effect of Spirulina platensis ingestion on the abnormal biochemical and oxidative stress parameters in the pancreas and liver of alloxan-induced diabetic rats. Pharm. Biol. 2017;55:1304–1312. doi: 10.1080/13880209.2017.1300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upasani CD, Balaraman R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous. Phytother. Res. 2003;17:330–334. doi: 10.1002/ptr.1135. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, et al. Association of dietary carotenoids intake with skeletal fluorosis in the coal-burning fluorosis area of Guizhou province. Biomed. Environ. Sci. 2018;31:438–447. doi: 10.3967/bes2018.057. [DOI] [PubMed] [Google Scholar]
- 60.Bai X, et al. Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. J. Biol. Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 61.Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Stahl W, Sies H. Lycopene: A biologically important carotenoid for humans? Arch. Biochem. Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 63.Colla LM, Oliveira Reinehr C, Reichert C, Costa JAV. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007;98:1489–1493. doi: 10.1016/j.biortech.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 64.Taiana D, Luciana P, Michele M, Badiale-Furlong E. Profile, antioxidant potential, and applicability of phenolic compounds extracted from Spirulina platensis. Afr. J. Biotechnol. 2015;14:2903–2909. doi: 10.5897/AJB2015.14926. [DOI] [Google Scholar]
- 65.Kanagaraj VV, Panneerselvam L, Govindarajan V, Ameeramja J, Perumal E. Caffeic acid, a phyto polyphenol mitigates fluoride induced hepatotoxicity in rats: A possible mechanism. BioFactors. 2015;41:90–100. doi: 10.1002/biof.1203. [DOI] [PubMed] [Google Scholar]
- 66.Ola-Davies OE. Ameliorative effect of gallic acid against sodium fluoride-induced hypertension and Hepato-renal complications in Wistar Rats. Afr. J. Biomed. Res. 2018;21:285–294. [Google Scholar]
- 67.Li Z, et al. Effects of culture conditions on the performance of Arthrospira platensis and its production of exopolysaccharides. Foods. 2020;11(14):2022. doi: 10.3390/foods11142020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madkour FF, Kamil AE-W, Nasr HS. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012;38:51–57. doi: 10.1016/j.ejar.2012.09.003. [DOI] [Google Scholar]
- 69.Bualuang A, Boonburapong B, Laloknam S. Optimization of Arthrospira platensis growth using organic culture medium. Asia Pac. J. Sci. Technol. 2022;27:1–9. [Google Scholar]
- 70.Michael A, Kyewalyanga MS, Lugomela CV. Biomass and nutritive value of Spirulina (Arthrospira fusiformis) cultivated in a cost-effective medium. Ann. Microbiol. 2019;69:1387–1395. doi: 10.1007/s13213-019-01520-4. [DOI] [Google Scholar]
- 71.Trinh DV, Nguyen PTH. Minimising the cost of Spirulina platensis culture medium using vinh hao natural mineral water. Chem. Eng. Trans. 2020;78:19–24. doi: 10.3303/CET2078004. [DOI] [Google Scholar]
- 72.Habiyakare T, et al. Dental fluorosis among people and livestock living on Gihaya Island in Lake Kivu Rwanda. One Health Outlook. 2021;3:23. doi: 10.1186/s42522-021-00054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dean HT. Classification of mottled enamel diagnosis. J. Am. Dent. Assoc. 1934;21:1421–1426. [Google Scholar]
- 74.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 75.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 76.Ni J, Sasaki Y, Tokuyama S, Sogabe A, Tahara Y. Conversion of a typical catalase from Bacillus sp. TE124 to a catalase-peroxidase by directed evolution. J. Biosci. Bioeng. 2002;93:31–36. doi: 10.1016/S1389-1723(02)80050-0. [DOI] [PubMed] [Google Scholar]
- 77.Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 78.He, F. BCA (bicinchoninic acid) protein assay. Bio-protocol. e44. 10.21769/BioProtoc.44. (2011)
- 79.Balasubramaniam P, Malathi A. Comparative study of hemoglobin estimated by Drabkin’s and Sahli’s methods. J. Postgrad. Med. 1992;38:8–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.