Abstract
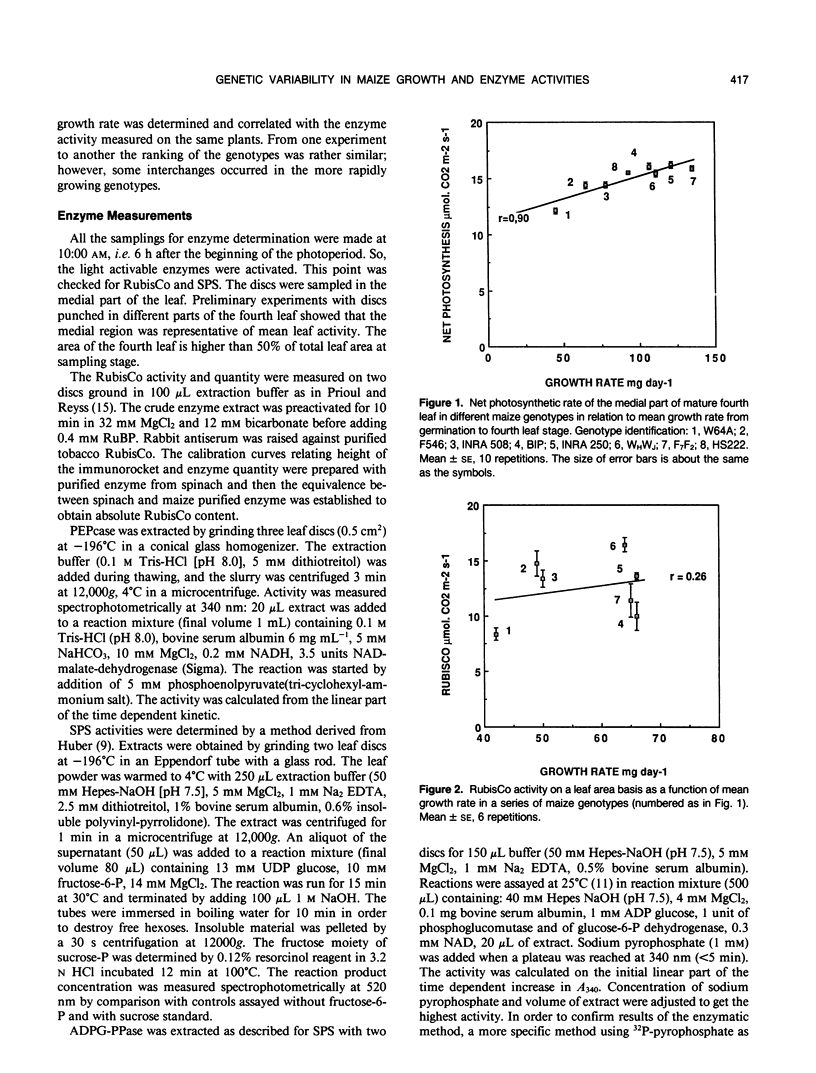
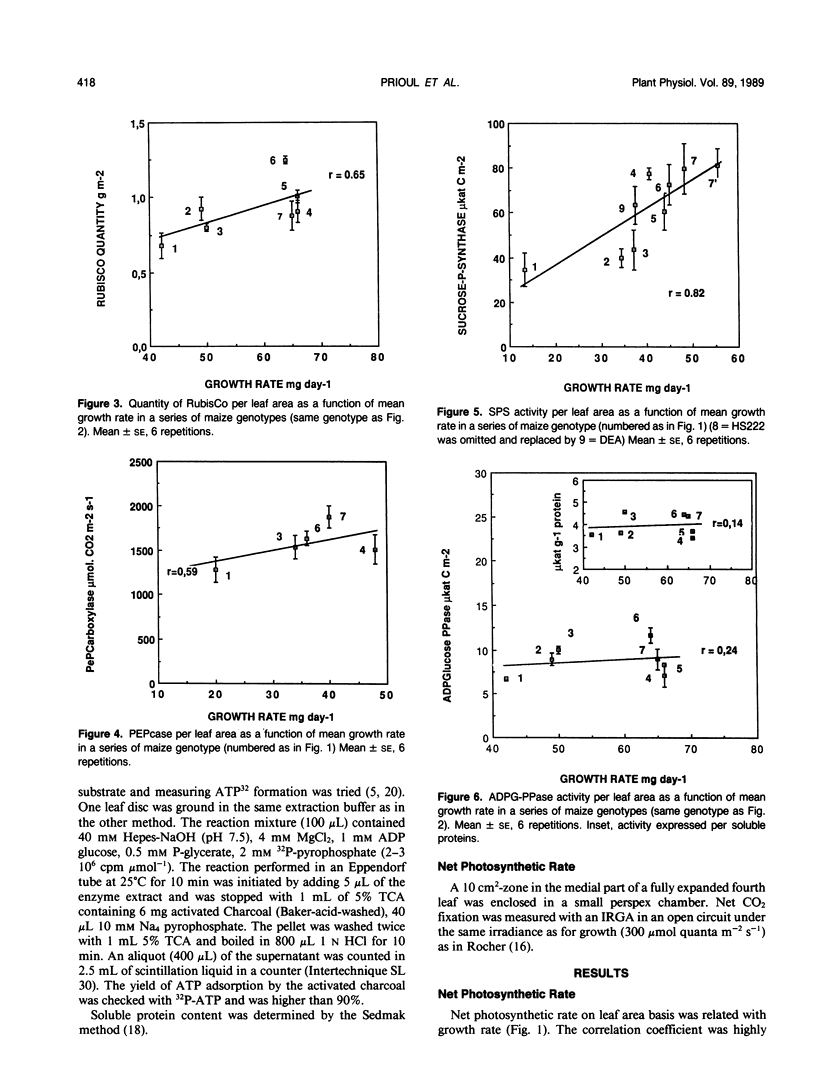
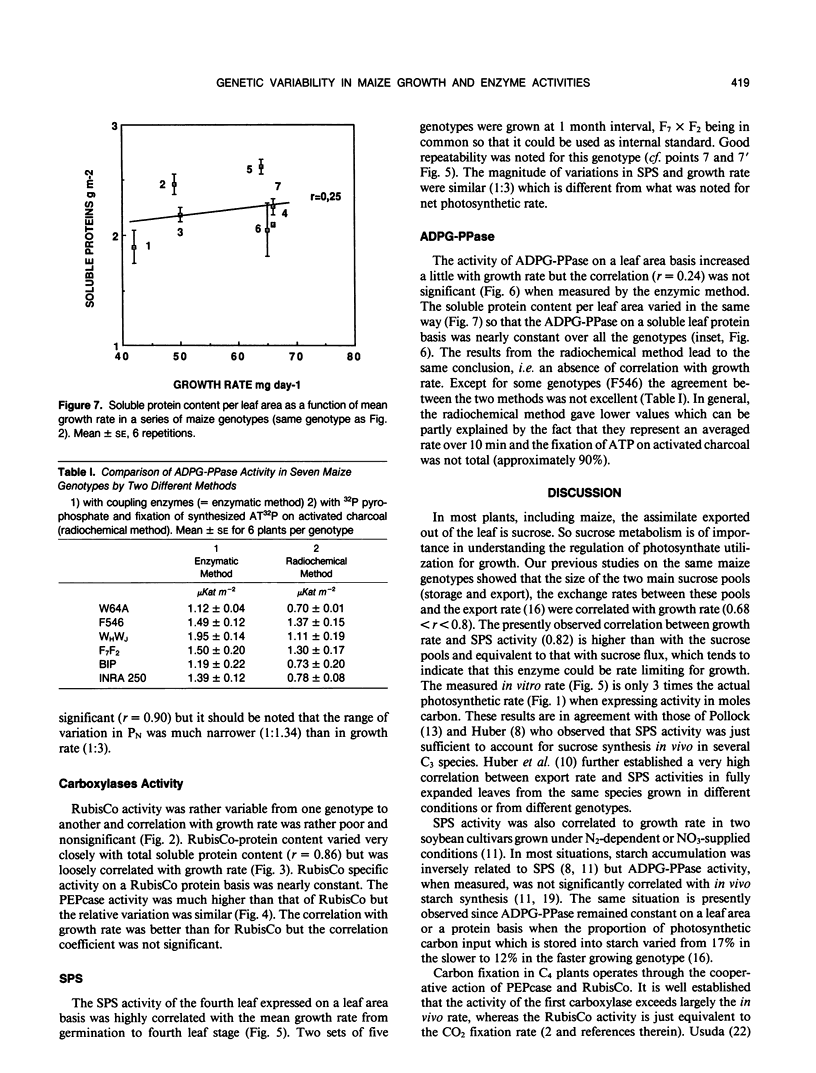
The net photosynthetic rate and the activities of ribulose 1,5 bisphosphate carboxylase (RubisCo), phosphoenolpyruvate carboxylase, sucrose-P-synthase, and ADP glucose-pyrophosphorylase, key enzymes of the leaf carbohydrate metabolism were compared in eight maize (Zea mays L.) genotypes presenting large differences in growth rate. The sucrose-P-synthase activity varied in the ratio 1 to 3 from the less active to the more active genotype and this variation was highly correlated with those in growth rate. ADP glucose pyrophosphorylase activity was not significantly different from one genotype to another whatever the basis for expression, leaf area, or soluble protein. The photosynthetic rate varied with similar amplitude (1:1) to the RubisCo activity or RubisCo quantity but the correlation with growth rate was highly significant for photosynthesis and nonsignificant for RubisCo or phosphoenolpyruvate carboxylase. So, in our series of genotypes the sucrose synthesis capacities as expressed by sucrose phosphate synthase activity seem to have a good predicting value for mean growth rate at a young stage.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crafts-Brandner S. J., Poneleit C. G. Carbon dioxide exchange rates, ribulose bisphosphate carboxylase/oxygenase and phosphoenolpyruvate carboxylase activities, and kernel growth characteristics of maize. Plant Physiol. 1987 Jun;84(2):255–260. doi: 10.1104/pp.84.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Huber S. C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983 Apr;71(4):818–821. doi: 10.1104/pp.71.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Rufty T. W., Kerr P. S. Effect of Photoperiod on Photosynthate Partitioning and Diurnal Rhythms in Sucrose Phosphate Synthase Activity in Leaves of Soybean (Glycine max L. [Merr.]) and Tobacco (Nicotiana tabacum L.). Plant Physiol. 1984 Aug;75(4):1080–1084. doi: 10.1104/pp.75.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Huber S. C., Israel D. W. Effect of N-source on soybean leaf sucrose phosphate synthase, starch formation, and whole plant growth. Plant Physiol. 1984 Jun;75(2):483–488. doi: 10.1104/pp.75.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Prioul J. L., Reyss A. Acclimation of Ribulose Bisphosphate Carboxylase and mRNAs to Changing Irradiance in Adult Tobacco Leaves: Differential Expression in LSU And SSU mRNA. Plant Physiol. 1987 Aug;84(4):1238–1243. doi: 10.1104/pp.84.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Silvius J. E., Chatterton N. J., Kremer D. F. Photosynthate partitioning in soybean leaves at two irradiance levels: comparative responses of acclimated and unacclimated leaves. Plant Physiol. 1979 Nov;64(5):872–875. doi: 10.1104/pp.64.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilatro S. R., Preiss J. Regulation of Starch Synthesis in the Bundle Sheath and Mesophyll of Zea mays L. : Intercellular Compartmentalization of Enzymes of Starch Metabolism and the Properties of the ADPglucose Pyrophosphorylases. Plant Physiol. 1987 Mar;83(3):621–627. doi: 10.1104/pp.83.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Mizuno M., Hayashi M. Partitioning of Nitrogen among Ribulose-1,5-bisphosphate Carboxylase/Oxygenase, Phosphoenolpyruvate Carboxylase, and Pyruvate Orthophosphate Dikinase as Related to Biomass Productivity in Maize Seedlings. Plant Physiol. 1984 Jul;75(3):665–669. doi: 10.1104/pp.75.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]


