Abstract
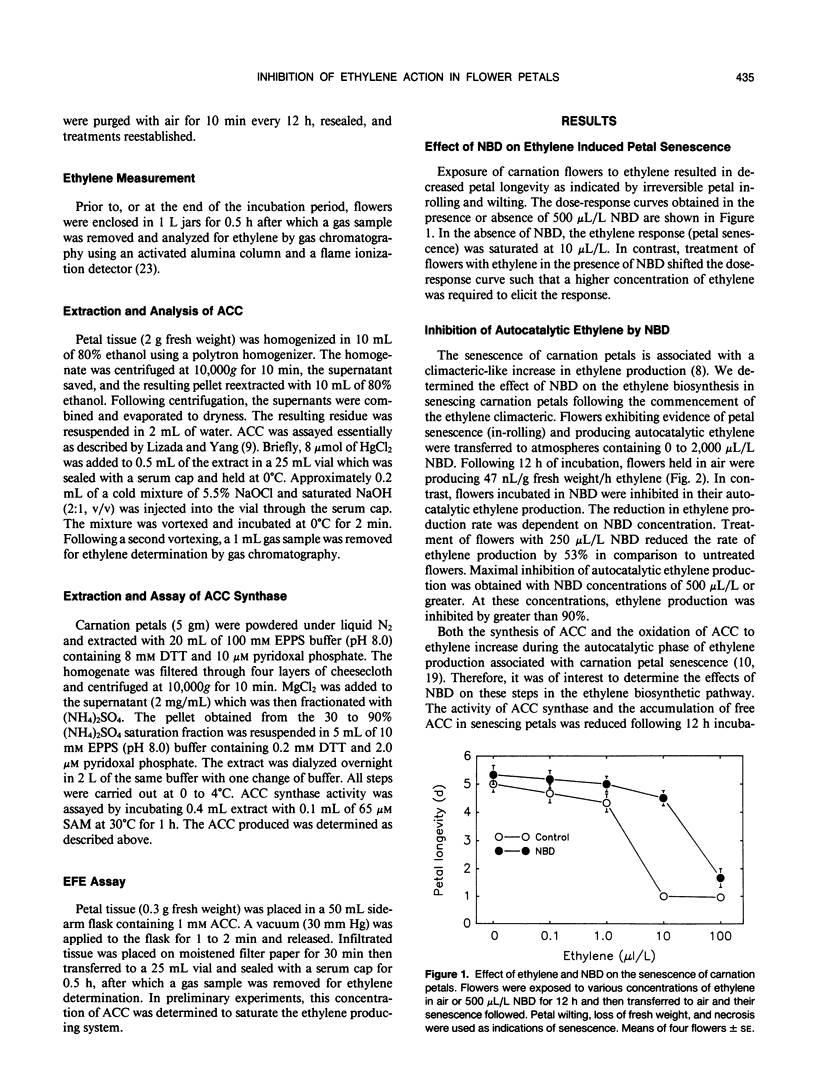
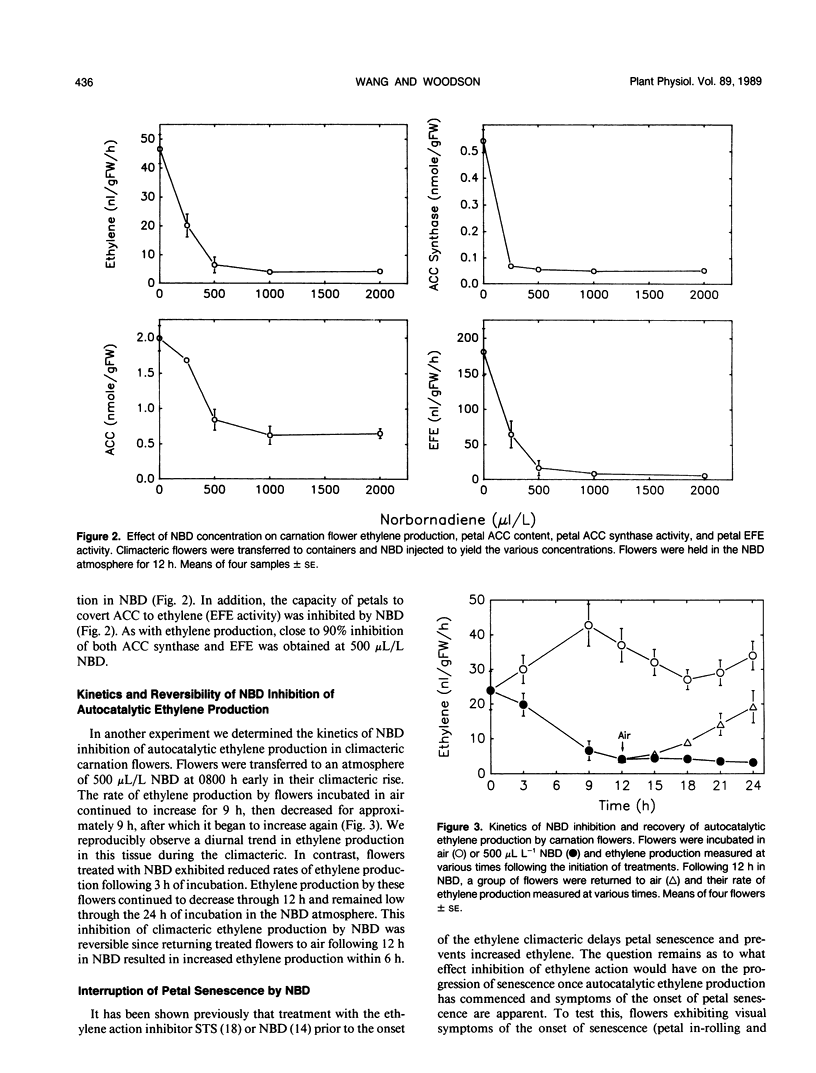
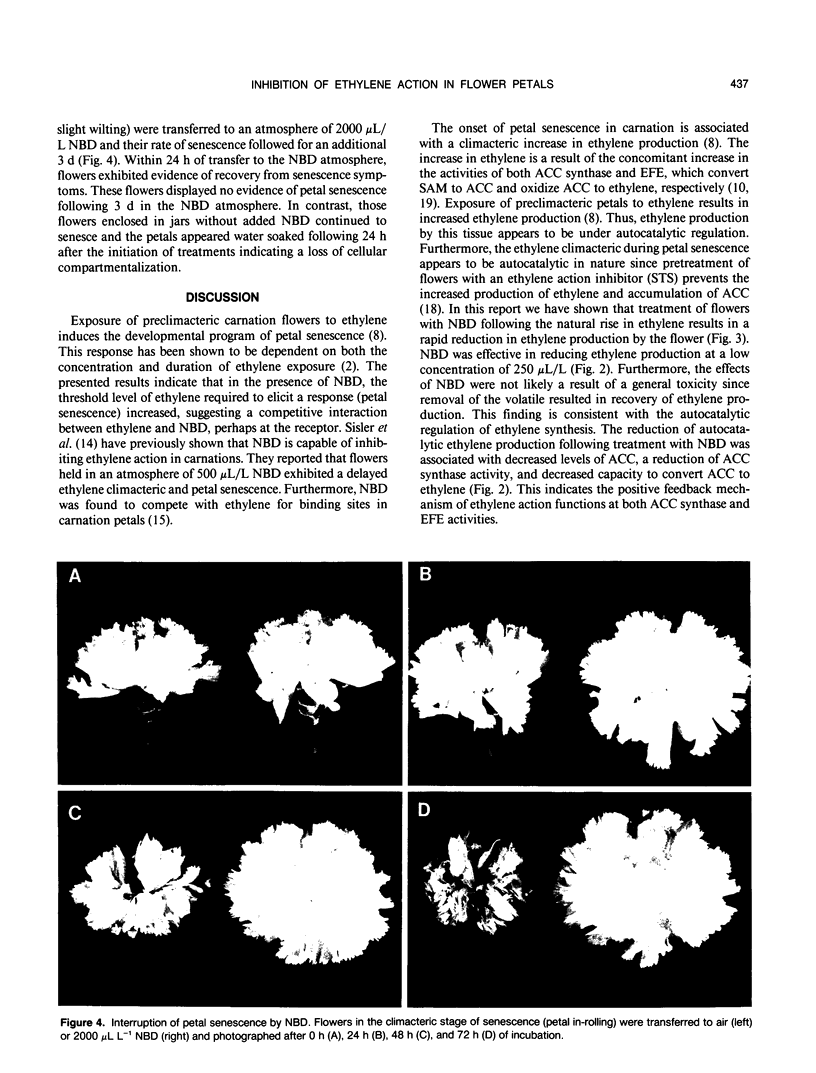
The inhibitory effects of the cyclic olefin 2,5-norbornadiene (NBD) on ethylene action were tested in carnation (Dianthus caryophyllus L. cv White Sim) flowers. Treatment of flowers at anthesis with ethylene in the presence of 500 microliters per liter NBD increased the concentration of ethylene required to elicit a response (petal senescence), indicating that NBD behaves as a competitive inhibitor of ethylene action. Transfer of flowers producing autocatalytic ethylene and exhibiting evidence of senescence (petal in-rolling) to an atmosphere of NBD resulted in a rapid reduction in ethylene production, petal 1-aminocyclopropane-1-carboxylic acid synthase activity, 1-aminocyclopropane-1-carboxylic acid content, and ethylene forming enzyme activity. Removal of NBD resulted in recovery of ethylene biosynthesis. These results support the autocatalytic regulation of ethylene production during the climacteric stage of petal senescence and suggest that continued perception of ethylene is required for maintenance of ethylene biosynthesis. The inhibition of ethylene action by NBD after the flowers had reached the climacteric peak was associated with interruption of petal senescence as evidenced by reversal of senescence symptoms. This result is in contrast to the widely held belief that the rate of petal senescence is fixed and irreversible once petals enter into the ethylene climacteric.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleecker A. B., Rose-John S., Kende H. An evaluation of 2,5-norbornadiene as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiol. 1987 Jun;84(2):395–398. doi: 10.1104/pp.84.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Riov J., Yang S. F. Effects of exogenous ethylene on ethylene production in citrus leaf tissue. Plant Physiol. 1982 Jul;70(1):136–141. doi: 10.1104/pp.70.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson W. R., Hanchey S. H., Chisholm D. N. Role of ethylene in the senescence of isolated hibiscus petals. Plant Physiol. 1985 Nov;79(3):679–683. doi: 10.1104/pp.79.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson W. R., Lawton K. A. Ethylene-induced gene expression in carnation petals : relationship to autocatalytic ethylene production and senescence. Plant Physiol. 1988 Jun;87(2):498–503. doi: 10.1104/pp.87.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]