Abstract
Background
Trihalomethanes (THMs) are the most dominant fraction of all the byproducts formed during chlorination of water. Disinfection by product (DBP) formation in water is a function of numerous factors, including pH, temperature, residual chlorine, source water characteristics, and organic matter. No study has determined the THM level in the drinking water supply of Addis Ababa, Ethiopia.
Methods
A cross-sectional design was conducted to collect water samples in the water supply distribution networks of Addis Ababa, Ethiopia. Twenty-one (21) sampling stations yielded a total of one hundred twenty (120) samples of drinking water. The sample handling and collection procedures were carried out in accordance with USEPA guidelines. A DB-5 capillary column was used to separate the THMs, which were detected using GC-ECD (gas chromatography-electron capture detector). Spectrophotometric and in situ methods were used for physicochemical parameters. Redundancy analysis (RDA) was used for data analysis of trihalomethanes and environmental variables using CANOCO 4.5.
Results
The mean concentration of total trihalomethanes in drinking water in Addis Ababa was 76.3 μg/L. The concentration of chloroform in the drinking water supply in Addis Ababa, Ethiopia, ranged between 4.03 and 79.4 μg/L. The mean total THMs in the Legedadi and Gefersa water supply systems were 77.4 μg/L and 69.66 μg/L, respectively. The residual chlorine, phosphates, UV absorbance at 254 nm, and combined chlorine had positive correlations with THM formation. However, electron conductivity had a negative correlation with THM formation.
Conclusions
Chloroform contributed the most to TTHMs in nearly all samples. The residual chlorine, UV absorbance, phosphate and hardness as calcium, and electron conductivity were found to be the main predictors determining the abundance and distribution of trihalomethanes. The monitoring and regulation of the THMs is required on a regular basis to analyse trends and guide the water treatment and distribution system.
Keywords: Trihalomethanes, Chlorination, Electron capture detector, Drinking water, Gas chromatography
1. Background
Chlorination is one of the most commonly used methods to disinfect drinking water and to control biofouling in water treatment utilities. The water treatment plants in Addis Ababa are designed as conventional water treatment facilities, employing six key processes: aeration, screening, coagulation, sedimentation, filtering, and chlorine disinfection [1]. Among the many chemical approaches for disinfection, chlorination is the most familiar method worldwide. This could be due to its [1] demonstrated effectiveness against a wide variety of bacteria [2], ideal oxidizing potential [3], accessibility at a relatively cheaper cost, and [4] capacity to provide residual chlorine throughout the water supply network, unlike UV disinfection [2,3]. However, numerous disinfection by-products (DBPs) are produced due to the interaction between chlorine and natural organic substances [4]. To date, more than a thousand halogenated DBPs have been reported [5]. The most well-known organic DBPs include trihalomethanes (THMs), haloacetonitriles, haloacetic acids, emerging organic DBPs and halo ketones (HKNs) [[6], [7], [8]]. The harmful inorganic DBPs, also recognized as oxy halide DBPs, include bromates (BrO3−), chlorite (ClO2−), and chlorate (ClO3−) [[9], [10], [11]]. Organic DBPs have attracted the attention of researchers due to their regular discovery and harmful effects [12]. Due to the presence of chlorination by products in the treated water, prolonged exposure to chlorinated water raises the risk of cancer, mutation, kidney and liver damage, retarded fetal growth, congenital disabilities, and possibly miscarriage [3,[13], [14], [15], [16], [17], [18], [19], [20]].
Disinfection by products, such as THMs and HAAs (haloacetic acids), are produced when chlorine undergoes a substitution reaction with organic matter, such as fulvic acids, humic acids, proteins, and amino acids [21,22]. DBP formation in water is a function of numerous factors, including pH, temperature, residual chlorine, source water characteristics, and organic matter [11] [3,11,23]. THMs are indicated as the most dominant fractions of all the by-products formed in the chlorination procedure. THMs are a class of DBPs that include chloroform (CHCl3), bromodichloromethane (CHCl2Br), bromoform (CHBr3) and chlorodibromomethane (CHClBr2) [24].
THMs were regulated shortly after their discovery in disinfected drinking water, with total trihalomethanes (TTHMs) having a maximum contaminant limit (MCL) of 100 μg/L [25]. The MCL for TTHMs was lowered to 80 μg/L by D-DBP rule stage 1, while the MCLs for haloacetic acids (HAAs), bromate, and chlorite were set at 60, 10, and 1000 μg/L, respectively [26].
The water treatment facilities in Ethiopia utilize chlorine to disinfect the water before it is distributed to the general public. However, DBPs in water supplies have not been measured by Ethiopian water utilities, particularly in Addis Ababa. Ethiopia's drinking water supply networks also do not have a system in place to monitor and regulate DBPs. Furthermore, the water treatment facilities in Ethiopia did not set the maximum allowed concentrations (MAC) for total THMs and additional DBPs. Therefore, this study aimed to explore the concentrations of THMs along with various water quality factors in the networks that provide drinking water in Addis Ababa, Ethiopia.
2. Methods
2.1. Study setting and period
Addis Ababa is the capital city of Ethiopia. Geographically, the city is located 9°01′29″ to the north and 38°44′48″ to the east. Addis Ababa is the largest city in Ethiopia, with a projected population of 3,434,000 in 2017 [27]. The city government of Addis Ababa obtains its water sources from both groundwater and surface water sources. There are three main dams used to collect run-off water to store in the dams, which serve as surface water sources for water supply. The Legedadi dam is located 18 km to the west of Addis Ababa, as are the Gefersa dam, 25 km to the east; the Dire dam, 10 km to the north of the Legedadi dam; and the Akaki groundwater (Akaki well field)) [28].
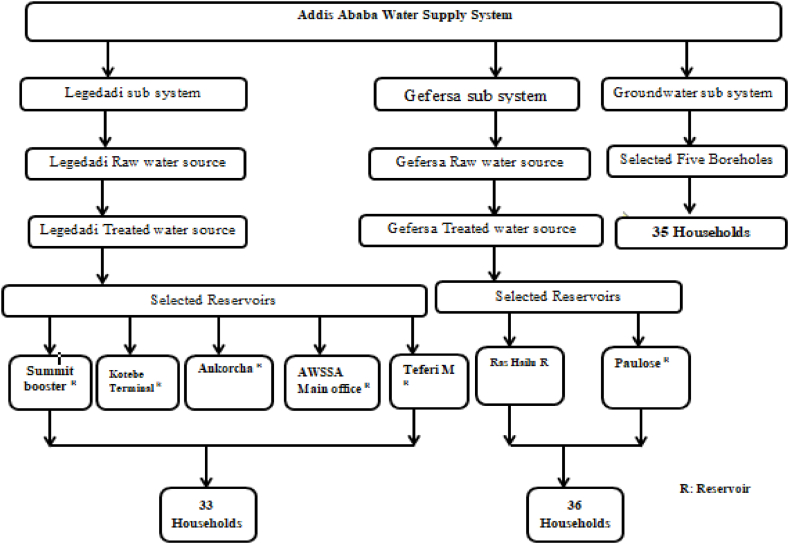
A total of seven surface water reservoirs, two surface water sources and seventy households (36 HHs and 34 HHs from the Gefersa and Legedadi water supplies, respectively) were selected for surface water sampling. For groundwater sampling, five boreholes and thirty-five households were chosen (Fig. 1, Fig. 2). Since the water sources are used just once a year and are distributed through a closed system, the samples taken at any time are believed to be consistent throughout the year. The closed system here implies no continuous filling of the dam. Here, the assumption is that the source water enters the dam once per year.. Samples were collected from June 1 to July 30, 2022.
Fig. 1.
Sampling points of the water samples in Addis Ababa, Ethiopia, 2022.
Fig. 2.
Selected sampling points for water collection, Addis Ababa, 2022.
2.2. Study design
A cross-sectional study design was conducted in the water supply distribution networks of Addis Ababa.
2.3. Sample size determination
In Addis Ababa, Ethiopia, distribution networks were used to gather 120 samples of drinking water from 21 sampling stations. Legedadi, Gefersa, and underground water sources were the three sampling locations (Fig. 1). Each sampling location yielded forty water samples. The samples were taken from untreated, conventionally treated waters and from different distribution network points of usage.
2.4. Location of sampling points
The primary requirements for choosing the sampling points were that they be spread out at different distances from the treatment facility, that at least one point represents the distribution system's extreme and that they all be supplied directly by the plant itself, excluding the influence of any re-chlorination facilities. Each locality must be taken into account uniquely when choosing sampling places, although the following standard criteria are frequently used [29]:
-
•
Sampling stations should be chosen so that the samples taken are representative of the various water sources used by the general public.
-
•
When choosing the number of sampling sites, it is important to take into account the linkages or branches in a piped distribution system as well as the distribution of the population.
-
•
Using strategically placed sampling locations, water from reservoirs and other sources must be able to be tested. The placement of the sampling stations should take into account the number of residents served by each water source in systems with several water sources [30].
2.5. Sample collection and storage for physicochemical analysis
Water samples were taken from three sampling areas in Addis Ababa, Ethiopia. A clean 350-mL plastic polyethylene bottle was used to collect the water samples and was carried to the laboratory in an icebox to avoid unusual changes in water quality. Prior to sampling, all bottles were washed and rinsed thoroughly with distilled water. Standard methods [31] were followed for sample collection and preservation (Table 1). The physicochemical analysis of water was performed in the Addis Ababa Sewerage and Sanitation Authority (AAWSA) laboratory.
Table 1.
Methods of determination for physicochemical analysis of water, Addis Ababa, Ethiopia, 2022.
2.6. Sample collection and storage for trihalomethane analysis
The USEPA protocol and practice for sample collection and handling were followed [32]. Duplicate water samples were properly collected from the raw water source, directly after chlorination (DAC), and from the distribution network and tap waters. The sample containers had a total volume of 125 mL and had a screw cap with Teflon-facing silicon septa. A sodium thiosulfate (Na2SO3 (0.5% w/v) solution was added to the sample containers (to quench residual chlorine). The sampling point was opened for approximately 3–5 min before sampling to ensure that the water came directly from the distribution system.
The sample container was filled in such a way to minimize the headspace so that no air bubbles passed through the sample as the bottle was filled and tightly sealed after collection. The sample containers were filled in such a way to minimize the headspace so that no air bubbles passed through the samples as the bottles were filled and tightly sealed. A sampling blank filled with THM-free reagent water was added to the list of samples to be analysed. Samples were transported to the laboratory using an ice bag and stored at 4 °C until analysis.
2.7. Chemicals
Certified reference material of EPA 501/601 THM calibration mix (200 μg/mL each of chloroform (CF), chlorodibromomethane (DBCM), bromodichloromethane (BDCM), and bromoform component in methanol) was purchased from Sigma‒Aldrich, Germany. Solvent (pentane) of GC grade was purchased from Sigma‒Aldrich, Germany.
2.8. Sample analysis
The concentrations of THMs in the collected samples were determined following EPA Method 551.1 with some modifications. In brief, 2 mL of pentane was vigorously shaken with 10 mL of the sample to extract the THMs. The extract was transferred into 2 mL vials, and 2 μL of it was injected into the GC-ECD system, which was placed in a Varian autoinjector model CP 8400 and then injected into a Varian CP-3800 gas chromatography (GC) system in the Ethiopian Agriculture Authority (EAA), quality and safety assessment centre, physicochemical laboratory, and services division.
The THM concentrations in the samples were determined using EPA Method 551.1 [33] with minor modifications. Ten millilitres of the water sample were violently mixed with 2 mL of pentane to extract the THMs. The extract was placed in 2 mL vials, and 2 μL of it was then injected into a Varian CP-3800 gas chromatography (GC) system in the Ethiopian Agriculture Authority's (EAA) quality and safety assessment centre, physicochemical laboratory, and services division. This system was mounted in a Varian autoinjector model CP 8400.
The THMs were separated by an Agilent 122–5032 GC Column DB-5 (30 m × 0.25 mm × 0.25 μm) capillary column and detected by an electron capture detector (ECD) set at 290°. The carrier gas was nitrogen set at a flow rate of 1.5 mL/min, while the makeup gas was nitrogen set at a flow rate of 1.5 mL/min, and the makeup gas was nitrogen set at a flow rate of 40–60 mL/min. The oven temperature was programmed at 40 °C isothermal for 5 min, then ramped to 250 °C at a rate of 15 °C and kept at this temperature for 2 min.
The matrix-matched calibration approach was used to calculate the concentrations of THMs using the previously indicated standard mixture of THMs. With the aforementioned setup, the GC was used for more than 12–14 h, and the background was constantly checked. After the baseline was stabilized, depending on the signal, the detector current was raised from 0.0 to 0.5–1.0 nA. As soon as the GC signal stabilized, the blank was run several times without any injection, and once the baseline stability had increased, 1 μL of pentane was injected for the THM measurement.
2.9. Quality control and assurance
Prior to analysing the samples that were collected, the applied analytical method, in accordance with the Eurachem guide, was confirmed in terms of linearity, recovery, minimum detection limit (MDL), and repeatability [34]. The smallest concentration of trihalomethanes at which a given level of confidence can detect them is known as the limit of detection (LOD). Matrix-matched samples of the four mixed standards were generated at a concentration of 10 μg/L (one tenth of the MAC; maximum permissible concentration) in seven independent replicates to calculate the LOD. The LOD was then established as LOD = 3 X SD after each sample was independently measured seven times, and the standard deviation (SD) of each measurement was calculated. Ten spiked samples are put through the complete analytical process, from extraction to analysis, as part of an LOD investigation [35].
During method verification, as internal quality control, blank samples and water samples fortified with THM compounds of interest at six working ranges were prepared as matrix-matched calibrants. As a result, three batches of matrix-matched calibrants were created over the course of three days (days 1–3). For determination of recovery and precision, nine [9] spiked samples each in triplicate were prepared by spiking blank samples at 0.5, 1.0 and 1.5 times the concentrations of the MACs. Each batch of verification samples is accompanied by matrix-matched reference standards fortified at the MAC level post spiked on the water matrix (after the sample has undergone all necessary preparations) and a true blank, a reagent blank, which is devoid of any target analytes, to prevent false-positive results and guarantee that the system is functioning properly. While test samples were created and analysed in duplicate, quality control samples were prepared and analysed in triplicate in this investigation (Table 2).
Table 2.
Method Verification parameters, Addis Ababa, Ethiopia, 2022.
| Parameter | Trihalomethanes (THMs) |
|||
|---|---|---|---|---|
| CHCl3 | CHCl2 Br | CHBr2 Cl | CHBr3 | |
| Retention time (min.± 5%) | 4.21 | 5.48 | 7.55 | 9.95 |
| Linearity correlation coefficients (r2) | 0.989 | 0.993 | 0.989 | 0.995 |
| Recovery (%) | 88.4–109.6 | 90.8–109.6 | 85.6–112.4 | 99.3–114.1 |
| MDLa or LOD (μg/L) | 1.37 | 1.73 | 1.92 | 1.67 |
| Repeatabilitya (%) | 6.18 | 3.58 | 6.79 | 6.41 |
MDL, Method Detection Limit; a for seven replicates.
2.10. Statistical analysis
The collected data were entered into Excel spreadsheets, validated, cleaned, and exported to Statistical Package for Social Sciences (SPSS) version 23 for analysis. A comparison of THM results from previous related studies was used (Table 3). Canonical correspondence analysis (CCA) or redundancy analysis (RDA) was used for data analysis of trihalomethanes and environmental variables using CANOCO 4.5 [2]. Detrended correspondence analysis (DCA) was used to check the gradient lengths. During the interpretation of the results, the statistical significance of a variable was based on a p value between 0.05 and <0.01.
Table 3.
Trihalomethanes levels found in drinking water samples in previous studies worldwide.
| Study drinking water samples, n | Site (country) | CHCl3(μg/l) | CHBrCl2 (μg/l) | CHCl2Br (μg/l) | CHBr3(μg/l) | TTHMs (μg/l) |
|---|---|---|---|---|---|---|
| Water samples, n = 3 [36] | water treatment plants (WTPs) in Nigeria | 950.57 | BD | BD | BD | 950.97 |
| Water samples, n = 1667 [5] | Water samples from twenty-three Egyptian governorates over a three-years period | 40.5 | 19.5 | 10 | 16.01 | 86.01 |
| Water samples, n = 175 [11] | Sea, open reservoir water samples, and soil in India | – | – | – | – | 151.62–198.25 |
| Water samples, n = 3 [37] | sea, river, and reservoir | – | – | – | – | 18.8 (river), 21.5 (reservoir) |
| Water samples, n = 35 [38] | Water samples were collected at the Riviere du Poste (RP) and Mont Blanc (MB), southern part of Mauritius | 20.3 | ||||
| samples from randomly selected taps in each water zone [39] | United Kingdom water company in the north of England | 36.6 | 8.0 | 2.8 | NS* | 46 |
| Water samples, n = 72 [40] | Hamadan Province, Iran, two water treatment plants | – | – | – | – | 10 to 26 |
| Two sampling sites were selected (drinking and nondrinking water samples [41] | Abadan, Khuzestan province (Iran) | – | – | – | – | 98.1 to 8.88 |
3. Results
3.1. Characteristics of raw water samples
The results of the characterization of raw water samples collected from the two treatment plants together with the groundwater samples are reported in Table 4. The data include determination of pH, temperature, combined chloride, UV absorbing (UV-254), total dissolved solids (TDS), and residual chlorine. The temperature ranged from 19.7 ± 2.5 to 22.7 ± 1.5, and the pH values of the raw water samples taken from the two water treatment plants were between 7.22 and 7.7. The chloride concentrations in the Gefersa and groundwater water samples were above the recommended limits [42]. It ranged from 4 ± 3.5 (Legedadi dam) to 10.9 ± 4.9 mg/L (Gefersa dam). The highest TDS level was detected from the groundwater source, which was 105 ± 4.5 mg/L.
Table 4.
Summary statistics for raw water samples collected from the two water treatment plants, Addis Ababa, Ethiopia, 2022.
| Type of water source | PH | Temperature ( | TDS (mg/L) | UV-254 (Abs/cm−1). | chloride (mg/L) | NO3-(mg/L) | Res. Cl (mg/L) (0.2–0.5 mg/L) |
|---|---|---|---|---|---|---|---|
| Legedadi RWa | 7.785 ± 0.28 | 22.7 ± 1.5 | 63 ± 3 | 89 ± 3.2 | 4 ± 3.5 | 6.1 ± 2.9 | 0.00 |
| Gefersa RW | 7.22 ± 0.28 | 19.7 ± 2.5 | 69 ± 4.2 | 93.6 ± 2.3 | 10.9±4.9%WSORL | 3.2 ± 4.1 | 0.00 |
| Groundwater source | 7.68 ± 0.05 | 19.54 ± 0.21 | 105 ± 4.5 | 85.24 ± 0.49 | 10.04 ± 0.22 | 0.53 ± 0.06 | 0.05 |
Raw water %WSORL = percentage of water source out of recommended limit.
3.2. Number of measurements below detection limits
The number of measurements recorded below the detection limits for CHCl3, CHCl2 Br, CHBr2 Cl, and CHBr3 were 28.3%, 37%, 57.5%, and 94.2%, respectively. The presence of certain THMs was below the LOD of the method employed (Table 5).
Table 5.
Detection number of trihalomethanes in drinking water in Addis Ababa, Ethiopia, 2022.
| Name of the variable | Number of measurements below detection limit (%) | Number of measurements above detection limit (%) |
|---|---|---|
| CHCl3 | 34 (28.3) | 86(71.7) |
| CHCl2Br | 76(63) | 44(37) |
| CHClBr2 | 69 ( 57.5) | 51 ( 42.5) |
| CHBr3 | 113( 94.2) | 7( 5.83) |
3.3. Trihalomethane concentration
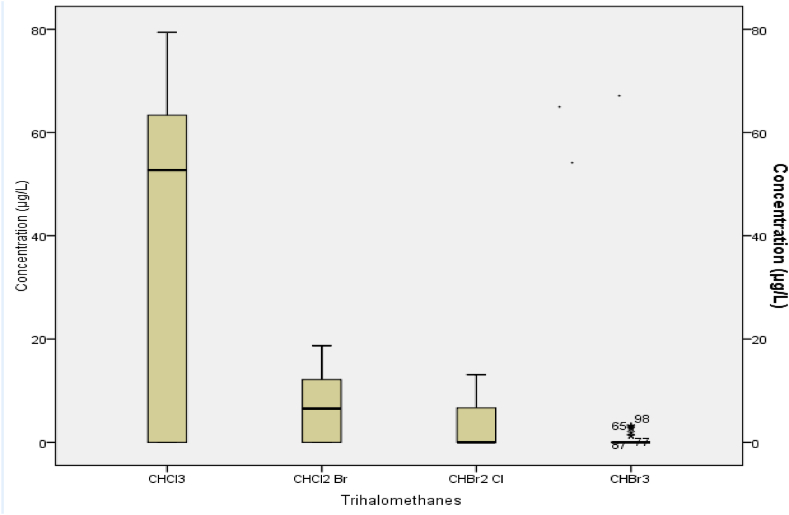
The mean concentrations of CHCl3, CHCl2 Br, CHBr2 Cl and CHBr3 in the drinking water were 54.9, 10.7, 7.70 and 3.02 μg/L, respectively. The maximum concentration of CHCl3 was 79.40 μg/L, and the minimum was 4.3 μg/L. The total mean concentration of trihalomethanes was 76.3 μg/L (Table 6, Fig. 3, Fig. 4).
Table 6.
Total trihalomethanes and drinking water supplies in Addis Ababa, Ethiopia, 2022.
| Mean Concentration μg/L (95% CI) | Min (μg/L) | Max (μg/L) | |
|---|---|---|---|
| CHCl3 | 54.93(50.97–58.53) | 4.3 | 79.40 |
| CHCl2 Br | 10.66(9.74–11.53) | 2.42 | 19 |
| CHBr2 Cl | 7.70(6.83–8.53) | 2.22 | 13.1 |
| CHBr3 | 3.02(2.83–3.21) | 2.7 | 3.21 |
| TTHMs | 76.31 |
Fig. 3.
Mean trihalomethanes in drinking water in Addis Ababa, Ethiopia, 2022.
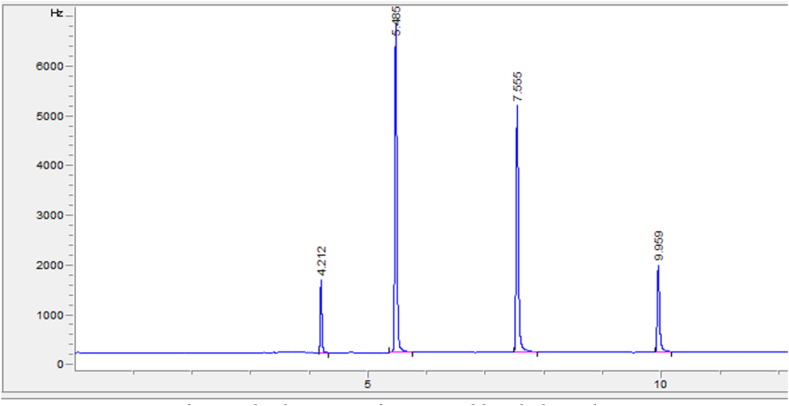
Fig. 4.
Chromatogram of a standard mixture of THMs, Addis Ababa, Ethiopia, 2022 Chromatogram for a water sample: CHCl3 (tR = 4.212); CHBrCl2 (tR = 5.485); CHBr2Cl (tR = 7.55); CHBr3 (tR = 9.959).
3.4. Distribution of each trihalomethane in the water supply system of Addis Ababa
The mean CHCl3 in the Gefersa reservoirs and Gefersa tap waters were 62.4 μg/L and 60.4 μg/L, respectively. The mean total THMs in the Gefersa reservoirs and Gefersa tap waters were 76.7 μg/L and 78.07 μg/L, respectively. Similarly, the mean CHCl3 values in the legedadi reservoirs and legedadi tap waters were 49.6 μg/L and 59.2 μg/L, respectively. The mean total THMs in the legedadi reservoirs and legedadi tap waters were 62.2 μg/L and 77.1 μg/L, respectively. The lowest THM concentration was recorded in the groundwater supply system (15.5 μg/L). In addition, the Gefersa water supply system recorded relatively higher mean levels of TTHMs compared to others. Very low bromoform was recorded only from the groundwater supply system (Table 7).
Table 7.
Comparison of each trihalomethane in the water supply system of Addis Ababa, Ethiopia, 2022.
| Gefersa reservoirs (95% CI) | Gefersa tap waters (95% CI) | Legedadi reservoirs (95% CI) | Legedadi tap water (95% CI) | Groundwater WS (95% CI) | |
|---|---|---|---|---|---|
| CHCl3 | 62.4(53.1–77.0) | 60.4(56.1–64.2) | 49.64(36.2–63.4) | 59.2(55.75–63.07) | 8.36(5.98–10.72) |
| CHBr Cl2 | 8.61(7.00–9.65) | 11.04(9.81–12.29) | 12.6(8.30–15.3) | 10.08(8.60–11.60) | 3.8(2.7–4.9) |
| CHClBr 2 | 5.69(1.13–8.04) | 6.63(5.29–7.93) | – | 7.80(6.68–8.95) | – |
| CHBr3 | – | – | – | – | 3.02(2.77–3.21) |
| TTHMs | 76.7 | 78.07 | 62.24 | 77.08 | 15.25 |
| Mean TTHMs | 77.4 | 69.66 | 15.25 | ||
*WS: water supply.
The highest concentration of TTHMs (97.4 μg/L) was observed in Gefersa tap water. Comparatively, the Legedadi water supply system contributed a lower concentration of THMs than the Gefersa supply system (Fig. 5).
Fig. 5.
Percentage contribution of each THM species to TTHMs. LRow = Legedadi row water, LR = Legedadi reservoir, LTW = Legedadi tap water, GTW= Gefersa tap water, GRW = Gefersa Reservoir water, and GDW = Groundwater.
3.5. Ordination analysis
The data set was first examined in CANOCO for Windows version 4.5 using a detrended correspondence analysis (DCA) to see if a linear or unimodal kind of response was evident along environmental gradients [43]. Since all environmental gradients were shorter than 2 standard deviation units, redundancy analysis (RDA) was then applied. Trihalomethanes were regarded as response variables in all RDA studies, while environmental factors were regarded as predictor variables (Table S2).
A preliminary analysis was performed to test multicollinearity in the environmental variables. Variables with a variance inflation factor of 5 were removed from the analysis (Table S1). Seven environmental aspects were selected as independent variables using a stepwise forward selection method. Prior to analysis, trihalomethanes and environmental data were log-converted [log(x+1)] to reduce variance. Using Monte Carlo permutations, the statistical significance of the eigenvalues and THM-environment correlations produced by the RDA was examined.
4. Results
Trihalomethanes appear to respond linearly to environmental gradients, according to the results of the detrended correspondence analysis (DCA), which indicated a gradient length less than 2 standard deviation units [43]. Trihalomethanes and the chosen environmental parameters were shown to be significantly correlated (p 0.05) for both the first axis and all canonical axes combined (Fig. 6). According to the first two axes of the RDA biplot of trihalomethanes and environmental variables, 99.6% of the variance in the trihalomethane data and 97.5% of the variation in the correlation and class averages of trihalomethanes with regard to the environmental variables were explained.
Fig. 6.
Redundancy analysis of Trihalomethanes and environmental variables of drinking water supply in Addis Ababa, Ethiopia.
The eigenvalues of the first two axes were 0.527 and 0.012, respectively. The trihalomethane-environment correlation in this ordination was 0.784 and 0.327 for the first two axes, respectively. An electron conductivity-related gradient was seen on the first axis of the RDA ordination. Trihalomethanes and this axis had a positive correlation (r = 0.784, p 0.05). The combined chlorine, calcium-based hardness, and UV absorbance were all defined by the second canonical axis.
5. Multivariate analysis
The initial two axes of the RDA biplots explained 99.6% of the variation in environmental data and 53.9% of the total variation in trihalomethanes (Table 8). With correlation coefficients of 0.78 and 0.33 for the first and second axes, respectively, the RDA ordination demonstrated a high link between trihalomethanes and environmental variables. Trihalomethanes and residual chlorine, UV absorbance, phosphate, and hardness as calcium had a positive correlation with trihalomethane species. THM species except bromoform had negative correlations with electron conductivity. Combined chlorine and turbidity had a positive relationship with Trihalomethanes. Furthermore, bromoform and combined chlorine do not show correlations (Fig. 6).
Table 8.
Detailed results of the redundancy analysis relating the core metrics of trihalomethanes to the environmental variables.
| Axes | 1 | 2 | 3 | 4 | Total variance |
|---|---|---|---|---|---|
| Eigenvalues: | 0.527 | 0.012 | 0.002 | 0.000 | 1.00 |
| Trihalomethanes environmental correlations: | 0.784 | 0.327 | 0.274 | 0.192 | |
| Cumulative percentage variance | |||||
| of Trihalomethanes data: | 52.7 | 53.9 | 54.1 | 54.1 | |
| Trihalomethanes- environmental relation: | 97.5 | 99.6 | 99.9 | 100 | |
| Sum of all eigenvalues | 1.00 | ||||
| Sum of all canonical eigenvalues | 0.541 | ||||
6. Discussion
This is the first study to assess the THM concentration in the water supply in Addis Ababa, Ethiopia. The typically obtained chromatogram depicts a sharp, well-separated, and resolved peak for each of the THM compounds (Fig. 4). Matrix-matched six-point calibrations were created (S 3–6). The limit of detection (LOD) was 1.67 μg/L. This figure is within the USEPA's detection thresholds, which for THMs range from 0.1 to 2.5 μg/L and vary across laboratories and time [44]. The correlation coefficients (r) were higher than 0.989 for all standards, which signifies a good linear response factor for the different THM compounds in the concentration range. The mean recovery ranged from 85.6 to 114.1%, which falls in the acceptable range (80%–120%) set by EPA method 551.1(45) and showed good accuracy of the method. The repeatability of the procedure (3.58%–6.79%) values were within the EPA method 551.1 guidelines. (<15%) [45] and showed the method's good precision.
The total mean concentration of trihalomethanes in water sources in Addis Ababa was 76.3 μg/L. This concentration is below the USEPA MCL of 100 μg/L [46] and the Shubra El-Khima Water Treatment Plant, Cairo, Egypt, which was between 29.07 and 86.01 μg/L(5). However, the THM values observed in this study were higher than those reported for studies in the cities of Hamadan and Tuyserkan in western Iran in 2016–2017, which were 47.5 μg/L(40). This dissimilarity could be due to differences in methodological and geographical characteristics. Certain THMs were present but at levels below the LOD of the method used. This may be because the water samples were not treated, which prevented them from being subjected to chlorination reactions. These results are comparable with those of similar studies performed in other nations [47,48].
The average number of THMs in the Gefersa reservoirs and Gefersa tap waters were 76.7 μg/L and 78.07 μg/L, respectively. Likewise, the average total THMs in the legedadi reservoirs and legedadi tap waters were 62.2 μg/L and 77.1 μg/L, respectively. These findings could be because drinking water is carried over long distances from the treatment plants to the reservoirs and then to local tap waters. To guarantee that there is residual chlorine in the water supply system, the water is chlorinated after each water booster pump. This chlorine addition increased the reaction time, which in turn increased the concentration of the various THM species and, ultimately, the concentration of TTHMs.
The mean total THMs in the Gefersa and Legedadi water supply systems were 77.4 μg/L and 69.66 μg/L, respectively. However, a smaller THM concentration was recorded in the groundwater supply system (15.5 μg/L). Very low bromoform was recorded only from the groundwater supply system. These findings are comparable with a related meta-analysis performed by Morris et al. [49], which showed that chlorinated surface water has significantly more chlorination by products than chlorinated groundwater (medians of 50.7 and 0.8 ppb, respectively). Therefore, the consumption of surface water was found to be an indirect proxy of exposure to disinfection by products used in associated studies [50,51].
In addition, there was a high THM concentration in the Gefersa water supply system compared to the Legedadi water supply system in this study. This could be because there is high organic matter in the dam due to the presence of high forestation coverage (allochthonous input – such as terrestrial DOM derived from vegetation), fish cultivation and decomposition of fishes and plant remnants. Furthermore, this treatment plant is located in the centre of an urban area and is likely to be affected by urbanization activities.
The measurements of bromodichloromethane (CHBrCl2), chloroform (CHCl3), dibromochloromethane (CHBr2Cl) and bromoform (CHBr3 Cl) in water samples showed that the only species detected was bromoform (CHBr3) in groundwater supplies (3.2 μg/L). This could be because the groundwater wells in the city are deepest (>100 m) and contain natural bromide in the water. This bromide might originate from the soil organic content (SOC), as indicated by a related study in which bromoform was the dominant THM produced during Cl2 treatment [52]. Furthermore, this distribution of THM species may be explained by the fact that bromine-carbon bonds are more resistant to dissociation than chlorine-carbon bonds due to lower dissociation energy [53].
CHCl3 was the most frequently detected species in the total THMs, with percentages ranging from 69.6% in Legedadi to 77.4% in the Gefersa water supply. CHBrCl2 was found at a percentage of 11.34% in the legedadi water supply. CHBrCl2 appeared at a percentage of 9.83% in the Gefersa water supply. These findings are comparable with those of previous researchers and demonstrate that the species with the greatest representation among all THMs is chloroform [4,5,40,54].
Fig. 5 shows different THM species in the drinking water of each area and their contribution to TTHMs. The highest concentration of CHCl3 was recorded from Gefersa (79.40 μg/L), while the lowest value was observed from groundwater sources (8.36 μg/L). This reflects the fact that the surface water in Addis Ababa contains higher levels of TOC and consumes higher amounts of chlorine for disinfection relative to groundwater sources. The pH values of the raw water samples were between 7.22 and 7.78, which is considered to be alkaline. This could be due to the addition of only Ca(OH)2 to the treatment plants. There is no practice of adding acids to reduce the pH in the treatment plants.
A fundamental understanding of the concentration of trihalomethanes is important to plan and implement effective trihalomethanes regulation intervention strategies in drinking water supplies [55]. In the present study, residual chlorine, UV absorbance, phosphate and hardness as calcium, electron conductivity, combined chlorine and turbidity were found to be the main predictors determining the abundance and distribution of trihalomethanes.
The THM species and residual chlorine had a strong positive correlation in this study. These findings were in line with a related study in Jordan and Iran showing that an increase in the value of residual chlorine has positive effects on the formation of THMs [56,57]. However, another study performed on the drinking water distribution of Ggaba, Kampala, Uganda [58] indicated that residual chlorine had a negative but significant correlation with trihalomethane formation. This could be due to differences in methodology and water characteristics. Similarly, a similar related study reported a strong positive correlation between UV absorbance at 254 nm (UV254) and the THM formation potential (THMFP) [59]. Related findings from Scottish and Jordan water treatment utilities [57,60] and several studies [56,[61], [62], [63]] showed a positive correlation between UV absorbance at 254 nm and total THM formation.
The presence of phosphate was significantly associated with the formation of trihalomethanes in this work. This might be due to the presence of phosphate in the plumbing systems (a certain proportion of which is ductile iron pipe (DCI) in the water supply network in Addis Ababa), which promoted a more significant growth of biofilm and led to an increased level of THMs [64]. In addition, the conversion of nonmicrobial carbon into microbial carbon DBP precursors by the biofilms under system conditions encouraged rapid bacterial regrowth and had noticeable effects on the kinetics of THM production, particularly when a high initial chlorine dose was used [65].
The total hardness as calcium had a positive correlation with THMs. This could be the result of the protein unfolding at the higher pH and possibly a lack of buffer to mitigate the pH effects of the protein/disinfectant [66]. THM formation showed negative correlations with electron conductivity. The causes for the inconsistency in the instances where THM and electron conductivity should be correlated may be related to the covariation in operational parameters or to the interaction between those parameters.
The combined chlorine was negatively associated with THM formation. These findings are similar to the studies in Gipuzkoa (Basque Country, Spain) [[67], [69], [70]]. The probable reason for the negative correlation is as follows: all-natural organic matter does not necessarily result in the formation of disinfectant by products [[68], [71]]. Similarly, there was a significant negative correlation between the amount of turbidity and THM formation. This could be because the water turbidity in the investigated treatment plants was not adequately high after the treatment process. In addition, bromoform and combined chlorine did not show correlations. This could be because bromoform was detected only in the groundwater supply in this study. This study used only UV 254 to estimate the organic constituents of the drinking water supplies, thus requiring further characterization of the total organic carbon in the water.
7. Conclusions
The concentrations of THMs in the drinking water of twenty-one sampling points were measured. Surface water supply networks have higher levels of total THMs than the groundwater supply. The average total THM content was lower than the US EPA's MCL. Chloroform contributed the most to TTHMs in nearly all samples, while bromoform contributed the least. In the present study, residual chlorine, UV absorbance, phosphate and hardness as calcium, and electron conductivity were found to be the main predictors determining the abundance and distribution of trihalomethanes.
The levels of THMs in the Addis Ababa drinking water supply system are generally below the US EPA and WHO drinking water guidelines. However, more attention should be given to TCM, BDCM and DBCM, as they pose high cancer risk even at low levels. Since drinking water containing THMs poses health hazards, precautions must be taken to keep these levels in control. Minimizing the levels of total organic carbon in the water supply sources should be targeted in the future. The monitoring and regulation of the THMs is required on a regular basis to analyse trends and guide the water treatment and distribution system.
Ethics approval and informed consent
Ethical approval was obtained from the Institutional Review Board of Natural and Computational Science College of Addis Ababa University.
Author contribution statement
Nebiyou Tafesse: conceived and designed the experiments; performed the experiments; analysed and interpreted the data; contributed reagents, materials, analysis tools or data; and wrote the paper.
Massimiliano Porcelli, Belachew Bacha Hirpessa; Janvier Gasana, R. K. Padhi, Sirak Robele, Argaw Ambelu: Conceived and designed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are also very grateful to the Addis Ababa Water and Sewerage Authority (AAWSA) for allowing us to collect the water samples and analyse the physicochemical parameters of the waters. We strongly acknowledge Armauer Hanson Research Institute (AHRI) for procurement and import of certified reference materials and solvents from Aldrich Company, Germany. Professor Dr. Daniel A. Enquobahrie from the School of Public Health, Washington University, and Zeleke Teferi from AAWSA are duly acknowledged. The authors would like to thank the Ethiopian Agriculture Authority (EAA), the Quality and Safety Assessment Centre, the Physicochemical Laboratory, the Services Division and the staff of the laboratory for providing the facilities to achieve this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19446.
List of abbreviations
- BDCM
bromodichloromethane
- DBCM
Dibromochloromethane
- DBPs
Disinfection By-products
- EC
electron conductivity
- ECD
Electron capture detector
- HAAs
Haloacetic Acids
- HKN
Halo ketones
- LOQ
Limit of Quantification
- MAC
Maximum allowable concentration
- MCL:
Maximum contaminant limit
- MDL:
Minimum Detection Limit
- SPSS
Statistical Package for Social Sciences
- TBM
Tribromomethane
- TCM
Trichloromethane
- TDS
total dissolved solids
- THMs
Trihalomethanes
- USEPA
United States Environmental Protection Agency)
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kanno G., Ashuro Z., Negassa B., Alembo A., Abate Z., Getahun B., et al. Sanitary Survey and drinking water quality performance of treat-ment plant: the case of Dilla Town, Ethiopia. Sci. Med. 2020;1:3–9. [Google Scholar]
- 2.Abdullah M.P., Yee L.F., Ata S., Abdullah A., Ishak B., Abidin K.N.Z. The study of interrelationship between raw water quality parameters, chlorine demand and the formation of disinfection byproducts. Phys. Chem. Earth, Parts A/B/C. 2009;34(13–16):806–811. [Google Scholar]
- 3.Padhi R., Subramanian S., Mohanty A., Satpathy K. Comparative assessment of chlorine reactivity and trihalomethanes formation potential of three different water sources. J. Water Proc. Eng. 2019;29 [Google Scholar]
- 4.Sadeghi H., Nasseri S., Yunesian M., Mahvi A.H., Nabizadeh R., Alimohammadi M. Trihalomethanes in urban drinking water: measuring exposures and assessing carcinogenic risk. J. Environ. Health Sci. Eng. 2019;17(2):619–632. doi: 10.1007/s40201-019-00374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishaqa E.-S.I., Radwan E.K., Ibrahim M., Hegazy T.A., Ibrahim M.S. Multiexposure human health risks assessment of trihalomethanes in drinking water of Egypt. Environ. Res. 2022;207 doi: 10.1016/j.envres.2021.112643. [DOI] [PubMed] [Google Scholar]
- 6.Radwan E.K., Barakat M.H., Ibrahim M.B. Hazardous inorganic disinfection byproducts in Egypt's tap drinking water: occurrence and human health risks assessment studies. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.149069. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen H.V.-M., Lee H.-S., Lee S.-Y., Hur J., Shin H.-S. Changes in structural characteristics of humic and fulvic acids under chlorination and their association with trihalomethanes and haloacetic acids formation. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148142. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim M.B., Radwan E.K., Moursy A.S., Bedair A.H. Humic substances as precursors for trihalomethanes yields upon chlorination. Desalinat. Water Treat. 2016;57(55):26494–26500. [Google Scholar]
- 9.Saradhi I., Sharma S., Prathibha P., Pandit G. Oxyhalide disinfection byproducts in packaged drinking water and their associated risk. Curr. Sci. 2015:80–85. [Google Scholar]
- 10.Michalski R. Inorganic oxyhalide by-products in drinking water and ion chromatographic determination methods. Pol. J. Environ. Stud. 2005;14(3) [Google Scholar]
- 11.Padhi R., Subramanian S., Satpathy K. Formation, distribution, and speciation of DBPs (THMs, HAAs, ClO2−, andClO3−) during treatment of different source water with chlorine and chlorine dioxide. Chemosphere. 2019;218:540–550. doi: 10.1016/j.chemosphere.2018.11.100. [DOI] [PubMed] [Google Scholar]
- 12.Righi E., Fantuzzi G., Predieri G., Aggazzotti G. Bromate, chlorite, chlorate, haloacetic acids, and trihalomethanes occurrence in indoor swimming pool waters in Italy. Microchem. J. 2014;113:23–29. [Google Scholar]
- 13.Komaki Y., Mariñas B.J., Plewa M.J. Toxicity of drinking water disinfection byproducts: cell cycle alterations induced by the monohaloacetonitriles. Environ. Sci. Technol. 2014;48(19):11662–11669. doi: 10.1021/es5032344. [DOI] [PubMed] [Google Scholar]
- 14.Du Y., Wang W.-L., He T., Sun Y.-X., Lv X.-T., Wu Q.-Y., et al. Chlorinated effluent organic matter causes higher toxicity than chlorinated natural organic matter by inducing more intracellular reactive oxygen species. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.134881. [DOI] [PubMed] [Google Scholar]
- 15.Avsar E., Avsar D.D., Hayta S. Evaluation of disinfection byproduct (DBP) formation and fingerprint in a swimming pool in Bitlis/Turkey: a case study. Environ. Forensics. 2020;21(3–4):375–385. [Google Scholar]
- 16.Kim D.-H., Park C.G., Kim Y.J. Characterizing the potential estrogenic and androgenic activities of two disinfection byproducts, mono-haloacetic acids and haloacetamides, using in vitro bioassays. Chemosphere. 2020;242 doi: 10.1016/j.chemosphere.2019.125198. [DOI] [PubMed] [Google Scholar]
- 17.Liu C., Deng Y.-L., Yuan X.-Q., Chen P.-P., Miao Y., Luo Q., et al. Exposure to disinfection byproducts and reproductive hormones among women: results from the Tongji Reproductive and Environmental (TREE) study. Environ. Res. 2022;209 doi: 10.1016/j.envres.2022.112863. [DOI] [PubMed] [Google Scholar]
- 18.Ristoiu D., von Gunten U., Mocan A., Chira R., Siegfried B., Haydee Kovacs M., et al. Trihalomethane formation during water disinfection in four water supplies in the Somes river basin in Romania. Environ. Sci. Pollut. Control Ser. 2009;16(1):55–65. doi: 10.1007/s11356-009-0100-1. [DOI] [PubMed] [Google Scholar]
- 19.Mashau F., Ncube E.J., Voyi K. Drinking water disinfection byproducts exposure and health effects on pregnancy outcomes: a systematic review. J. Water Health. 2018;16(2):181–196. doi: 10.2166/wh.2018.167. [DOI] [PubMed] [Google Scholar]
- 20.Tafesse N., Porcelli M., Gari S.R., Ambelu A. Prevalence and trends of drinking water disinfection byproducts-related cancers in Addis Ababa, Ethiopia. Environ. Health Insights. 2022;16 doi: 10.1177/11786302221112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson A., Gonsior M., Harir M., Hertkorn N., Schmitt-Kopplin P., Powers L., et al. Molecular changes among nonvolatile disinfection byproducts between drinking water treatment and consumer taps. Environ. Sci. J. Integr. Environ. Res.: Water Res. Technol. 2021;7(12):2335–2345. [Google Scholar]
- 22.Wu Q.-Y., Liang Z.-F., Wang W.-L., Du Y., Hu H.-Y., Yang L.-L., et al. Nonvolatile disinfection byproducts are far more toxic to mammalian cells than volatile byproducts. Water Res. 2020;183 doi: 10.1016/j.watres.2020.116080. [DOI] [PubMed] [Google Scholar]
- 23.Grellier J. Comment on “disinfection byproducts in drinking water and evaluation of potential health risks of long-term exposure in Nigeria”. J. Environ. Public Health. 2018;2018 doi: 10.1155/2018/1901429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung A.Q. University of Nevada; Las Vegas: 2019. The Effects of Ventilation on Trihalomethanes Removal by Spray Aeration. [Google Scholar]
- 25.Li R.A., McDonald J.A., Sathasivan A., Khan S.J. A multivariate Bayesian network analysis of water quality factors influencing trihalomethanes formation in drinking water distribution systems. Water Res. 2021;190 doi: 10.1016/j.watres.2020.116712. [DOI] [PubMed] [Google Scholar]
- 26.Yang L., Chen X., She Q., Cao G., Liu Y., Chang V.W.-C., et al. Regulation, formation, exposure, and treatment of disinfection byproducts (DBPs) in swimming pool waters: a critical review. Environ. Int. 2018;121:1039–1057. doi: 10.1016/j.envint.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Central Statistical Authority E. Population Projection of Ethiopia for All Regions. 2013. [Google Scholar]
- 28.MCE NrbZAa . 2015. Alternative Power Source for Pumping Stations at the Reservoirs and Wells Existing Assessment Report. Addis Ababa. [Google Scholar]
- 29.Kirmeyer G.J., Martel K. 2001. Pathogen Intrusion into the Distribution System: American Water Works Association. [Google Scholar]
- 30.Organization WH . 2010. Drinking Water Quality. [Google Scholar]
- 31.Dirican S. Assessment of water quality using physico-chemical parameters of Çamlıgöze Dam Lake in Sivas, Turkey. Ecologia. 2015;5(1):1–7. [Google Scholar]
- 32.Clesceri L.S., Eaton A.D., Rice E. American Public Health Association, American Water Works Association, and the Water Environment Association; Washington, DC: 2005. Standard Methods for Examination of Water and Wastewater Method 5210B. [Google Scholar]
- 33.Organization WH . fourth ed. 2017. Guidelines for Drinking-Water Quality: First Addendum to the. [PubMed] [Google Scholar]
- 34.Skandaraja S.S. 2015. Lesson 16: Determination of Water Quality Parameters Such as Temperature, Colour, Turbidity and Total Solids by Physical Methods. [Google Scholar]
- 35.USEPA . 2016. Quick Guide to Drinking Water Sample Collection. [Google Scholar]
- 36.Munch D.J., Hautman D.P. Method 551.1: determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid‒liquid extraction and gas chromatography with electron-capture detection. Methods Determinat. Org. Compd. Drink. Water. 1995;1358:14–19. [Google Scholar]
- 37.Bertil M., Örnemark U. LGC; Teddington, Middlesex, UK: 2014. The Fitness for the Purpose of Analytical Methods: a Laboratory Guide to Method Validation and Related Topics. A Laboratory Guide to Method Validation and Related Topics. [Google Scholar]
- 38.Magnusson B. 2014. The Fitness for the Purpose of Analytical Methods: a Laboratory Guide to Method Validation and Related Topics. Eurachem; 2014. [Google Scholar]
- 39.Benson N.U., Akintokun O.A., Adedapo A.E. Disinfection byproducts in drinking water and evaluation of potential health risks of long-term exposure in Nigeria. J. Environ. Public Health. 2017;2017 doi: 10.1155/2017/7535797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padhi R., Subramanian S., Mohanty A., Satpathy K. Monitoring chlorine residual and trihalomethanes in the chlorinated seawater effluent of a nuclear power plant. Environ. Monit. Assess. 2019;191(7):1–13. doi: 10.1007/s10661-019-7611-0. [DOI] [PubMed] [Google Scholar]
- 41.Uppeegadoo A., Yive N.C.K., Gopaul K. Determination of Trihalomethanes in Drinking Water in Southern Mauritius. Univ. Mauritius Res. J. 1999;3:109–114. [Google Scholar]
- 42.Keegan T., Whitaker H., Nieuwenhuijsen M., Toledano M., Elliott P., Fawell J., et al. Use of routinely collected data on trihalomethane in drinking water for epidemiological purposes. Occup. Environ. Med. 2001;58(7):447–452. doi: 10.1136/oem.58.7.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadali A., Rahmani A., Asgari G., Leili M., Norouzi H.A., Naghibi A. The assessment of trihalomethanes concentrations in drinking water of Hamadan and Tuyserkan cities, Western Iran and its health risk on the exposed population. J. Res. Health Sci. 2019;19(1) [PMC free article] [PubMed] [Google Scholar]
- 44.Kujlu R., Mahdavianpour M., Ghanbari F. Multiroute human health risk assessment from trihalomethanes in drinking and nondrinking water in Abadan, Iran. Environ. Sci. Pollut. Control Ser. 2020;27(34):42621–42630. doi: 10.1007/s11356-020-09990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alemu Z.A., Teklu K.T., Alemayehu T.A., Balcha K.H., Mengesha S.D. Physicochemical quality of drinking water sources in Ethiopia and its health impact: a retrospective study. Environ. Sys. Res. 2015;4(1):1–8. [Google Scholar]
- 46.Van den Brink P.J., Van den Brink N.W., Ter Braak C.J. Multivariate analysis of ecotoxicological data using ordination: demonstrations of utility on the basis of various examples. Australas. J. Ecotoxicol. 2003;9(2):141–156. [Google Scholar]
- 47.Kaufman J.A., Wright J.M., Evans A., Rivera-Núñez Z., Meyer A., Narotsky M.G. Disinfection byproduct exposures and the risk of musculoskeletal birth defects. Environ. Epidemiol. 2020;4(1) doi: 10.1097/EE9.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikolaou A.D., Lekkas T.D., Golfinopoulos S.K., Kostopoulou M.N. Application of different analytical methods for determination of volatile chlorination byproducts in drinking water. Talanta. 2002;56(4):717–726. doi: 10.1016/s0039-9140(01)00613-0. [DOI] [PubMed] [Google Scholar]
- 49.Xie Y. 2003. Disinfection Byproducts in Drinking Water: Formation, Analysis, and Control: CRC press. [Google Scholar]
- 50.Zhao R-s, Lao W-j, Xu X-b. Headspace liquid-phase microextraction of trihalomethanes in drinking water and their gas chromatographic determination. Talanta. 2004;62(4):751–756. doi: 10.1016/j.talanta.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 51.Andreola R., Reche P.M., Tono G., de Souza Bido G., Felipe D.F., Mannigel A.R., et al. Influence of cleaning time in household reservoirs on trihalomethane formation in treated water. J. Water Resour. Protect. 2019;11(11):1389–1397. [Google Scholar]
- 52.Morris R., Audet A., Angelillo I., Chalmers T., Mosteller F. Chlorination, by chlorination by products, and cancer: a meta-analysis (Am J Public Health (1992) 82 (955-963. Am. J. Publ. Health. 1993;83(9):1257. doi: 10.2105/ajph.82.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaves R.S., Salvador D., Nogueira P., Santos M.M., Aprisco P., Neto C., et al. Assessment of water quality parameters and their seasonal behaviour in a Portuguese water supply system: a 6-year monitoring study. Environ. Manag. 2022;69(1):111–127. doi: 10.1007/s00267-021-01572-w. [DOI] [PubMed] [Google Scholar]
- 54.Doyle T.J., Zheng W., Cerhan J.R., Hong C.-P., Sellers T.A., Kushi L.H., et al. The association of drinking water source and chlorination byproducts with cancer incidence among postmenopausal women in Iowa: a prospective cohort study. Am. J. Publ. Health. 1997;87(7):1168–1176. doi: 10.2105/ajph.87.7.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padhi R. Carbonaceous DBP (THMs and HAAs) formation during Cl 2 and ClO 2 treatment of aqueous soluble fractions of soil derived natural organic matter. Environ. Sci. J. Integr. Environ. Res.: Water Res. Technol. 2022;8(3):597–606. [Google Scholar]
- 56.Rasheed S., Hashmi I., Zhou Q., Kim J., Campos L. Central composite rotatable design for optimization of trihalomethane extraction and detection through gas chromatography: a case study. Int. J. Environ. Sci. Technol. 2023;20(2):1185–1198. [Google Scholar]
- 57.Bujar D.H., Vezi D., Ismaili M., Shabani A., Reka A.A. Variation of trihalomethanes concentration in Tetova's drinking water in the autumn season. Middle East J. Sci. Res. 2013;16(6):814–821. [Google Scholar]
- 58.Cotruvo J.A. WHO guidelines for drinking water quality: first addendum to the fourth edition. J. - Am. Water Works Assoc. 2017;109(7):44–51. 2017. [Google Scholar]
- 59.Babaei A.A., Atari L., Ahmadi M., Ahmadiangali K., Zamanzadeh M., Alavi N. Trihalomethanes formation in Iranian water supply systems: predicting and modelling. J. Water Health. 2015;13(3):859–869. doi: 10.2166/wh.2015.211. [DOI] [PubMed] [Google Scholar]
- 60.Saidan M., Rawajfeh K., Fayyad M. Investigation of factors affecting THMs formation in drinking water. Am. J. Environ. Eng. 2013;3(5):207–212. [Google Scholar]
- 61.Nshemereirwe A., Zewge F., Malambala E. Evaluation of formation and health risks of disinfection byproducts in drinking water supply of Ggaba waterworks, Kampala, Uganda. J. Water Health. 2022;20(3):560–574. doi: 10.2166/wh.2022.272. [DOI] [PubMed] [Google Scholar]
- 62.Edzwald J.K., Becker W.C., Wattier K.L. Surrogate parameters for monitoring organic matter and THM precursors. J. - Am. Water Works Assoc. 1985;77(4):122–132. [Google Scholar]
- 63.Valdivia-Garcia M., Weir P., Frogbrook Z., Graham D.W., Werner D. Climatic, geographic and operational determinants of trihalomethanes (THMs) in drinking water systems. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep35027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chowdhury S., Champagne P., McLellan P.J. Models for predicting disinfection byproduct (DBP) formation in drinking waters: a chronological review. Sci. Total Environ. 2009;407(14):4189–4206. doi: 10.1016/j.scitotenv.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Nikolaou A.D., Arhonditsis G., Golfinopoulos S.K., Lekkas T.D. Predicting the formation of trihalomethanes and haloacetic in surface waters by linear regression models. Epidemiology. 2002;13(4):172. [Google Scholar]
- 66.Li B., Qu J., Liu H., Zhao X. Formation and distribution of disinfection byproducts during chlorine disinfection in the presence of bromide ion. Chin. Sci. Bull. 2008;53(17):2717–2723. [Google Scholar]
- 67.Jang H.-J., Choi Y.-J., Ro H.-M., Ka J.-O. Effects of phosphate addition on biofilm bacterial communities and water quality in annular reactors equipped with stainless steel and ductile cast iron pipes. J. Microbiol. 2012;50(1):17–28. doi: 10.1007/s12275-012-1040-x. [DOI] [PubMed] [Google Scholar]
- 68.Abokifa A.A., Yang Y.J., Lo C.S., Biswas P. Investigating the role of biofilms in trihalomethane formation in water distribution systems with a multicomponent model. Water Res. 2016;104:208–219. doi: 10.1016/j.watres.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.David D. University of Missouri-Columbia; 2014. The Effects of Alkalinity, Hardness, and pH on the Formation Potential of Disinfection Byproducts. [Google Scholar]
- 70.Abilleira E., Goñi-Irigoyen F., Aurrekoetxea J.J., Cortés M.A., Ayerdi M., Ibarluzea J. Swimming pool water disinfection byproducts profiles and association patterns. Heliyon. 2023;9(2) doi: 10.1016/j.heliyon.2023.e13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohammadi A., Miri M., Ebrahimi A., Khorsandi H., Nemati S. Monitoring of THMs concentration in Isfahan water distribution system and zoning by GIS, a case study in the center of Iran. Iran. J. Health, Saf. Environ. 2016;3(1):421–427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.