Abstract
The use of synthetic dyes in the textile industry is mostly non-degradable, which are carcinogenic and pollute the environment severely. Natural dyes have gained significant attention recently due to their potential to mitigate the environmental challenges associated with synthetic colorants. This investigation is centered around the extraction of natural dyes sourced from mahogany trees and the exploration of environmentally friendly techniques for coloring jute fabric. The derived dyes were procured from distinct segments of the mahogany tree: namely, the bark, fruits, and wood remnants. Employing an aqueous extraction methodology, inherent coloring agents were meticulously separated and subsequently applied to jute fabric subsequent to appropriate mordanting employing a variety of mordant categories. An exhaustive assessment encompassing wash, light, rubbing, and perspiration resistance was conducted on jute fabric that was subjected to dyeing using three distinct variants of mahogany tree-derived dyes. Notably, jute fabric treated with wood wastage-sourced dye exhibited commendable to exceptional resistance properties. The efficacy of this dyeing process was further substantiated through diverse characterization techniques, inclusive of scanning electron microscopy (SEM) and Fourier-transform infrared spectroscopy (FTIR), which unequivocally affirmed the successful bonding of mahogany-derived dyes onto the surface of the jute fabric. The textile industry, particularly dyeing operations that use large, designed colors and synthetic chemicals, is wreaking havoc on the sea-going environment by dumping emissions directly into bodies of water. Synthetic colors are commonly used to dye jute fabric, which has major health and environmental consequences. Therefore, concerning the environmental challenges, the dyeing of jute fabric using naturally extracted dyes from mahogany trees can be a suitable alternative to synthetic dyes in the textile industry.
Keywords: Mahogany tree, Jute fabric, Sustainability, Natural dye, Mordant
1. Introduction
The viewpoint of our contemporary fashion has been swiftly shifting as cloth production and coloring tactics grow. Natural coloring has been employed since antiquity, utilizing a variety of coloring sources until the invention and commercialization of synthetic dyes such as direct dyes, dispersion dyes, reactive dyes, azo dyes, and so on [1]. Due to their widespread availability and affordability, a significant portion of textile manufacturers have shifted towards the utilization of synthetic dyes. Nevertheless, the adoption of synthetic dyes carries inherent health hazards and exerts a substantial toll on the environment [2]. As a result, natural dyes are unbeatable as a viable alternative to synthetic colors [3]. Due to the availability of natural colors, researchers have been compelled to publish a variety of research studies on extraction and their uses, which have been described in a few well-researched review publications [4]. People are particularly interested in using textile items colored with natural dyes because they are concerned about eco-friendly organic products. Natural pigments used in textile dyeing are also a sustainable option because natural ingredients are often biocompatible and consistent with the natural environment [5].
Researchers in the field of textiles are in a continuous pursuit of innovative botanical sources capable of producing natural dyes and pigments. This endeavor aims to effectively address the environmental concerns that prevail in relation to the predominant use of synthetic or organic dyes [6]. Correspondingly, our own efforts have been directed towards harnessing natural coloring agents from diverse sources such as tulsi leaves [7], Butea monosperma plants [8], black rice [9], gardenia yellow [10], Alkanna tinctoria roots [11], Coral Jasmine flower [12] and neem leaves extractions [13]. However, it is important to acknowledge that, despite the promising aspects of natural dyes, certain limitations persist. These limitations encompass their non-reproducibility, elevated costs (especially in achieving vibrant and consistent hues), relatively lower color yield, and inadequate resistance properties, particularly when sourced from botanical components [14]. In our pursuit of viable solutions to address these challenges, we encountered a noteworthy plant named mahogany (Swietenia mahagoni). This botanical specimen boasts an extensive historical background in the realm of timber production. The mahogany tree, belonging to the genus Swietenia and a member of the Meliaceae family of pantropical chinaberries, comprises three distinct tropical hardwood species native to the Americas. These trees yield a distinctively straight-grained, reddish-brown wood that is widely recognized as mahogany [15]. Therefore, it can be a potential source for natural dye extraction. Many researchers extracted natural dyes from the mahogany tree. In line with this, Mamun et al. [16] have previously documented the extraction of mahogany seed pods for dyeing nylon fabrics, circumventing the need for any mordant. Similarly, Lutfi et al. [17] have extracted natural dyes from mahogany barks and applied them in the dyeing process of batik fabric. Additionally, Mamun et al. [18] employed naturally derived dyes from mahogany seed pods to color polyester fabric. Haque et al. [19] extensively investigated the extraction of rubiadin pigment from Swietenia mahagoni and its subsequent application in dying silk fabric, utilizing metallic mordants. Consequently, our own endeavors encompassed the extraction of dyes from discarded mahogany wood segments, subsequently employed in the dyeing of nonwoven organic cotton fabric [20]. Certainly, the distinction between the previous study involving the dyeing of nonwoven cotton fabric and our present research focused on jute fabric. In the aforementioned study, the dye extraction specifically encompassed the utilization of mahogany wood wastage, with subsequent application onto diverse organic cotton fabric materials. This differentiation highlights the unique focus and methodology of our current investigation, where our efforts are centered on the extraction of natural dyes from various components of the mahogany tree, subsequently directed towards the dyeing of jute fabric. By addressing this novel avenue of exploration, we aim to contribute valuable insights into the utilization of mahogany-derived dyes for jute fabric dyeing, thereby expanding the scope of potential applications and enriching the field of natural dye research.
The application of natural colors to jute fabric is a relatively underexplored area, with limited research dedicated to this aspect. Investigations into natural dye utilization on jute fabric have been limited thus far. In particular, dyeing of bleached jute fabric has been explored through the use of both single and binary combinations of aqueous extracts. Notably, red sandalwood extracts have been employed alongside aqueous extracts derived from other natural dye sources like manjistha, jackfruit wood, marigold, sappan wood, and babool, with varying proportions [21]. Jute fabric were colored with an aqueous tea extract with tannins as the primary colorant species [22]. Manjistha, annatto, ratanjot, and babool natural dyes were standardized and used to dye on jute fabric [23]. Accordingly, Samanta et al. [24] studies the dyeing of jute fabric using the natural color extracted from red sandal wood. In another study, jute fabrics were dyed using jackfruit wood extract and at pH 11 for pre-mordanted sample shown good color fastness ratings [25]. An investigation focused on dyeing bleached jute fabric using diverse combinations of aqueous extracts was conducted. This encompassed varying proportions of red sandalwood extract combined with aqueous extracts from other natural dye sources such as manjistha, jackfruit wood, marigold, sappan wood, and babool. The dyeing process included a pre-mordanting step utilizing myrobolan followed by subsequent pre-mordanting with aluminum sulfate [26]. Both jute fabric and natural dyes are beneficial to the environment. However, no research has been done on utilizing mahogany tree for the coloration of jute fabric.
This research endeavors to dye bleached jute fabric employing natural dyes sourced from various parts of the mahogany tree, including its bark, fruits, and wood wastages. An in-depth exploration was conducted into the wash, light, rubbing, and perspiration fastness properties of the dyed jute fabric, utilizing three distinct types of mahogany tree-derived dyes. Significantly, jute fabric subjected to wood waste-derived dye exhibited notably favorable fastness characteristics, ranging from good to excellent. Consequently, the utilization of naturally extracted hues from mahogany trees for coloring jute fabric emerges as a promising eco-conscious alternative to synthetic dyes within the textile industry, contributing positively to environmental concerns.
2. Experimental details
2.1. Materials
A 100% pure plain weave grey jute fabric with a weight of 260 g/m2 was sourced from the Bangladesh Jute Research Institute in Dhaka, Bangladesh. The mahogany bark, fruits, and wood wastages employed for natural dye extraction were procured from Shariatpur, Dhaka, Bangladesh. Chemical mordanting agents, namely ferrous sulfate (FeSO4•7H2O; CAS Number: 7782-63-0), potassium aluminum sulfate (KAl (SO4)2•12H2O; CAS Number: 7784-24-9), and stannous chloride (SnCl2; CAS Number: 7772-99-8), were utilized, while glauber's salt (Na2SO4•10H2O; CAS Number: 7757-82-6) was employed to enhance dye exhaustion onto the fabric. All chemicals were acquired from Dye Star Chemical Company Ltd., Dhaka, Bangladesh.
2.2. Dye extractions
Mahogany barks (Fig. 1A) and fruits (Fig. 1B) were collected from mahogany trees using knife. At the same time mahogany wastage (Fig. 1C) was collected from the furniture industry and washed them with normal water to remove dust. Then these raw materials were cut to make small pieces. The pieces were kept directly under the sunlight to make them dry. After drying, these were grinded separately to make fine powder and stored in different three pots. Again, dust was removed from grinded powder with the help of fine strainer. Then the powder was ready for extraction. By the following aqueous extraction method, the dye components were extracted separately from mahogany three samples (bark, fruits, and wastages). The extraction was done with the fixed amount of grinded powder with a liquor ratio of 1:10 at 90 °C for 60 min. After cooling the solution, the extracted dyes were filtered twice with fine filter paper to get liquid dye solution and stored these three dyes solution (from bark, fruits, and wastages) for further use. The different stages of dye extractions from mahogany sample to dye solution are shown in Fig. 1.
Fig. 1.
Extraction of dye solution from (A) mahogany bark, (B) mahogany fruits and (C) mahogany wood wastages.
2.3. Bleaching, mordanting and dyeing of jute fabric
The grey jute fabric was bleached in a closed bath for 80 min at a temperature of 85 and the material to liquor ratio was 1:20. The chemicals used in the bleaching process was hydrogen peroxide (H2O2) = 1 ml/L, sodium hydroxide (NaOH) = 1 g/L, trisodium phosphate (Na3PO4) = 5 g/L, non-ionic detergent = 5 ml/L, and sodium silicate (Na2SiO3) = 8 g/L. Then bleached jute fabric was washed with cold water and neutralized using acetic acid (2 ml/L) and again washed with water then kept it to dry in air.
Mordants play a pivotal role in binding dyes to fabrics, simultaneously enhancing color uptake quality and imparting improved color and light fastness. In the process of mordanting involving multiple mordanting agents, the jute fabric underwent treatment with various mordanting chemicals, following a fundamental procedure. The mordanting process was executed on the jute fabric, employing 5% of the fabric weight with a liquor ratio of 1:30, at a temperature of 80 °C for a duration of 50 min. The selection of mordanting agents encompassed ferrous sulfate (FeSO4.7H2O), potassium aluminum sulfate (KAl (SO4)2.12H2O), and stannous chloride (SnCl2), organized into combinations such as ferrous sulfate-alum = (2.5 + 2.5) % and ferrous sulfate-alum-tin = (2 + 2 + 1) %. In addition, identical percentages of single metal mordants were employed both for comparison and to ascertain the optimized mordanting conditions. Following the mordanting process, the jute fabric underwent a cold water wash and was subsequently dried using a dryer.
A total of 15 jute fabric samples underwent individual dyeing processes utilizing an oscillating sample dyeing machine, at a temperature of 90 °C for 60 min, and a material to liquor ratio of 1:20. Following experimentation with different combinations, the optimized dyeing recipe was established for the jute fabric. This encompassed a dye solution of 10% on the weight of fabric (owf), complemented by a wetting agent at 2 g/L, a levelling agent at 2 g/L, and a sequestering agent at 1 g/L. To enhance dye exhaustion, sodium sulfate (Na2SO4) at 5 g/L was introduced during the dyeing process. Subsequent to dyeing, the dyed jute fabric was subjected to a cold-water wash and subsequently dried using a dryer. The detailed sequence of bleaching, mordanting, and dyeing steps for the jute fabric is elucidated in Fig. 2.
Fig. 2.
A process curve of step-by-step jute fabric dyeing using natural dye.
2.4. Measurement of coloring properties
The color intensity (K/S) of the dyed jute fabric samples was quantified utilizing the Data Color 400 Spectrophotometer under illuminant D65 and 10° observers. For each sample, a four-ply opaque view was obtained by folding it twice, followed by automated calculation of the spectrophotometric value. The initial naturally colored sample served as the standard, while the subsequent batch sample was analyzed. The computation of the K/S value followed the Kubelka-Munk theory and was executed utilizing Equation (1) [27]. The K/S value was measured in five different places of each dyed sample and the mean value was reported. The standard deviation and coefficient of variation percentage (CV%) was also calculated to identify the uniformity of dyeing. The resistance of color to various factors including wash, perspiration, rubbing (both dry and wet conditions), and light was assessed following ISO standards: ISO 105-CO3:2013, ISO 105-E04:1994, ISO 105-X12:2016, and ISO 105-BO2:2013 [28]. To explore the surface morphology of both dyed and undyed jute fabric, scanning electron microscopy (SEM) images were acquired. The Hitachi VP-SEM SU1510 was employed, operating at an accelerating voltage of 10.0 kV, to capture SEM images for analysis. The Fourier transform infrared spectroscopy (FTIR) of mahogany dye powder, dyed jute fabric and undyed jute fabric were measured to identify the existence of free functional group as well as the interactions between dye and jute fabric. The FTIR spectra were recorded using the FT/IR-4700 spectrometer, made in Japan, with a wavelength range of 500–4000 cm−1 [29].
| (1) |
3. Results and discussion
Jute fabric offers intrinsic benefits such as its renewable nature, good biocompatibility, moderate moisture retention, strong thermal and acoustic insulation capabilities, and low cost. It is primarily used to create sackings and hessians, which are currently employed as packing materials. To address the increased awareness of health and cleanliness, eco-friendly jute fabric with high added value is being developed [30]. As a result, coloration of jute fabric using natural dye is very much important. In this research, jute fabric was dyed using the different part of the mahogany tree including bark, fruits and wood wastages. According to our previous study, the types and amount of mordant was selected [31]. The bleached jute fabric was mordanted using single metal mordant and one binary combination of metal mordant which is shown in Table 1. The reason behind the use of binary combination of metal mordant is to create a more sustainable and environmentally friendly approach of natural dyeing. Some single metal mordants can be hazardous to human health and the environment [32]. The amount of potentially dangerous mordanting compounds can be lowered while still attaining the required color and colorfastness by adopting a mixed metal method. The use of a mixed metal mordant has the benefit of producing a larger spectrum of colors [33]. Different metals can react with the dye in various ways, producing in a variety of colors and shades. Combining metals can result in more complex and more subtle colors than using a single metal mordant [34]. Another benefit of mixed metal mordants is that they might increase the color fastness properties. The resultant connection with the fiber can be stronger by utilizing a mix of metals, resulting in less fading and improved resilience to washing and light exposure [35]. After mordanting, three types of mahogany dye solution were then applied on to the mordanted jute fabric. Fifteen dyed jute fabric specimens were meticulously crafted, with the selection of dye solution and mordant types serving as the basis for differentiation. The corresponding dyed shades of the jute fabric, corresponding to each sample number, are comprehensively documented in Table 1.
Table 1.
Shade of the dyed jute fabric.
| Serial Number | Mordant types and amount | Dye solution type | Sample number | Dyed sample |
|---|---|---|---|---|
| 1. | Ferrous sulfate (5%) | Mahogany bark | 1 |  |
| 2. | Alum (5%) | 2 |  |
|
| 3. | Tin (5%) | 3 |  |
|
| 4. | Ferrous sulfate + alum (2.5 + 2.5)% | 4 |  |
|
| 5. | Ferrous sulfate + alum + tin (2 + 2 + 1)% | 5 |  |
|
| 6. | Ferrous sulfate (5%) | Mahogany fruits | 6 |  |
| 7. | Alum (5%) | 7 |  |
|
| 8. | Tin (5%) | 8 |  |
|
| 9. | Ferrous sulfate + alum (2.5 + 2.5)% | 9 |  |
|
| 10. | Ferrous sulfate + alum + tin (2 + 2 + 1)% | 10 |  |
|
| 11. | Ferrous sulfate (5%) | Mahogany wood wastages | 11 |  |
| 12. | Alum (5%) | 12 |  |
|
| 13. | Tin (5%) | 13 |  |
|
| 14. | Ferrous sulfate + alum (2.5 + 2.5)% | 14 |  |
|
| 15. | Ferrous sulfate + alum + tin (2 + 2 + 1)% | 15 |  |
The inherent fabric-dye affinity plays a pivotal role in influencing the color strength (K/S) of a dyed fabric. In a pursuit to discern enhanced color yield, the dyed jute fabric samples underwent assessment to determine their color strength, employing equation (1). The aggregated color strength means values, corresponding to each sample number, are vividly depicted in Fig. 3. Here, all the dyed samples which used single mordant combination were shown almost the same values of color strength. On the other hand, the mixed combination of metal mordanting samples showed higher values of color strength. This was because all dyes were extracted from the different parts of the same mahogany tree and the mordanting agent behavior was same. However, for all dyed samples, the single mordanted dyed jute fabric showed less K/S values compared with mixed mordant combinations. This phenomenon can be attributed to the utilization of mixed mordanting combinations, which has led to amplified dye uptake percentages and a broader spectrum of colors [33]. Here, sample 14 had the highest color strength value of 3.816. The reason could be the darker color was extracted using the mahogany wood wastage. As depicted in Fig. 3, it is evident that the dyed jute fabric exhibits vibrant and vivid colors in their respective produced shades, owing to the utilization of natural dyes [36].
Fig. 3.
Color strength values of different dyed jute fabric.
For the purpose of comprehensive color property comparison among the diverse dyed jute fabric samples, measurements were conducted for L*, a*, b*, c*, and h* values, encompassing both sets of samples. These values have been meticulously documented and are presented in Table 2. The mean, standard deviation, and CV% of K/S values were also calculated and presented in Table 2. Here, the values of L* was higher in sample 3 which was 75.10 and lower in sample 14 which was 58.45. It indicated that sample 14 was darker than other dyed fabrics. The values of a* was higher in sample 15, which was 10.43, and lower in sample 3 which was 5.80. It indicated sample 15 has redder than the other samples. The value of b* was higher in sample 14, which was 24.21, and lower in sample 3 which was 16.19. It indicated sample 14 has yellower than the other samples. The chroma, c* values and the hue, h* values for all dyed samples were in the consistent range. It confirmed that the purity or saturation and the shade or tone were nearly same for both dyed jute fabric. The mean value of the K/S was higher for the sample 14 and it was 3.816. According to the color characteristics result, our optimized dyed sample 14 was darker reddish yellow. The standard deviation was 0.017 and the CV% was 0.43%. It also indicated that the produced dyed fabric had uniform shade. The similar phenomenon was found when the wool cloth was colored using Rhizoma coptidis extract [37]. In addition, the highest CV% of the dyed sample was found in sample 12 and it was 1.73, which means the produced dyed fabric was not uniform in shade. According to Table 2, the more uniform dyed shade was produced in sample 14.
Table 2.
Color characteristics value of the dyed jute fabrics using mahogany dyes.
| Sample Number | L* | a* | b* | c* | h* | K/S (mean) | Standard deviation (K/S) | CV% (K/S) |
|---|---|---|---|---|---|---|---|---|
| 1 | 73.75 | 5.92 | 17.12 | 17.32 | 68.12 | 2.06 | 0.04 | 1.66 |
| 2 | 71.14 | 6.02 | 17.15 | 18.38 | 67.32 | 2.02 | 0.03 | 1.42 |
| 3 | 75.10 | 5.80 | 16.19 | 16.42 | 65.51 | 1.94 | 0.03 | 1.57 |
| 4 | 65.21 | 7.20 | 20.09 | 21.03 | 69.32 | 3.00 | 0.01 | 0.38 |
| 5 | 67.10 | 6.80 | 18.93 | 20.42 | 68.51 | 2.93 | 0.04 | 1.18 |
| 6 | 73.10 | 6.21 | 17.42 | 17.98 | 68.68 | 2.09 | 0.03 | 1.44 |
| 7 | 71.58 | 5.93 | 16.75 | 18.04 | 66.85 | 2.02 | 0.02 | 1.14 |
| 8 | 74.46 | 6.10 | 16.22 | 16.32 | 65.92 | 1.91 | 0.02 | 1.13 |
| 9 | 68.79 | 6.02 | 17.92 | 19.49 | 67.61 | 2.72 | 0.03 | 1.07 |
| 10 | 68.09 | 6.45 | 18.32 | 20.10 | 68.21 | 2.76 | 0.03 | 0.87 |
| 11 | 73.95 | 5.98 | 17.89 | 17.90 | 69.12 | 2.19 | 0.04 | 1.54 |
| 12 | 71.43 | 6.43 | 17.69 | 18.98 | 67.56 | 2.11 | 0.04 | 1.73 |
| 13 | 74.10 | 6.50 | 16.46 | 16.89 | 66.25 | 1.96 | 0.02 | 1.06 |
| 14 | 58.45 | 9.36 | 24.21 | 24.65 | 72.43 | 3.82 | 0.02 | 0.43 |
| 15 | 60.32 | 10.43 | 23.32 | 23.14 | 70.90 | 3.55 | 0.03 | 0.85 |
Here, L* = lighter/darker; a* = redder/greener; b* = yellower/bluish; c* = chromaticity; h* = hue saturation; K/S = color strength.
To understand the interactions between mahogany dyes and jute fabrics, several characterizations were performed on the undyed and optimized dyed jute fabric. The surface morphology and modifications to the fiber surface structure of jute fabric before and after dyeing was observed using SEM images. Understanding the connection between fiber structure and dye absorption and optimizing dyeing procedures to obtain desired color and surface qualities in jute fabric was evaluated based on this information. The type of dye used, its concentration, and the manner of dyeing can all have an impact on how much the surface of colored jute fabric changes. The distribution of the dye throughout the fiber, including the dye's penetration into the fiber structure was also studied using SEM. When the SEM used on jute fabric, it disclosed the information about the surface of the fiber, including the existence of surface imperfections and the impacts of dyeing. Here, the undyed jute fabric was often very uneven on the surface, with various surface flaws and variances in surface morphology from fiber to fiber (Fig. 4A). Pits, cracks, and surface roughness were observed on it. These surface flaws were linked to variances in the fiber structure; however, the processing technique and the environmental circumstances in which the fiber was formed may also have an impact. On the other hand, the changes in the fiber surface were seen when mahogany dyed jute fabric was examined under the SEM (Fig. 4B). The presence of a mahogany dye on the surface of a fiber was result in an increase in surface roughness as well as the filling of surface defects such as fractures and pits. This was due to the dye molecule adsorption on the fiber surface, which was the cause changes in the chemistry and topography of the jute fabric surface [38].
Fig. 4.
SEM images of (A) undyed, and (B) dyed jute fabric.
The technique of FTIR was used to analyze the chemical composition of materials. Here, the FTIR disclosed data about the functional groups present in the mahogany dye powder, undyed jute fabric and dyed jute fabric (Fig. 5). In case of mahogany dye powder, the characteristics small peak at 3704 cm−1 was due to the –OH stretching, which means that alcoholic group was present in the dye powder. The asymmetric and symmetric CH2 stretching vibrations were observed at 2924 cm−1 and 2856 cm−1, respectively [16]. The notable peak observed at 2360 cm−1 was attributed to the stretching vibrations of O C O. The dye powder displayed a distinct peak at 1736 cm−1, indicative of C O carbonyl stretching, which can be attributed to the presence of acetyl and carboxyl moieties originating from hemicellulose. Furthermore, a peak at 1594 cm−1 was identified, corresponding to N–O stretching vibrations, implying the presence of ligneous substances [18]. The other peaks appeared at 1269 cm−1 and 1033 cm−1 regions appeared due to the C–O stretching vibrations of the mahogany dye powder [39]. In addition, the FTIR spectra of mahogany powder dyed and undyed jute fabric was differed due to the presence of different chemical compounds in the samples. Here, the FTIR spectrum of undyed jute fabric showed the characteristic peaks associated with the cellulose and lignin present in the fiber. On the other hand, the FTIR spectrum of mahogany powder dyed jute fabric showed additional peaks associated with the presence of mahogany dye molecules on the fiber surface. It was clear from Fig. 5; the dyed jute fabric had more peaks than the undyed fabric and the new appeared peaks were consistent with the mahogany dye powder. It also confirmed the successful interactions between jute fabric and mahogany dyes. The similar phenomena were observed in the dyed fabric using mahogany trees by previous researchers [18].
Fig. 5.
FTIR spectra of mahogany dye powder, dyed and undyed jute fabric.
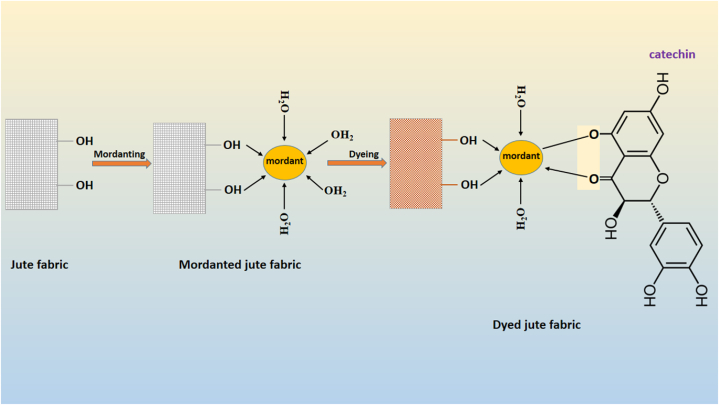
Mahogany tree belongs to the genus Swietenia. Several studies find out the chemical constitute present in the mahogany trees are flavonoids, catechin and epicatechin [40,41]. Whenever the components of catechin are oxidized, it develops the color of reddish brown. Therefore, the several chemical constitute of mahogany trees are responsible for producing the natural color [42]. In the absence of mordant application, many natural dyes exhibit limited affinity for various types of textile materials. The majority of naturally derived dyes require the introduction of a mordant to establish an interaction between the textile fabric and the extracted colorant or natural pigment molecules. The application of a mordant not only influences the color and texture of the blank samples but also facilitates the formation of metal complexes with both the fabric and the dyes. Upon mordanting, the fabric becomes endowed with metal salts that attract dye molecules, resulting in their bonding to the textile material. This process establishes a bridging link between the primary functional group of the colorant agent and the fabric, accomplished through the generation of coordinating complexes.
Jute fabric's affinity for natural colors is quite low. The dye extracted from mahogany trees are brownish red. The introduction of a metallic mordant into a coloring solution has been observed to induce a pronounced and abrupt alteration in color. This phenomenon arises from the integration of the metal atom into the dye's electron system, which is characterized by electron delocalization. Metallic mordants possess comparatively lower energy levels, hence their addition to a delocalized electron system leads to a reduction in the overall energy state. This intricate process contributes to the significant shift in color observed upon the interaction between the dye and metallic mordant. This behavior was connected to the hue's absorption and, thus, to its color [36]. The potential process and mechanism underlying the dyeing of jute fabric with mahogany tree-derived dyes in the presence of metallic mordants are depicted in Scheme 1. This scheme illustrates the interplay between the metal-dye complex and the fabric, as well as the formation of metal complexes between the dyes and the metallic mordant. In this context, the majority of metallic mordants engage in a chemical interaction with specific mordantable functional groups inherent in the extracted natural color. This interaction results in bonding through mechanisms such as covalent or hydrogen bonds, facilitated by various intermolecular forces. Notably, analogous mechanisms have been observed by prior researchers as well [36,43].
Scheme 1.
The possible formation and fixation mechanism between jute fabric and mahogany dyes in the presence of mordant.
The dyed jute fabric samples were subjected to color fastness to wash, light, rubbing and perspiration. The summarization of the test result is presented in Table 3. Evidently, the utilization of natural dyes in conjunction with mordants has yielded desirable wash fastness properties, falling within an acceptable range. This observation underscores the successful interaction between the mahogany-derived dye particles and the jute fabric. The robust wash fastness signifies the establishment of a strong bond between the dyed particles and the fabric, indicative of a harmonious and effective dye-fabric interaction facilitated by the mordant. Different mordant with variable concentration shown different fastness properties. Here, the sample 14 have more fastness properties than others. The solution used in here was from mahogany wastage and the mordant was ferrous sulfate-alum. The assessment of fastness ratings unveiled that the highest dye extraction occurred from mahogany wastage. Notably, the fastness ratings for color fastness to light, rubbing, perspiration, and washing ranged between 4 and 5. It is crucial to recognize that the velocity of dye diffusion and the internal state of the dye within the fiber exert significant influence on the washing fastness attribute of natural dyes. This underscores the critical role of dye diffusion dynamics and dye-fiber interactions in determining the washing fastness characteristics of natural dyes [44]. During washing, it was seen that the white cloths had a slight color stain. The findings reveal that the jute fabric, when dyed using extracted dyes and subjected to mordanting, displayed a subtle shift in shade, discernible through the standard greyscale. Notably, in terms of rubbing fastness, the rating for dry color change surpassed that of wet color change, with the outcome achieving a favorable rating (4–5 and 4). This discrepancy in rubbing fastness may be attributed to the presence of water-soluble dye components, which could facilitate their relatively easier detachment from the fabric, thereby contributing to the observed variations in rubbing fastness characteristics [36]. The examination of fastness properties revealed that mordanting significantly enhances color depth. This enhancement is attributed to the mordanting process, which introduces additional binding sites for the dye molecules. This phenomenon can be rationalized by considering the presence of ionizable groups in natural colorants, such as –OH and CO2H. These –OH groups, in particular, exhibit solubility in aqueous solutions due to their susceptibility to reduction within anionic environments. Mordants containing metal ions possessing unoccupied orbitals of suitable energy levels can initiate the formation of complexes, referred to as chelates, involving the fabric, mordant, and dye molecule. This chelation process induces a shift in the natural color and proves especially effective when applied to natural fabrics. The synergy between these factors accounts for the heightened color depth achieved through mordanting [45]. Therefore, the jute fabric dyed with mahogany tree extracted dyes were in the acceptable range of fastness properties.
Table 3.
Color fastness to rubbing, perspiration, wash and light of different dyed jute fabric.
|
Sample Number |
Rubbing Fastness |
Perspiration Fastness |
Wash Fastness |
Light Fastness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline |
Acid |
Color change | Color staining |
|||||||||||
| Dry | Wet | Color change | Color staining | Color change | Color staining | Wool | Acrylic | Polyester | Nylon | Cotton | Acetate | |||
| 1 | 3–4 | 3 | 4 | 4–5 | 4–5 | 3–4 | 4–5 | 4 | 3–4 | 4–5 | 3–4 | 3–4 | 4 | 4 |
| 2 | 3–4 | 3–4 | 4 | 4–5 | 4–5 | 4 | 4–5 | 4 | 4 | 4–5 | 3–4 | 3–4 | 4 | 3–4 |
| 3 | 4 | 3 | 4–5 | 4 | 4–5 | 4 | 4 | 4 | 4 | 4–5 | 4 | 3–4 | 4 | 3–4 |
| 4 | 4 | 3–4 | 4 | 4–5 | 4 | 4–5 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4 | 4–5 | 4 |
| 5 | 4 | 3 | 4–5 | 4 | 4 | 4 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| 6 | 3–4 | 3–4 | 4 | 4–5 | 4 | 4 | 4–5 | 4 | 4 | 4 | 4 | 3–4 | 4 | 3–4 |
| 7 | 3 | 2–3 | 4–5 | 4 | 4–5 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4 | 3–4 | 4 | 4 |
| 8 | 4 | 3 | 4–5 | 4 | 4–5 | 4 | 4 | 4 | 4–5 | 4–5 | 4 | 4 | 4 | 3–4 |
| 9 | 3–4 | 3 | 4–5 | 4–5 | 4–5 | 4 | 4–5 | 4 | 4 | 4–5 | 4 | 3–4 | 4 | 3–4 |
| 10 | 4 | 3–4 | 4–5 | 4–5 | 4 | 4–5 | 4–5 | 4 | 4 | 4–5 | 4–5 | 4 | 4 | 4 |
| 11 | 3–4 | 3 | 4–5 | 4 | 4–5 | 4 | 4–5 | 4 | 4 | 3–4 | 4 | 3–4 | 4 | 4 |
| 12 | 4 | 3 | 4 | 3–4 | 4–5 | 4 | 4 | 4 | 4 | 4–5 | 4 | 3–4 | 4 | 4 |
| 13 | 4 | 3–4 | 4–5 | 4 | 4–5 | 4 | 4–5 | 4 | 4 | 4–5 | 4 | 4–5 | 4 | 4 |
| 14 | 4–5 | 4 | 4–5 | 4–5 | 4 | 4 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| 15 | 4 | 4 | 4–5 | 4–5 | 4 | 4 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
4. Conclusion
Extracted dyes from mahogany bark, fruits, and wood wastage provide a good to excellent color fastness on dyed jute fabric. Among the three dye solutions derived from mahogany tree, the wood wastage produced superior results on jute fabric compared with others. Based on the color characteristic values, the optimized sample was achieved through the utilization of mahogany wood wastage in combination with the ferrous sulfate-alum mordant. This optimal configuration yielded the highest color strength, measured at 3.816, with a remarkably low standard deviation of 0.017 and a coefficient of variation (CV%) of merely 0.43%. Furthermore, the fastness ratings for color fastness to light, rubbing, perspiration, and washing were consistently high, ranging between 4 and 5. These favorable results underscore the potential of natural colors extracted from the mahogany tree as a viable substitute for synthetic dyes. The comprehensive findings support the viability of mahogany-derived natural dyes as an environmentally friendly and sustainable alternative within the realm of dyeing practices. Based on the results and observations, the hypothesis was that the lignin found in jute fabrics and the colorants from mahogany were where most of the color binding takes place. Natural dyes are known to have a strong affinity for lignin, which can also serve as a binding agent. In addition, when employed as mordants, ferrous sulfate and alum can have a synergistic effect. This mixture was improved the dye's absorption and fixing onto the jute fabrics, producing colors that are deeper and brighter. The mordants also have a strong binding capacity and can effectively trap the dye molecules, preventing them from leaching or fading over time. This attribute contributes to the enhanced color characteristics observed when using the mixed mordant. To preserve the globe from dyeing industrial risks, the modern world is increasingly turning to natural dyes. Dyes are eco-friendly and safe only when they are efficiently biodegradable, with no health risks and eco-mark. It is evident that mahogany tree dyes are among those that have such beneficial characteristics. Based on the results and other benefits, it can be concluded that mahogany dyes have a greater potential for use in the textile industry, particularly in the application of jute fabric dyeing.
Author contribution statement
Md Abu Bakar: Conceived and designed the experiments; Performed the experiments.
Md. Ikramul Islam: Analyzed and interpreted the data; Wrote the paper.
Rony Mia: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Taosif Ahmed: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data.
Sharif Tasnim Mahmud: Performed the experiments; Analyzed and interpreted the data.
Gazi Farhan Ishraque Toki: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Md. Anamul Haque: Conceived and designed the experiments; Performed the experiments.
Data availability
All data generated or analyzed during this study are included in this study.
Funding
This work was supported by the Chinese Government CSC Scholarship Program (CSC Number: 2019SLJ019821) at Wuhan Textile University, Wuhan 430200, People's Republic of China.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to express thank and gratitude towards National Institute of Textile Engineering and Research, University of Dhaka, Dhaka 1000, Bangladesh, for providing machineries and equipment on time during the research work.
References
- 1.Sk S., Mia R., Haque M., Shamim A.M. Review on extraction and application of natural dyes. Textile Leather Rev. 2021;4(4):218–233. doi: 10.31881/TLR.2021.09. [DOI] [Google Scholar]
- 2.Rahman M., et al. Synthesis and investigation of dyeing properties of 8-hydroxyquinoline-based azo dyes. J. Iran. Chem. Soc. 2021;18(4):817–826. doi: 10.1007/s13738-020-02070-2. [DOI] [Google Scholar]
- 3.Rehman F.U., et al. Microwave-assisted exploration of yellow natural dyes for nylon fabric. Sustainability. 2022;14(9):5599. doi: 10.3390/su14095599. [DOI] [Google Scholar]
- 4.Uddin M.A., et al. Textile colouration with natural colourants: a review. J. Clean. Prod. 2022 [Google Scholar]
- 5.Mia R., et al. Natural dye extracted from Triadica sebifera in aqueous medium for sustainable dyeing and functionalizing of viscose fabric. Clean. Eng. Technol. 2022;8 doi: 10.1016/j.clet.2022.100471. [DOI] [Google Scholar]
- 6.Jiang H., et al. Eco-friendly dyeing and finishing of organic cotton fabric using natural dye (gardenia yellow) reduced-stabilized nanosilver: full factorial design. Cellulose. 2022;29(4):2663–2679. doi: 10.1007/s10570-021-04401-9. [DOI] [Google Scholar]
- 7.Mia R., Sk M.S., Oli Z.B.S., Ahmed T., Kabir S., Waqar M.A. Functionalizing cotton fabrics through herbally synthesized nanosilver. Clean. Eng. Technol. 2021;4 doi: 10.1016/j.clet.2021.100227. [DOI] [Google Scholar]
- 8.Adeel S., Abbas S., Habib N., Ticha M.B., Batool F., Mia R. Innovative isolation of colorant from Butea monosperma for surface-modified silk dyeing. Surf. Innovat. 2022;40(XXXX):1–13. doi: 10.1680/jsuin.22.01016. [DOI] [Google Scholar]
- 9.Haque M.A., et al. Sustainable dyeing and functionalization of wool fabrics with black rice extract. Resour. Environ. Sustain. 2022;7 doi: 10.1016/j.resenv.2021.100045. [DOI] [Google Scholar]
- 10.Jiang H., et al. Fabrication and stabilization of green nanosilver using gardenia yellow natural dyes for efficient degradation of bacteria. Environ. Prog. Sustain. Energy. 2022 doi: 10.1002/ep.14048. [DOI] [Google Scholar]
- 11.Adeel S., Liaqat S., Hussaan M., Mia R., Ahmed B., Wafa H. Environmental friendly bio-dyeing of silk using Alkanna tinctoria based Alkannin natural dye. Ind. Crop. Prod. 2022;186 doi: 10.1016/j.indcrop.2022.115301. [DOI] [Google Scholar]
- 12.Adeel S., Ahmad S., Habib N., Mia R., Ahmed B. Coloring efficacy of Nyctanthes Arbortristis based yellow natural dye for surface-modified wool. Ind. Crop. Prod. 2022;188 doi: 10.1016/j.indcrop.2022.115571. [DOI] [Google Scholar]
- 13.Hasan R., Nishi S.I., Mia R., Islam M.M., Hasan M.M., Ahmed F. Ecofriendly functionalization of jute–cotton blended yarn using Azadirachta Indica leaves. Environ. Technol. Innovat. 2023;29 doi: 10.1016/j.eti.2022.102959. [DOI] [Google Scholar]
- 14.Affat S.S. Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: a review. Univ. Thi-Qar J. Sci. 2021;8(1):130–135. https://www.jsci.utq.edu.iq/index.php/main/article/view/790 [Google Scholar]
- 15.Venketeswaran S., Dias M., Sultanbawa F., Weyers U. vol. 30. Springer; 1988. Tissue culture studies on mahogany tree, Sweitenia; pp. 147–153. (Somatic Cell Genetics of Woody Plants). [Google Scholar]
- 16.Mamun A.A., Mahbubul Bashar M., Khan S., Roy M.N., Hossain M.M., Khan M.A. Mordant-free dyeing of nylon fabric with mahogany (Swietenia mahagoni) seed pods: a cleaner approach of synthetic fabric coloration. Textil. Res. J. 2022;92(17–18):3111–3119. doi: 10.1177/00405175211050526. [DOI] [Google Scholar]
- 17.lutfi I., Fatoni R., Fatimah S. The application of mahagony bark (Swietenia mahagony L.) for natural dyeing. Adv. Sustain. Sci. Eng. Technol. 2020;2(no. 1) doi: 10.26877/asset.v2i1.6017. [DOI] [Google Scholar]
- 18.Al Mamun M.A., Hossain M.M., Khan M.A. Dyeing of polyester fabric with natural colorants extracted from mahogany (Swieteniamahagoni) seed pods. J. Eng. Sci. 2020;11(1):37–42. doi: 10.3329/jes.v11i1.49545. [DOI] [Google Scholar]
- 19.Haque M.A., Khan G., Razzaque S., Khatun K., Chakraborty A.K., Alam M.S. Extraction of rubiadin dye from Swietenia mahagoni and its dyeing characteristics onto silk fabric using metallic mordants. Indian J. Fibre Text. Res. 2013;38:280–284. http://nopr.niscpr.res.in/handle/123456789/21433 [Google Scholar]
- 20.Mia R., Bakar M.A., Islam M.R., Ahmed T. Eco-friendly coloration from mahogany wood waste for sustainable dyeing of organic nonwoven cotton fabric. Results Eng. 2023;17 doi: 10.1016/j.rineng.2023.101032. [DOI] [Google Scholar]
- 21.Samanta A.K., Agarwal P., Singhee D., Datta S. Application of single and mixtures of red sandalwood and other natural dyes for dyeing of jute fabric: studies on colour parameters/colour fastness and compatibility. J. Text. Inst. 2009;100(7):565–587. doi: 10.1080/00405000802125246. [DOI] [Google Scholar]
- 22.Deo H., Desai B. Dyeing of cotton and jute with tea as a natural dye. Color. Technol. 1999;115(7‐8):224–227. doi: 10.1111/j.1478-4408.1999.tb00360.x. [DOI] [Google Scholar]
- 23.Chattopadhyay S., Pan N., Roy A., Saxena S., Khan A. Development of natural dyed jute fabric with improved colour yield and UV protection characteristics. The J. Textile Inst. 2013;104(8):808–818. doi: 10.1080/00405000.2012.758352. [DOI] [Google Scholar]
- 24.Samanta A., Agarwal P., Datta S. Physico-chemical studies on dyeing of jute textiles with natural dye extracted from red sandalwood. J. Inst. Eng. (India)—Textile Eng. 2006;87:16–26. [Google Scholar]
- 25.Samanta A.K., Agarwal P. Dyeing of jute and cotton fabrics using jackfruit wood extract: Part I—effects of mordanting and dyeing process variables on colour yield and colour fastness properties. Indian J. Fiber Textil Res. 2007;32(4):466–476. http://nopr.niscpr.res.in/handle/123456789/336 [Google Scholar]
- 26.Samanta A., Agarwal P. Application of mixture of red sandal wood and other natural dyes for dyeing of jute fabric-studies on dye compatibility. Int. Dyer. 2008;192(3):37–41. [Google Scholar]
- 27.Chowdhury M., et al. A feasibility study to analyze the behavior of heat settings on the cleaner production of knitted fabrics. Clean. Eng. Technol. 2022;7 doi: 10.1016/j.clet.2022.100429. [DOI] [Google Scholar]
- 28.Sk M.S., et al. Antimicrobial performance of silver–copper–zeolite microparticle-treated organic cotton fabric using versatile methods. Surf. Innovations. 2022;40(XXXX):1–8. [Google Scholar]
- 29.Khan M.T., AL Mamun M.A., Rony M., Anchang X., Rashi̇d M.M. Effect of different solvent systems on fiber morphology and property of electrospun PCL nano fibers. Tekstil ve Mühendis. 2021;28(122):61–76. [Google Scholar]
- 30.Acha B.A., Marcovich N.E., Reboredo M.M. Physical and mechanical characterization of jute fabric composites. J. Appl. Polym. Sci. 2005;98(2):639–650. doi: 10.1002/app.22083. [DOI] [Google Scholar]
- 31.Banna B.U., et al. Effectiveness of dyeing with dye extracted from mango leaves on different fabrics by using various mordants. North Am. Acad. Res. 2019;2(10):123–143. doi: 10.5281/zenodo.3519880. USA. [DOI] [Google Scholar]
- 32.Repon M.R., Islam M.T., Mamun M.A.A. Ecological risk assessment and health safety speculation during color fastness properties enhancement of natural dyed cotton through metallic mordants. Fashion Textiles. 2017;4:1–17. doi: 10.1186/s40691-017-0109-x. [DOI] [Google Scholar]
- 33.Safapour S., Mazhar M., Abedinpour S. Broadening color shade range of Rubia tinctorum L. Natural colorants on wool fibers via combination of metal mordants: color characteristics and fastness studies. J. Nat. Fibers. 2023;20(1) doi: 10.1080/15440478.2022.2157923. [DOI] [Google Scholar]
- 34.Khan S.A., et al. Mixed metal mordant dyeing of wool using root extract of Rheum emodi (Indian Rhubarb/Dolu) J. Nat. Fibers. 2015;12(3):243–255. doi: 10.1080/15440478.2014.919893. [DOI] [Google Scholar]
- 35.Safapour S., Rather L.J. Assessment of colorimetric and fastness properties of Prangos ferulacea (Jashir) dyed wool yarns in conjunction with mixed metal mordant combinations via reflectance spectroscopy. J. Nat. Fibers. 2023;20(1) doi: 10.1080/15440478.2022.2134267. [DOI] [Google Scholar]
- 36.Ayele M., Tesfaye T., Alemu D., Limeneh M., Sithole B. Natural dyeing of cotton fabric with extracts from mango tree: a step towards sustainable dyeing. Sustain. Chem. Pharm. 2020;17 doi: 10.1016/j.scp.2020.100293. [DOI] [Google Scholar]
- 37.Ke G., Yu W., Xu W. Color evaluation of wool fabric dyed with Rhizoma coptidis extract. J. Appl. Polym. Sci. 2006;101(5):3376–3380. doi: 10.1002/app.24033. [DOI] [Google Scholar]
- 38.Hossain A.N.-U., Sela S.K., Hasan N., Rahman H., Hassan M.N., Alam S.M.M. Surface modification of naturally dyed jute fabric to ameliorate its multifunctional properties and electrical conductivity. Vacuum. 2023;207 doi: 10.1016/j.vacuum.2022.111612. [DOI] [Google Scholar]
- 39.Sartape A.S., Patil S.A., Patil S.K., Salunkhe S.T., Kolekar S.S. Mahogany fruit shell: a new low-cost adsorbent for removal of methylene blue dye from aqueous solutions. Desalinat. Water Treat. 2015;53(1):99–108. doi: 10.1080/19443994.2013.839404. [DOI] [Google Scholar]
- 40.Athomo A.B.B., et al. Chemical composition of African mahogany (K. ivorensis A. Chev) extractive and tannin structures of the bark by MALDI-TOF. Ind. Crop. Prod. 2018;113:167–178. doi: 10.1016/j.indcrop.2018.01.013. [DOI] [Google Scholar]
- 41.Falah S., Suzuki T., Katayama T. Chemical constituents from Swietenia macrophylla bark and their antioxidant activity. Pakistan J. Biol. Sci. 2008;11(16):2007–2012. doi: 10.3923/pjbs.2008.2007. 2012. [DOI] [PubMed] [Google Scholar]
- 42.Gala S., Sumarno S., Mahfud M. Comparison of microwave and conventional extraction methods for natural dyes in wood waste of mahogany (Swietenia mahagoni) J. Appl. Eng. Sci. 2020;18(4):618–623. doi: 10.5937/jaes0-23695. [DOI] [Google Scholar]
- 43.Zhang Y., Zhou Q., Rather L.J., Li Q. Agricultural waste of Eriobotrya japonica L.(Loquat) seeds and flora leaves as source of natural dye and bio-mordant for coloration and bio-functional finishing of wool textile. Ind. Crop. Prod. 2021;169 doi: 10.1016/j.indcrop.2021.113633. [DOI] [Google Scholar]
- 44.Jothi D. Extraction of natural dyes from African marigold flower (Tagetes erecta L.) for textile coloration. Autex Res. J. 2008;8(2):49–53. http://www.autexrj.org/No2-2008/0275 [Google Scholar]
- 45.Mihalick J.E., Donnelly K.M. Using metals to change the colors of natural dyes. J. Chem. Educ. 2006;83(10):1550. doi: 10.1021/ed083p1550. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this study.