Abstract
Since the majority of reactive dyes only have a moderate affinity for cotton, significant amounts of electrolytes are frequently needed to cause tiredness. As a result, wastewater contains significant amounts of salt and dye, and the increasing salinity of the rivers has an effect on the delicate biochemistry of aquatic life. The aim of the study was to find a sustainable dyeing process for cotton knit fabric using EPTMAC (2, 3-epoxypropyl trimethyl ammonium chloride) as a cationic agent and comparison of the cationic dyeing process (salt free dyeing) with the regular dyeing process (dyeing with salt). For this purpose, cotton knit fabric samples were dyed with reactive dyes following salt free process and with salt. Afterwards, color fastness (wash and rubbing), spectrophotometric evaluation, bursting strength test, analysis of dye bath discharge water and Scanning Electron Microscope (SEM) image of the dyed samples were carried out. Moreover, water consumption was also evaluated for the both cationic and regular dyeing process. In terms of color fastness, cationized dyed fabric showed no change to a slight loss in depth (rating of 4–5) for both wash and rubbing fastness. From the spectrophotometric evaluation, it was found that cationized dyed fabric appeared darker and less yellowish tone. Moreover, in case of bursting strength, cationized black, hot pink, and light pink colored fabrics possessed bursting strengths of 287 kPa, 337 kPa, and 440 kPa, correspondingly. After analysis of dye bath discharge water, Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Total Dissolved Solids (TDS) value of regular colored water samples were 45%, 39%, 54% greater than that of cationized dyed water samples respectively. Cationized dyed water value for Dissolved Oxygen (DO) was 6.39 mg/l, which was within the acceptable limit. The SEM image asserted that the cationized colored samples had consistent dye dispersion, greater adhesion, and no dye anomalies. Considering water consumption, it was found that 37%, 27% and 23% less amount of water required for dyeing dark, medium and light shade of cationized samples due to fewer washes after dyeing and elimination of fixing steps. In addition of that, total cost of cationic dyeing process was less due to less chemical consumption, less utility use, shorter process time and less amount of dyes needed. Cationic dyeing process is a sustainable practice of dyeing cotton fabric with reactive dyes that offers numerous advantages when compared to the regular dyeing process with less cost consumption and low amount of environmental pollution.
Keywords: Sustainable, Eco-friendly, Cationic dyeing process, Profitability
1. Introduction
Cotton fibers are used extensively in the textile industry because of their excellent hydrophilicity, absorbency, bio - compatibility and good comfort properties [1]. Because of their vivid shade, wide color spectrum, adaptable application methods, and all-around good color fastness characteristics of the dyeing that emerges, reactive dyes are the most commonly used dyes for dyeing cotton [2,3]. However, some problems, such as insufficient dye utilization, excessive electrolyte usage, and large wastewater discharge volumes are always present when using reactive dyes [4]. The use of reactive dyes for cotton dyeing is growing in popularity, which has brought environmental concerns to light. Cotton only has a moderate affinity for the majority of reactive dyes, hence large doses of electrolytes, such as NaCl or Na2SO4 (40–100 gpl), are often required for exhaustion. This means that for some colors, dye bath exhaustion and fixing can still be as low as 50%. Therefore, wastewater has a high salt and dye content, which has a negative impact on the ecosystem [5]. Due to these problems, this class of dyes is the least environmentally friendly; the delicate biochemistry of aquatic life is impacted by the high BOD/COD ratios and increased salinity of the rivers. It is possible to estimate the overall amount of pollution produced by the usage of reactive dyes given that their production and use total more than 80,000 tons annually [6]. The cotton dyeing business must therefore address the critical challenges of increasing dye use and reducing the use of salts [7]. Depending on the specific reactive dye used and the quantity of shade, alkali-generated dye hydrolysis, which invariably comes before dye-fiber fixation, has a fixing effectiveness of 50–70% [8]. Due to the fact that between 30 and 50% of the applied reactive is not covalently linked to the fiber and is instead discharged as colored effluent, the presence of both hydrolyzed dye and unfixed dye in such effluent poses major issues. The rigorous wash-off process used in reactive dyeing on cellulosic fibers, which is necessary to remove hydrolyzed dye as well as electrolyte and other auxiliaries, is responsible for a large amount of the overall dyeing expenses (50%) and effluent load [9]. Large amounts of wastewater are produced during the reactive dyeing of cellulosic fabrics as a result of both the dyeing process and, more significantly, the multiple-stage washing procedures required for wash-off. Depending on the method used to apply the reactive dyes to the cellulose fibers, the large amounts of wastewater produced by this process contain a variety of auxiliaries in addition to the hydrolyzed/unreacted dye and electrolyte. Such effluent has a high concentration of electrolytes, a bright color, and a high organic content. Additionally, it exhibits exceptional biodegradation resistance [10]. Reactive dyeing waste water frequently has high BOD and COD values, is high in salinity, and contains cotton fragments because it comprises both soluble and insoluble organic residues from both hydrolyzed and unreacted forms of reactive dye as well as from dyeing auxiliaries. Because of the colors' innately high stability, treating such wastewater is challenging from an environmental and financial point of view [11]. Because they generate unwelcome dye effluents, textile businesses produce a lot of wastewater that is dumped into the environment, which is one of the main environmental pollution issues. Because of the massive amounts of wastewater that are released, environmental pollution is a severe issue as a result of re-dyeing methods carried out to suit specific color requirements [12].
Awais Khatri et al. looked into ways to make the dyeing process more environmentally friendly, including developing reactive dyes, altering dyeing equipment and procedures, chemically modifying cotton fiber before dyeing, using biodegradable organic compounds in dyebath formulation, and effluent treatment procedure [13]. Tianjie Niu et al. investigated the multifunctional alteration of cotton fabrics for salt-free dyeing, resistance to wrinkle, and bacterial inhibition utilizing (Polyaminopropyl Biguanide [PHMB]), which was determined to be environmentally hazardous and carcinogenic [14]. Sanjit Acharya et al. demonstrated that treating cotton with CHPTAC (3-chloro-2-hydroxylpropyl) trimethyl-ammonium chloride boosted color take-up characteristics and produced unparalleled coloring without the expansion of salt. This reduces the amount of severely darkened and salted material that is released into the environment, saving water, salt, dyes, and other auxiliaries while enhancing dye uptake and color strength [15]. Muhammad Ismail Ab Kadir, Mohd Rozi Ahmad, and Asmida Ismail employed cationic surfactants to show the effects of treating cotton and silk with Sargassum Sp. On both treated and untreated cotton and silk, concurrent mordanting techniques using cetyl trimethyl ammonium bromide (CTAB) were used during the exhaustion process for 60 min at 85°. In fabrics treated with CTAB, the color intensity and dye take-up were better. The measurement of the outcome was carried out in accordance with the MS ISO standard [16]. A water-saving and salt-free reactive dyeing of cotton garments in non-aqueous media system was investigated with the goal of resolving the issues that occurred in conventional cotton dyeing with reactive dyes, such as low dye uptake, excessive salt consumption, and huge wastewater-emission. Investigations were conducted to determine how pickup rate, alkali concentration, and fixation temperature affected the colour yield (K/S value) and levelness of dyed cloth. Additionally, a preliminary analysis of the dyeing mechanism in non-aqueous media systems was conducted [17]. When dyeing cotton fibres, reactive dye chemistry is essential. In order to shed light on the adaptability of salt-free reactive dyeing for sustainable environmental development, the impact of dye chemistry on the dyeing characteristics of cotton fibres in the suggested ethanol-carbon tetrachloride-water ternary solvent system was explored in detail [18]. The effectiveness of the Cationic Dyeing Process Agent CIBAFIX WFF was evaluated by Gopalakrishnan Mariappan, and it was deemed to be preferable to contrasting and conventional or existing techniques for responsive coloring of cotton. In comparison to deeper colors, the results were better in lighter shades. Additionally, the results for color fastness and color strength were good and less expensive than the conventional dyeing procedure [19]. The textile industry is changing, according to Sofia Willis, who also discussed recent worries about material coloring and treatment, new developments, and inexpensive coloring techniques. The use of waterless technology and the opacity of chemical substances could help protect the environment for future generations [20]. Cotton cationization is one of the best solutions to the aforementioned issue. However, in order for cationization to be successful on an industrial scale, it must be done via an exhaust process and be adaptable to the several dye chemistries currently used in the sector [21]. Although numerous studies on the cationic dyeing process have been conducted, the use of EPTMAC (2,3-epoxypropyl trimethylammonium chloride) as a cationic agent and comparison of its effectiveness with conventional dyeing process constitute an original study that carried out the novelty of the work. The primary goals of the study are to find a salt-free reactive dyeing method that is sustainable for cotton knit fabrics using EPTMAC (2,3-epoxypropyl trimethylammonium chloride) as cationic agent and to compare the cationic dyeing method (salt-free dyeing) to the traditional dyeing method (salt-based dyeing) through color fastness (wash and rubbing), spectrophotometric evaluation, bursting strength test, analysis of dye bath discharge water, SEM image of the dyed samples and assessment of water and cost consumption.
Cotton primarily consists of cellulose. Because the hydroxyl groups on cellulose are partially ionized, cotton picks up negative charges when it comes into contact with water. As a result, cotton fibers are electrostatically repelled by negatively charged, sulfonated reactive pigments. Anionic dyes must first overcome a significant unfavorable charge barrier in order to absorb on cotton fibers. The traditional methods of reactively dyeing cotton require high quantities of electrolytes, such as sodium chloride and sodium sulfate, to prevent the accumulation of negative charges and lessen the solubility of colors. The fixing rate of reactive dyes is still only relatively high even when electrolytes are provided, especially when high dye concentrations are used. Because of this, dye bath effluent typically contains too much salt and color that hasn't been disseminated, which is detrimental for the environment [21]. A quick and easy way to encourage dye-fiber affinity without adding salt in the dye bath is to pre-treat cotton before dyeing it. Positive charges that were applied to cotton fiber during cationization make anionic dyes more substantive. Cationization is a chemical procedure that converts the cotton's natural hydroxyl (-OH) coloring sites into cationic (positively charged) dyeing sites. Reactive dyeing at neutral pH without an electrolyte is also made possible by treating cellulose with cationic nucleophilic polymers (Fig. 1). The color values rise when more dye is used to colour cationic cotton. Because of the robust dye-fiber interactions brought about by the cationization process used to color cotton, little to no rinsing is required after washingThe affinity of reactive colors for cotton can be considerably increased by adding cationic groups to cotton fibers. Ionic interactions between reactive dyes and cationized cotton have the potential to enhance dye uptake, reduce or eliminate the need for electrolytes, reduce dye washing off, and consume less water and energy. The pretreatment of cotton with cations may minimize the environmental issues brought on by salt and color in effluent [22].
Fig. 1.
Attraction and Repulsion mechanism of cotton fiber towards reactive dye [23].
2. Materials and methods
2.1. Materials
In case of both cationic and traditional dyeing processes, lycra single jersey (100% cotton) fabrics of 160 GSM were used for dyeing with reactive dyes (hot, cold and medium brands). A wide range of chemical compounds such as Jingen DT HLF (wetting agent), Lubricar SB (detergent), Lubricar SB (anti-creasing agent), Lufibrol 2UD (sequestering agent), Stabicar OR (stabilizer), NaOH and soda ash (alkali), H2O2 (bleaching agent), Ban 480L (peroxide killer), Cellusoft L Ultra (bio polishing agent), Neutra acid, Jin Leveco CL 225 (levelling agent), Fortazalate QSE (soaping agent) were utilized. In the cationic dyeing method, EPTMAC (2, 3-epoxypropyl trimethyl ammonium chloride) was utilized as the cationic agent, whereas in the conventional dyeing process, gluber salt and Nearfix RTS (fixing agent) were used.
2.2. Methods
2.2.1. Cationization of cotton fabric with EPTMAC
The cationic agent used in this investigation to cationize cellulose fibrils was EPTMAC (2,3-epoxypropyl trimethyl ammonium chloride). There are various methods for attaching quaternary ammonium groups to cellulose by chemical reactions, such as using 2,3-epoxypropyl trimethyl ammonium chloride (EPTMAC) to do so. The other end of the cationic reagent, which has quaternary ammonium salt as its cationic end, is an epoxy group. Cellulose fibrils undergo a cationic alteration as a result of an etherification reaction between their alkali activated hydroxyl group and the epoxy group of EPTMAC (Fig. 2) [24].
Fig. 2.
Cationization of cellulose fibrils using EPTMAC cationic agent [25].
2.2.2. Salt free cationic dyeing process of cotton fabric
The cationic coloring process is used in mass production and is comparable to a cold padding approach. There were four dosing motors, an immersion tank, squeezing rollers, input and output rollers for fabric, and rollers for applying pressure to the wet fabric. The immersion tank was receiving a mixture of chemicals and water that had passed through a heat exchanger with a temperature range of 18 °C–22 °C. A frame (batcher) was used to catch the fabric after it had passed through the immersion tank. In order to prevent any air from coming into contact with the fabric, the batcher was covering it with plastic. After that, depending on the proportion of the fabric's shade, the cloth was rotated for 4–6 h.
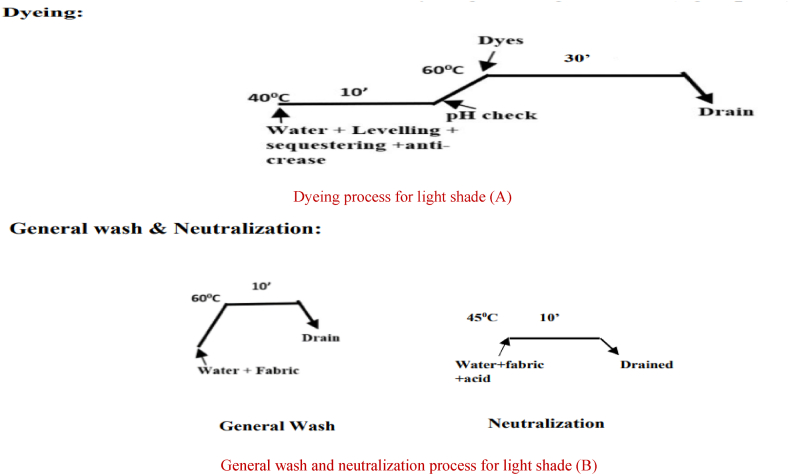
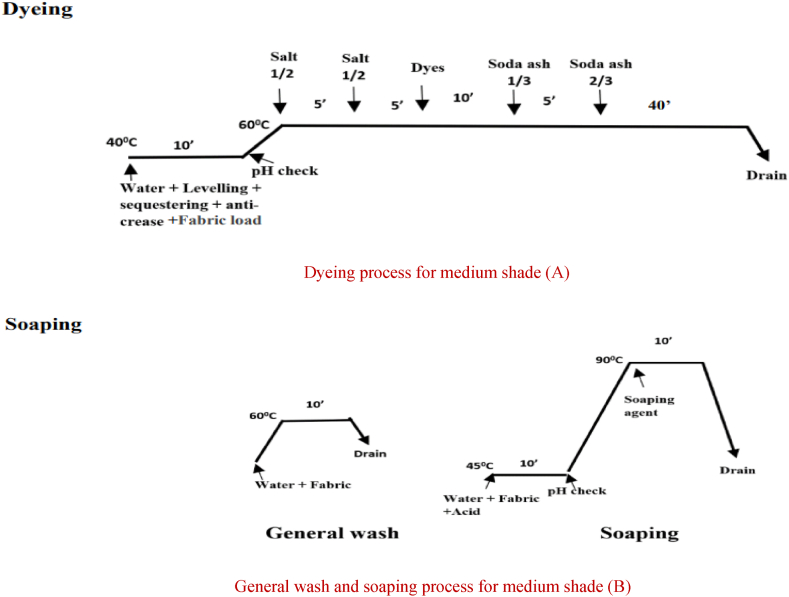
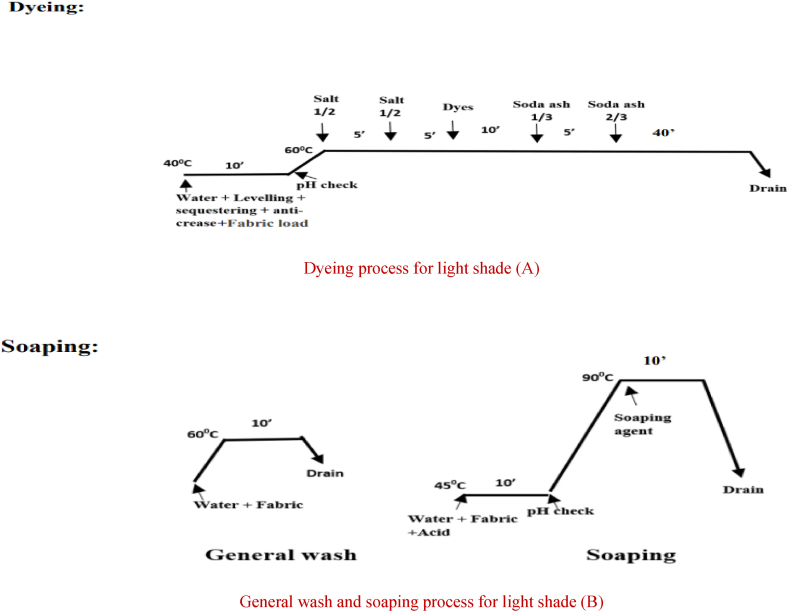
After that, anti-creasing and leveling agents were applied to the water in the lab dyeing machine before the fabric was dyed a dark color. The fabric was then loaded and ran for 10 min at 40 °C. The pH was then measured, and the temperature was then elevated to 60 °C. After adding the dyes, the bath was agitated for 10 min. Liquid alkali was added for fixation, and it was let to run for 30 min. The dye bath water was drained after that. It used to take 10 min of acid neutralization at 45 °C to remove dye from fabric. The sample was then washed twice. First, the sample was washed in water for 10 min at 60° Celsius. Next, soaping was used to wash the colored samples for 10 min at 75° Celsius and then water was drained. The same process was used for medium and light shades without the addition of liquid alkali for fixing. The alkali that was used to cationize the cotton cloth triggered and completed the fixing process for dark, medium and light shade shown in Fig. 3, Fig. 4, Fig. 5.
Fig. 3.
Dyeing, general wash and soaping process of cationized fabric for dark shade (Black)
Dyeing process for dark shade (A)
General wash and soaping process for dark shade (B).
Fig. 4.
Dyeing, general wash and soaping process of cationized fabric for medium shade (Hot Pink)
Dyeing process for medium shade (A)
General wash and soaping process for medium shade (B).
Fig. 5.
Dyeing, general wash and neutralization process of cationized fabric for light shade (Light Pink)
Dyeing process for light shade (A)
General wash and neutralization process for light shade (B).
2.2.3. Regular dyeing process of cotton fabric using salt
Anti-creasing and leveling ingredients were added to the water in the lab dyeing machine when dyeing bleached cotton cloth using a traditional dyeing procedure with a dark color. The fabric was then loaded and heated to 40° for 10 min. After measuring the pH, the temperature was raised to 60 °C. After adding gluber salt, the machine ran for 10 min. The colors were added, and the bath was stirred for 10 min. Two additions of soda ash were made for fixing. Mild alkali was introduced first and run for 10 min; the remaining alkali was then added and run for 40 min the dye bath's water was drained. After that, the sample was rinsed three times. The sample was first cleaned in water heated to 60° Celsius for 10 min. The colored sample was then twice soaped for 10 min at 90° Celsius and drained. Finally, at pH 4.5 fixing chemical was applied to the cloth and treated for 10 min at 45 °C before the water was drained. For medium and light, the sample underwent two rinses. The sample was first washed for 10 min in water that had been heated to 60° Celsius. The colored sample was then drained after being soaped for 10 min at 90° Celsius. No fixing agent was utilized in this instance to set the dye, neither for the medium nor the light shades shown in Fig. 6, Fig. 7, Fig. 8.
Fig. 6.
Dyeing, general wash, soaping and fixing process of conventional colored fabric for dark shade (Black)
Dyeing process for dark shade (A)
General wash and soaping process for dark shade (B)
Fixing process for dark shade (C).
Fig. 7.
Dyeing general wash and soaping process of conventional colored fabric for medium shade (Hot Pink)
Dyeing process for medium shade (A)
General wash and soaping process for medium shade (B).
Fig. 8.
Dyeing, general wash and soaping process of conventional colored fabric for light shade (Light Pink)
Dyeing process for light shade (A)
General wash and soaping process for light shade (B).
2.2.4. Color fastness to wash and rubbing
The dyed samples from both processes were cut into precise measurements and stitched to many fibers to test the color fastness to washing. After that, washing is carried out using a specialized ISO 105C06 procedure. The samples were then visually evaluated using grey scales for shade shift and staining intensity. It was decided to use the ISO 105 X 12:1993 method to test color fastness to rubbing. Samples were inserted under the finger into the crock meter and then rubbed 5 cm in either direction. 10 strokes in 10 s on a dry crocking cloth followed by 10 strokes on a wet (100% pickup) crocking fabric. The fabric is then examined using a grey scale to measure the shade shift.
2.2.5. Evaluation of colored samples using spectrophotometry
Delta E (or E), denoting the shift in color, was calculated using the three-dimensional axes L a b and the Hunter Lab color space. An ellipse with every recognized hue was produced by the ratio of opposite combinations. The % of a specific color that was red, green, yellow, or blue as well as how light or dark it was, as an alternative. For colors that were discussed more frequently, the Delta E was lower. Color was measured in L, a, and b using a Spectrophotometer and the Hunter Lab color scale. The axis was scaled from left to right, starting with all green. One unit of green was swapped out for one unit of red when the composition shifted to the right. As one moved to the right, more red replaced more green. It was fully red at the far right. It started out as merely green drops. One drop of green was subtracted and one drop of red was added as it moved to the right. More and more green was being replaced by red as it swung to the right. The ratio of red to green in the container changed, but the number of droplets didn't. The square of each reading's distance had to be done (to assure a positive number) in order to compute the Delta E variation from the produced color to the target. The Delta E variance was then calculated by adding together all of the squares of those readings and taking the square root of that amount (Fig. 9) [26].
Fig. 9.
CIE Lab color space-color opponent model [26].
2.2.6. Measurement of bursting strength
The flexible diaphragm used in the ISO 139308-1 standard kind of bursting strength test was used to stack the fabric, and the pressure in the fabric was determined by measuring how much liquid was behind the diaphragm. Through a circular clipping ring, a test specimen was clipped over a long diaphragm. The fabric and the diaphragm were stretched as a result of expanding fluid strain applied to the underside of the diaphragm. Up until the test example blows, the fluid volume was expanded at a constant rate for each unit of time. Additionally, the bursting distension and bursting strength were assessed.
2.2.7. Scanning electron microscope (SEM)
A strong beam of high-energy electrons is used by the scanning electron microscope (SEM) to produce a variety of indications at the surface of solid materials. The signals from the electron-sample interaction reveal information about the sample's organic structure, relative orientation of its component parts, and exterior texture [27].
2.2.8. Fourier transform infrared Radiation (FTIR)
The FTIR employs interferometry to record data about a material placed in an IR beam. The Fourier transform produces spectra that analysts can use to identify the material. Spectral patterns aid in sample identification since molecules have unique IR fingerprints. The Fourier transform converts the detector output into an interpretable spectrum. The FTIR produces spectra with patterns that reveal structural information [28].
2.2.9. Salt-free and traditional dye bath discharge water
Tests on the biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS), and dissolved oxygen (DO) were conducted to evaluate the dye bath discharge water of both the salt-free and traditional dyeing processes. To decide on the dye bath discharge water, the visual assessment dye bath water for both processes was also 2 assessed (Table 1).
Table 1.
Acceptable range of ETP water [29].
| Parameters | Range |
|---|---|
| pH | 5.5–9 |
| Electric Conductivity (EC) | <1800 |
| Dissolve Oxygen (DO) | 4.5–8 |
| Chemical Oxygen Demand (COD) | <250 |
| Biological Oxygen Demand (BOD) | <120 |
| Total Dissolve Solids (TDS) | <1900 |
2.2.10. Water consumption and cost analysis
One of the most essential ingredients for the dyeing industry is water. It is difficult to think or operate any form of factory without water. The water layer is gradually eroding away. Finding a dyeing method that used less water was therefore necessary. Six colors' water usage statistics were collected and analyzed for both cationic and traditional dyeing processes. Cost is regarded as a key consideration for any business. Profitability is the primary consideration in our nation, regardless of the technology or procedure used. As a result, the whole process cost of a specific process must be justified before any technology is proposed. Both salt-free and conventional dyeing methods were used to color six different samples.
3. Results and discussion
3.1. Assessment of color fastness to wash
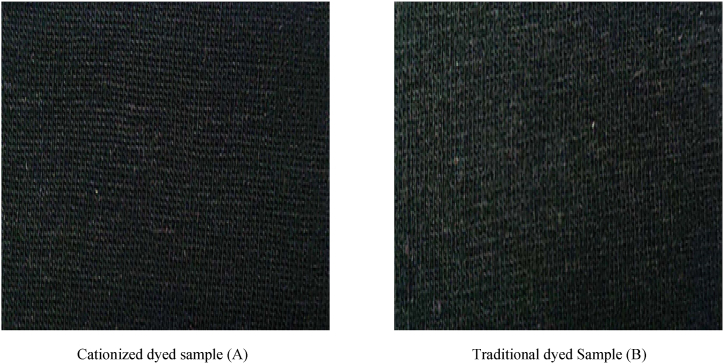
The shade change rating on a grey scale was shown in Table 2 for samples of regular dyed cloth and cationized cotton colored fabric. Black (dark shade), hot pink (mid shade), and light pink (light shade) were among the hues that were put to the test. The standard black colored cloth and the materials used to measure color differences both had a minor to noticeable loss in depth (rating of 3–4), which was a good to fair outcome. The black dyed fabric that had been cationized had a minor reduction in depth (rating of 4), which was a positive outcome. The usual dyed cloth for the hot pink samples had a tiny decrease in depth (rating of 4), which was a satisfactory outcome. The cationized cotton's depth (rating of 4–5) showed no change or a minor loss, which indicated excellent to good results. For light pink samples, the standard colored cloth showed outstanding to good results with no change or a minor loss in depth (rating of 4–5). This was a fantastic outcome for the cationized cotton, which showed no alteration (rating of 5). Better results were seen for all three shades of cationized colored fabric with compared to regular dyed fabric in Fig. 10, Fig. 11, Fig. 12.
Table 2.
The grey scale rating for color fastness to wash of regular and cationized cotton dyed samples.
|
Color |
Dyed fabric Samples |
Color Staining |
Shade Change | |||||
|---|---|---|---|---|---|---|---|---|
| Polyester | Cotton | Nylon | Acrylic | Acetate | Wool | |||
| Black (Dark shade) | Regular | 4–5 | 3 | 3–4 | 3–4 | 4–5 | 4 | 3–4 |
| Cationized cotton | 4–5 | 3–4 | 4 | 4 | 5 | 4 | 4 | |
| Hot pink (Medium shade) | Regular | 5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4 |
| Cationized cotton | 5 | 4–5 | 5 | 5 | 5 | 5 | 4–5 | |
| Light pink (Light shade) | Regular | 5 | 4–5 | 5 | 4–5 | 5 | 4–5 | 4–5 |
| Cationized cotton | 5 | 5 | 5 | 5 | 5 | 4–5 | 5 | |
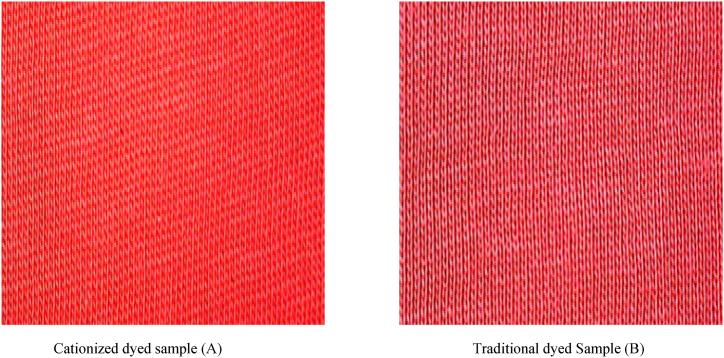
Fig. 10.
Cationized and traditional dyed samples for dark shade
Cationized dyed sample (A) Traditional dyed Sample (B).
Fig. 11.
Cationized and traditional dyed samples for medium shade
Cationized dyed sample (A) Traditional dyed Sample (B).
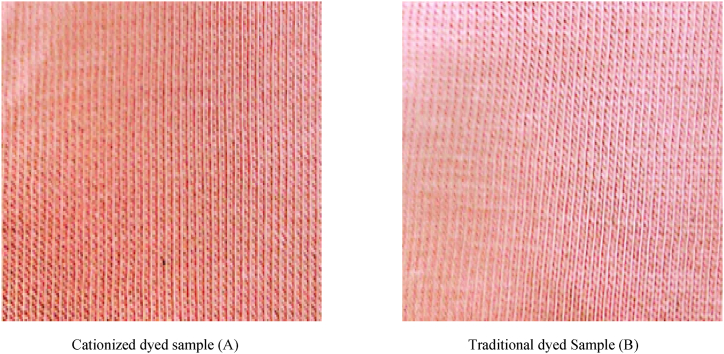
Fig. 12.
Cationized and traditional dyed samples for light shade
Cationized dyed sample (A) Traditional dyed Sample (B).
According to samples of both regularly dyed and cationized cotton colored fabric, Table 2 shows the grey scale scores for color stains on multifiber fabrics. The findings were usually great, with only a small discrepancy between the two fabric samples. Despite this, the data table demonstrates that cationized dyed fabric outperformed ordinary dyed fabric significantly for dark and medium hues. Because cationized fabric typically had a stronger affinity for the dyes than non-cationized fabric did. Cationized colored fabric had a higher dye pick up % than non-cationized dyed fabric as a result [30].
3.2. Assessment of color fastness to rubbing
The shade changes caused by the rubbing of regularly dyed cloth and cationized fabric are rated on a grey scale in Table 3. On cloth that had been dyed black, the findings of the tests for fastness on both dried and wet fabric samples were very dissimilar. The dry rubbing scores for the dark shade for samples of ordinary dyed fabric and samples of cationized dyed fabric were 4 (minor loss in depth) and 4–5 (no change to slight loss), respectively. The wet rubbing scores, however, were 2–4 (slight loss in depth to considerable loss in depth). Similar results were found for light pink, which had values of 5 (no change) and 4–5 (no change to small loss) for both dried and wet rubbing. Therefore, the color fastness test using rubbing demonstrated that cationized cotton colored fabric differs significantly from conventional dyed fabric for both dried and wet rubbing when the color was black (dark shade), and that the difference for the color hot pink (medium shade) was considerable. Light pink (light shade) showed no difference in this dried and wet rubbing test. It is possible to explain the resemblance between cationic dyeing and conventional light pink dyeing methods by the fact that a specific dye or dyeing method is not necessarily required to achieve a light shade of pink. The factors of the dyeing environment, such as temperature and length, as well as the concentration and combination of the dye's constituents, are more significant. As a result, in the case of light pink coloring, the colour fastness to rubbing between cationic dyeing and traditional dyeing processes might not have a significant effect [31]. Regular fabric often makes covalent bonds with the dyes, but cationized fabric typically develops both ionic and covalent bonds. As a result, the ionic bonds were stronger and generated excellent results in the rubbing test compared to the covalent bond of regularly dyed cloth found in Fig. 10, Fig. 11, Fig. 12 [32].
Table 3.
The grey scale rating for color fastness to rubbing of regular and cationized cotton dyed samples.
| Color | Dyed fabric Samples | Dry Rubbing | Wet Rubbing |
|---|---|---|---|
| Black (Dark shade) | Regular | 4 | 2–3 |
| Cationized cotton | 4–5 | 4 | |
| Hot pink (Medium shade) | Regular | 4–5 | 4 |
| Cationized cotton | 4–5 | 4–5 | |
| Light pink (Light shade) | Regular | 5 | 4–5 |
| Cationized cotton | 5 | 4–5 |
3.3. Spectrophotometry evaluation of dyed samples
The spectrophotometric assessment of the color differences between the samples of conventional dyed fabric and the samples of cationized dyed fabric was performed, and deviations were computed using the CIE L*a*b* color system with E or DE values. Light sources D65 and msTL84 or A were used.
Fig. 13 compares cationized materials colored with a dark hue to typical dyed samples under several light sources, including D65 and A. Traditional dyed fabrics had greater DL* values than cationized dyed textiles, indicating that they were lighter. In comparison to the other negative values—namely, Da*, Db*, and Dc*—the conventional colored samples of dark shade were less reddish, yellowish, and bright in tone. For light sources D65 and A, total colour deviations were DE 4.41 and DE 4.36, respectively. Because the cationized dyeing technique binds colors to the fabric through both ionic and covalent interactions, resulting in a higher proportion of dye uptake than the standard dyeing mechanism, the ordinary black colored cloth was lighter than the cationized black colored cloth. Using the same number of dyes on cationized dark colored fabric, a darker colour was discovered (Table 4) [33].
Fig. 13.
The bar diagram of regular black dyed fabric sample under different light sources.
Table 4.
The spectrophotometric evaluation of Regular dyed fabric samples for black (dark shade).
| Samples (Color) | Light source | DL* | Da* | Db* | Dc* | DE |
|---|---|---|---|---|---|---|
| Black (Dark shade) | D65 | 7.49 | −1.28 | −3.14 | −1.05 | 4.41 |
| A | 6.98 | −1.81 | −3.83 | −2.43 | 4.36 |
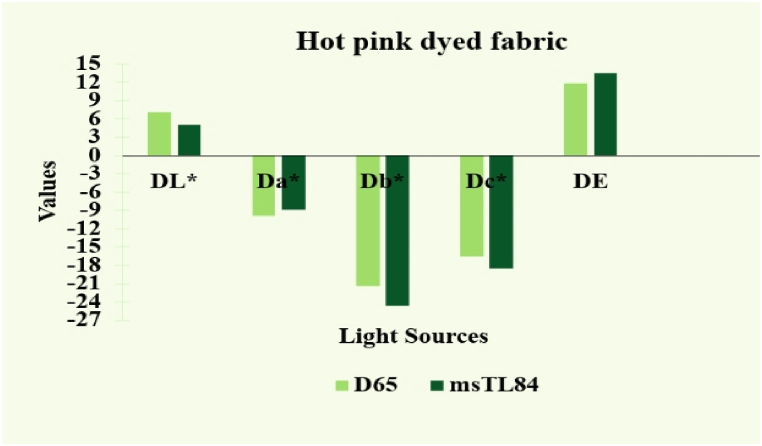
Fig. 14 compares cationized materials dyed with a medium shade to conventional dyed samples under various light sources, including D65 and MsTL84. Traditional dyed samples had higher DL* values than cationized dyed textiles, indicating that they were lighter. In contrast to the other negative values—namely, Da*, Db*, and Dc*—the traditional colored samples of medium shade were less reddish, yellowish, and less bright in tone. Total colour variations were DE 11.88 and DE 13.47 for light sources D65 and MsTL84, respectively. The ordinary hot pink colored cloth was lighter than the cationized hot pink colored cloth because the cationized dyeing technique attaches colors to the fabric through both ionic and covalent interactions, resulting in a higher proportion of dye uptake than the normal dyeing mechanism. Using the same amount of dyes, a richer colour was discovered on cationized medium colored fabric (Table 5). [33].
Fig. 14.
The bar diagram of regular Hot pink dyed fabric sample under different light source.
Table 5.
The spectrophotometric evaluation of Regular dyed fabric samples for hot pink (medium shade).
| Samples (Color) | Light source | DL* | Da* | Db* | Dc* | DE |
|---|---|---|---|---|---|---|
| Hot pink (Medium shade) | D65 | 7.05 | −9.90 | −21.41 | −16.56 | 11.88 |
| msTL84 | 5.05 | −8.85 | −24.54 | −18.49 | 13.47 |
Fig. 15 shows a comparison of cationized materials dyed with a light shade to traditional dyed samples under different light sources, including D65 and MsTL84. Traditional dyed fabrics had greater DL* values than cationized dyed textiles, implying that they were lighter. In contrast to the other negative values (Da*, Db*, and Dc*), the traditional colored medium shade samples were less reddish, yellowish, and bright in tone. For light sources D65 and MsTL84, total colour deviations were DE 0.94 and DE 0.97, respectively. The regular light pink colored cloth was lighter than the cationized light pink colored cloth because the cationized dyeing approach attaches colors to the fabric through both ionic and covalent interactions, resulting in a higher proportion of dye uptake than the normal dyeing mechanism. On cationized light colored fabric, a deeper colour was discovered using the same amount of dyes (Table 6). [33].
Fig. 15.
The bar diagram of regular Light pink dyed fabric sample under different light sources.
Table 6.
The spectrophotometric evaluation of Regular dyed fabric samples for light pink (light shade).
| Samples (Color) | Light source | DL* | Da* | Db* | Dc* | DE |
|---|---|---|---|---|---|---|
| Light pink (Light shade) | D65 | 0.58 | −1.69 | −0.09 | −1.63 | 0.94 |
| msTL84 | 0.51 | −1.73 | −0.17 | −1.66 | 0.97 |
3.4. Assessment of bursting strength
The bursting strengths of traditional black, hot pink, and light pink dyed fabrics were 260 kPa, 320 kPa, and 387 kPa, respectively in Fig. 16. Cationized cotton black, hot pink, and light pink fabrics had bursting strengths of 287 kPa, 337 kPa, and 440 kPa, correspondingly. The graph unmistakably demonstrates that of the two fabric samples, the light pink cloth had the highest bursting strength when compared to the black fabric. Black and hot pink had discrepancies between ordinary colored and cationized cotton dyed fabrics that were under 20 kPa and under 30 kPa, respectively. However, the difference for light pink was noticeable because it was greater than 50 kPa. Compared to black and hot pink fabric, light pink fabric has the highest bursting strength. This is probably because light-colored fabrics, whether cationized or conventionally dyed, go through a less severe dyeing procedure than standard fabrics and because lighter samples need less chemicals to dye than darker ones [34].
Fig. 16.
Graphical representation of bursting strength test between regular and cationic dyed samples.
3.5. Scanning electron microscope (SEM) analysis
The microstructure of both the ordinary dyed cloth and the cationized dyed fabric can be seen in the Scanning Electron Microscopy (SEM) images in Fig. 8 (500x and 2000x). Dye dispersion and dye-fiber adhesion between cationized and traditional reactive dyed samples can be evaluated using SEM images. This information was critical for determining the dyeing process's effectiveness and colour fastness. In comparison to traditional dyed samples, SEM pictures revealed a consistent dispersion of dye on the surface of the cationized fibres. It was owing to the formation of positive charges on the fibre surface using a cationic chemical, which increased attraction for negatively charged cotton fibres. According to the SEM pictures in Figure [17(A,B,C,D)], the cationized colored samples displayed consistent dye dispersion, higher adherence, and no dye anomalies when compared to conventional dyed samples [35].
Fig. 17.
SEM images (500x and 2000x) of the dark shade between cationic dyed samples and regular dyed samples
SEM image (500x) of cationic dyed samples (A) SEM image (500x) of regular dyed samples (B)
SEM image (2000x) of cationic dyed samples (C) SEM image (2000x) of regular dyed samples (D).
3.6. Fourier transform infrared Radiation (FTIR) analysis
Fig. 18 is a graphical representation of the infrared spectrum of the dark shade of cationic dyed samples, where the vertical axis represents the percentage of transmittance and the horizontal axis represents the wave number. Here several significant peaks were visible in the infrared spectrum. One peak indicated C–H stretching, with a peak point of roughly 2900-3000 cm-1, while another represented O–H stretching, with a peak point of around 3300 cm-1. The other peaks reflected N–H bending, C–N stretching, and C O stretching about 1550–1560 cm-1 and 1650-1670 cm-1, respectively. Thus, the existence of reactive dyes in the cationized sample was confirmed by FTIR analysis [36].
Fig. 18.
The infrared spectrum of the dark shade of cationic dyed samples.
3.7. Analyses between salt-free and traditional dye bath discharge water
It can be seen from Table 7, Table 8 that the average water test result value for cationic dyeing water was better than usual. This variation was mostly brought on by the use of salt. The environment's increasingly bad water quality was caused by salt. It raised the EC, BOD, COD, TDS, and other parameters. As BOD and COD grew, the amount of dissolved oxygen available to aerobic species like fish and other aquatic organisms dropped. It put the life of the aerobic creature in danger. Since the water quality was good for cationic dyeing, less chemical was needed for treatment and utilities were only needed to convert the water into an appropriate range limit, saving the ETP cost. Because cationized dyed samples have a significant attraction to anionic dyes, they were able to absorb more dye molecules from the dye bath. More dye molecules reacted with the fiber as a result. Salt was employed to boost the anionic dyes' affinity because conventional dyeing had a lower affinity, although the rise was just average or modified. The remaining dye molecules are wasted because it can only react with 60–65% of them. More than 80–85% of the colors in the bath can be absorbed by the cationic technique, resulting in reduced dye waste, less effluent, and ultimately, cost savings [37].
Table 7.
Analytical report of regular dye bath discharge water (dark shade).
| Stage | pH | TDS (mg/l) | EC (μs/cm) | DO (mg/l) | COD (mg/l) | BOD5 (mg/l) |
|---|---|---|---|---|---|---|
| Bleaching | 11.3 | 2980 | 5130 | 7.65 | 1317 | 610 |
| Dye bath | 11.8 | 9780 | 13820 | 2.69 | 1842 | 870 |
| Bath drain | 10.7 | 4740 | 8540 | 3.76 | 1354 | 590 |
| Hot Wash | 8.5 | 3620 | 4120 | 4.15 | 1017 | 460 |
| Soaping 1 | 7.6 | 1910 | 1760 | 4.53 | 823 | 370 |
| Soaping 2 | 7.3 | 1150 | 930 | 5.09 | 497 | 210 |
Table 8.
Analytical report of cationized dye bath discharge water (dark shade).
| Stage | pH | TDS (mg/l) | EC (μs/cm) | DO (mg/l) | COD (mg/l) | BOD (mg/l) |
|---|---|---|---|---|---|---|
| Bleaching | 10.2 | 2010 | 4010 | 6.24 | 596 | 240 |
| Dye bath | 10.9 | 3650 | 6300 | 6.89 | 1186 | 480 |
| Bath drain | 9.4 | 1940 | 3140 | 7.65 | 923 | 320 |
| Hot wash | 8.23 | 1060 | 1120 | 6.39 | 527 | 230 |
| After soaping | 7.3 | 540 | 490 | 6.90 | 319 | 140 |
3.7.1. Biological oxygen demand (BOD)
Since high levels of BOD show that the water is considerably contaminated with organic pollutants, including sewage or agricultural runoff, which could induce eutrophication and be hazardous to aquatic life, they are a crucial indication of the health of waterbodies. Table 9 showed that the BOD value of a regular colored water sample was 45% higher than that of a cationized dyed water sample. For aquatic life, ordinary colored water was thus 45% more dangerous [37].
Table 9.
Average waste water reduction.
| Title | pH | TDS (mg/l) | EC (μs/cm) | DO (mg/l) | COD (mg/l) | BOD(mg/l) |
|---|---|---|---|---|---|---|
| Regular dye bath water | 9.54 | 4030 | 5716 | 4.65 | 1142 | 518 |
| Cationized dye bath water | 9.2 | 1840 | 3012 | 6.82 | 710 | 282 |
| Reduction | 54% | 47% | 46% | 39% | 45% |
3.7.2. Chemical oxygen demand (COD)
The COD measures the amount of oxidized organic and inorganic components in water. It acts as a gauge for the degree of contaminant buildup in water bodies as well as the efficiency of wastewater treatment techniques. Table 9 showed that the COD value of a regular colored water sample was 39% greater than that of a cationized dyed water sample. In other words, naturally colored water was 39% riskier for aquatic life [37].
3.7.3. Total dissolved solids (TDS)
The total concentration of all inorganic and organic components contained in a liquid, most typically water, is referred to as TDS. Minerals, salts, metals, and organic molecules that have dissolved in the water all contain these elements. Table 9 shows that the overall TDS reduction between catonized colored water and ordinary colored water was 54%. Due to the large amount of salt utilized during this method, the TDS value was greater in conventional dyeing water. This salt dissolves as a solid in the water, increasing the salinity and making the water uninhabitable for aquatic life [37].
3.7.4. Dissolved oxygen (DO)
Dissolved oxygen (DO) is the term for the amount of oxygen that was either dissolved in the water or that was readily available to aquatic life. For high-quality water, a sufficient level of dissolved oxygen was necessary. When the water's dissolved oxygen content dropped below 5.0 mg/l, aquatic life became vulnerable. Healthy water should normally have dissolved oxygen concentrations more than 6.5–8 mg/l. Table 9 shows that the cationized dyed water value was 6.39 mg/l, which was within the acceptable limit, and that the ordinary dyed water did value was 3.34 mg/l, which was below standard. Here, the testing result for cationized colored water is nearly 100% better than that for conventional dyed water.water that is too salty for aquatic life [37].
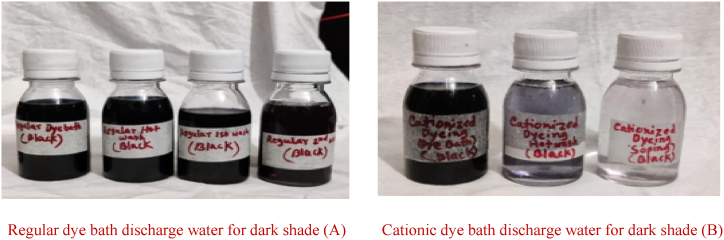
3.7.5. Visual assessment of dye bath discharge water
Fig. 19, Fig. 20, Fig. 21 showed that, for dark, medium, and light shades, the water in the cationic water bottles was clearer than that in the conventional ones. This occurred as a result of the fabric being cationized, which absorbed additional color molecules from the dye solution and strengthened covalent bonds. As a result, less unfixed or poorly attached colour molecules scaled off after washing than in ordinary fabric. After the dyeing, hot washing, and soaping were finished, the water was cleaner than it was in standard fabric.
Fig. 19.
Visual assessment of the dark shade between regular and cationic dye bath discharge water
Regular dye bath discharge water for dark shade (A) Cationic dye bath discharge water for dark shade (B).
Fig. 20.
Visual assessment of the medium shade between regular and cationic dye bath discharge water
Regular dye bath discharge water for medium shade (A) Cationic dye bath discharge water for medium shade (B).
Fig. 21.
Visual assessment of the light shade between regular and cationic dye bath discharge water
Regular dye bath discharge water for light shade (A) Cationic dye bath discharge water for light shade (B).
3.7.6. Analysis of water consumption between cationic and regular dyeing process
One of the most essential ingredients for the dyeing industry is water. It is difficult to think or operate any form of factory without water. The water layer is gradually eroding away. Therefore, a less water-intensive dyeing procedure must be developed. Table 10 shows that less water was required for the cationic process than for the conventional approach. This occurred as a result of the procedure being shorter, requiring fewer washes after dyeing, and using less liquor than usual. Water savings are also a result of the removal of fixing steps. Because washing darker colors required more wash stages, double soaping, and a higher liquor ratio than washing lighter shades (such as Pink/Yucca), darker shades (like Black) result in superior water savings than lighter shades (such as Pink/Yucca). However, cationic dyeing did not require any additional washes or a higher liquid ratio than conventional dyeing. As a result, the conventional method required more water than the cationic process did [38].
Table 10.
Analysis of water consumption between cationic and regular dyeing process.
| Color Name | Water required for cationic dyeing process (liter/kg) | Water required for regular dyeing process (liter/kg) | Remark |
|---|---|---|---|
| Black | 57 L/kg | 89 L/kg | 37% less water consumption in cationic dyeing process |
| Black | 54 L/kg | 85 L/kg | 36% less water consumption in cationic dyeing process |
| Wine | 51 L/kg | 72 L/kg | 27% less water consumption in cationic dyeing process |
| Navy | 50 L/kg | 69 L/kg | 28% less water consumption in cationic dyeing process |
| Pink | 41 L/kg | 53 L/kg | 23% less water consumption in cationic dyeing process |
| Yucca | 44 L/kg | 55 L/kg | 20% less water consumption in cationic dyeing process |
3.7.7. Cost comparison of the cationic and conventional dyeing processes
Cost is actually regarded as a key component for any business. Profitability is the first priority in any business, regardless of the technology or procedure used. As a result, the whole process cost of a specific process must be justified before any technology is proposed. It is evident from Table 11 that the overall cost of the cationic dyeing process is lower than the Regular. It is because less chemicals are consumed, fewer resources are used, the process takes place faster, and fewer dyes are required to get the desired shade. Less dye is required because there is less dye waste or less dye that has been hydrolyzed (Table 11).
Table 11.
Cost comparison between cationic and regular dyeing process.
| Color | Cost Of Chemical (BDT)/Kg of Fabric |
Cost Of Dyes (BDT)/Kg of Fabric |
Cost Of Utility (BDT)/Kg of fabric |
Total Cost (BDT) |
||||
|---|---|---|---|---|---|---|---|---|
| Cationic Dyeing | Regular Dyeing | Cationic Dyeing | Regular Dyeing | Cationic Dyeing | Regular Dyeing | Cationic Dyeing | Regular Dyeing | |
| Black | 28.07 | 32.86 | 29.83 | 34.98 | 5.34 | 9.66 | 63.24 | 77.5 |
| Black | 28.07 | 32.86 | 28.66 | 31.67 | 5.62 | 9.83 | 62.35 | 74.36 |
| Hot Pink | 25.24 | 28.06 | 19.02 | 21.68 | 5.29 | 8.36 | 49.55 | 58.1 |
| Hot Pink | 25.24 | 28.06 | 14.87 | 17.64 | 5.07 | 8.17 | 45.18 | 53.87 |
| Light Pink | 19.31 | 21.87 | 6.98 | 8.03 | 4.83 | 6.91 | 31.12 | 36.81 |
| Light Pink | 19.31 | 21.87 | 2.87 | 3.98 | 4.61 | 6.12 | 26.79 | 31.97 |
3.7.8. Key findings
The environmentally friendly cationic coloring technique eliminated the need for salt as an electrolyte. For the cationic coloring wash-off process, less water was required. The results of the Spectrophotometer test demonstrated that the highest level of dye fixing was accomplished using the cationic dyeing method. Water pollution was least evident in the test results for cationic dye bath discharge water. When the dye bath water was visually inspected using the cationic dyeing procedure, there was little dye hydrolysis. The cationic dyeing process significantly lowers costs. The sustainable cationic dyeing process places a high priority on environmental protection.
3.7.9. Limitations
The cost savings for light shade cationized dyed samples are not as significant as those for medium shade and dark shade. Furthermore, if the batching or rotating time is increased when using a cationic dyeing procedure, the fabric's strength may suffer.
4. Conclusion
People are now more concerned than ever with protecting the environment, and the textile business is transforming as a result of customer demand for eco-friendly goods. The environment is severely harmed by the textile industry's regular dyeing process, but the harm can be reduced by adopting the cationization method. The amount of alkali, chemicals, and salts saved by making the fabric cationic prior to dyeing is significant, and the dyeing time is also reduced. Compared to the regular technique, this one improves productivity, reduces costs, and uses less color. Because only 50–60% of the dye in the regular cotton dyeing process can be absorbed, the remaining dye is wasted, and the BOD, COD, and TDS values are better than those of the regular dyeing method, which is beneficial for aquatic life. The results of the tests comparing the cationic and regular dyeing processes shown how the cationic method is superior to the regular. Even while the cationization procedure yields superior results, this is only true for the deep hues; the outcome for light shades is less than ideal. But in the near future, it might be resolved, and we might fully transition to a sustainable textile dyeing sector.
Declarations
Author contribution statement
Joyjit Ghosh*
Conceived and designed the experiments; Performed the experiments; Analyzed
and interpreted the data and Wrote the paper.
Nishat Sarmin Rupanty:
Analyzed and interpreted the data; Contributed reagents, materials, analysis tools
or data and Wrote the paper.
5. Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Joyjit Ghosh, Email: joyjit.te@aust.edu.
Nishat Sarmin Rupanty, Email: sharminnishat489@gmail.com.
References
- 1.Rattee I.D. Reactive dyes for cellulose 1953–1983. Rev. Prog. Coloration Relat. Top. 1984 Jun;14(1):50–57. [Google Scholar]
- 2.Lewis D.M., Vo L.T. Dyeing cotton with reactive dyes under neutral conditions. Color. Technol. 2007 Oct;123(5):306–311. [Google Scholar]
- 3.Ristic N., Ristic I. Cationic modification of cotton fabrics and reactive dyeing characteristics. Journal of Engineered fibers and fabrics. 2012 Dec;7(4) [Google Scholar]
- 4.Ma W., Jz Y. The Proceedings of the 3rd International Conference on Functional Molecules. 2002. Development of functional polymers in modification of cotton for improving dyeability of reactive dyes. [Google Scholar]
- 5.Periyasamy A.P., Dhurai B., Thangamani K. Salt-free dyeing–a new method of dyeing on lyocell/cotton blended fabrics with reactive dyes. Autex Res. J. 2011 Mar 1;11(1):14–17. [Google Scholar]
- 6.Hessel C., Allegre C., Maisseu M., Charbit F., Moulin P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007 Apr 1;83(2):171–180. doi: 10.1016/j.jenvman.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Allègre C., Moulin P., Maisseu M., Charbit F. Treatment and reuse of reactive dyeing effluents. J. Membr. Sci. 2006 Feb 1;269(1–2):15–34. [Google Scholar]
- 8.Burkinshaw S.M., Salihu G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 6 analysis of conventional models that describe the manner by which inorganic electrolytes promote reactive dye uptake on cellulosic fibres. Dyes Pigments. 2019 Feb 1;161:595–604. [Google Scholar]
- 9.Burkinshaw S.M., Kabambe O. Attempts to reduce water and chemical usage in the removal of bifunctional reactive dyes from cotton: Part 2 bis (vinyl sulfone), aminochlorotriazine/vinyl sulfone and bis (aminochlorotriazine/vinyl sulfone) dyes. Dyes Pigments. 2011 Feb 1;88(2):220–229. [Google Scholar]
- 10.Burkinshaw S.M., Kabambe O. Attempts to reduce water and chemical usage in the removal of reactive dyes: Part 1 bis (aminochlorotriazine) dyes. Dyes Pigments. 2009 Dec 1;83(3):363–374. [Google Scholar]
- 11.Nieminen E., Linke M., Tobler M., Vander Beke B. EU COST Action 628: life cycle assessment (LCA) of textile products, eco-efficiency and definition of best available technology (BAT) of textile processing. J. Clean. Prod. 2007 Sep 1;15(13–14):1259–1270. [Google Scholar]
- 12.Jeong S., Lim J., Hong S.I., Kwon S.C., Shim J.Y., Yoo Y., Cho H., Lim S., Kim J. A framework for environmental production of textile dyeing process using novel exhaustion-rate meter and multi-layer perceptron-based prediction model. Process Saf. Environ. Protect. 2023 Jul 1;175:99–110. [Google Scholar]
- 13.Khatri A., Peerzada M.H., Mohsin M., White M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015 Jan 15;87:50–57. [Google Scholar]
- 14.Niu T., Wu Y., Zhai X., Sun D., Fang L., Zhang X. Investigation on multifunctional modification of cotton fabrics for salt-free dyeing, resisting crease and inhibiting bacteria. Colloids Surf. A Physicochem. Eng. Asp. 2022 Sep 5;648 [Google Scholar]
- 15.Acharya S., Abidi N., Rajbhandari R., Meulewaeter F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose. 2014 Dec;21:4693–4706. [Google Scholar]
- 16.Ab Kadir M.I., Ahmad M.R., Ismail A. The effect OF CATIONIC SURFACTANT treatment ON the DYEABILITY OF COTTON and silk fabrics with natural dye from BROWN SEAWEEDS SARGASSUM SP. Int. J. Appl. Nat. Sci. 2016;5:19–28. [Google Scholar]
- 17.An Y., Ma J., Zhu Z., Hu M.G., Shao J. Study on a water-saving and salt-free reactive dyeing of cotton fabrics in non-aqueous medium of liquid paraffin system. J. Text. Inst. 2020 Oct 2;111(10):1538–1545. [Google Scholar]
- 18.Wang A., Sheng D., Zhang C., Gong J., Fu Z., Wang Y., Li W., Xia L., Xu W. Salt-free reactive dyeing of cotton fibers in a ternary solvent system with different reactive dye chemistries. Cellulose. 2023 Jan;30(1):463–479. [Google Scholar]
- 19.Kannan M.S., Gobalakrishnan M., Kumaravel S., Nithyanadan R., Rajashankar K.J., Vadicherala T. Influence of cationization of cotton on reactive dyeing. Journal of Textile and Apparel, Technology and Management. 2006;5(2):1–6. [Google Scholar]
- 20.Binet F., Coste-Manière I., Decombes C., Grasselli Y., Ouedermi D., Ramchandani M. Fast fashion and sustainable consumption. Fast fashion, fashion brands and sustainable consumption. 2019:19–35. [Google Scholar]
- 21.Nallathambi A., Rengaswami G.D. Industrial scale salt-free reactive dyeing of cationized cotton fabric with different reactive dye chemistry. Carbohydrate polymers. 2017 Oct 15;174:137–145. doi: 10.1016/j.carbpol.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Tarbuk A., Grancaric A.M., Leskovac M. Novel cotton cellulose by cationisation during the mercerisation process—Part 1: chemical and morphological changes. Cellulose. 2014 Jun;21:2167–2179. [Google Scholar]
- 23.Wang L., Ma W., Zhang S., Teng X., Yang J. Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydrate Polymers. 2009 Oct 15;78(3):602–608. [Google Scholar]
- 24.Correia J., Rainert K.T., Oliveira F.R., de Cássia Siqueira Curto Valle R., Valle J.A. Cationization of cotton fiber: an integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose. 2020 Oct;27:8527–8550. [Google Scholar]
- 25.Ren J.L., Sun R.C., Liu C.F., Chao Z.Y., Luo W. Two-step preparation and thermal characterization of cationic 2-hydroxypropyltrimethylammonium chloride hemicellulose polymers from sugarcane bagasse. Polym. Degrad. Stabil. 2006 Nov 1;91(11):2579–2587. [Google Scholar]
- 26.Farnand S.P. Using [Delta] E metrics for measuring color difference in hard copy pictorial images. InColor Imaging VIII: Processing, Hardcopy, and Applications. 2003 Jan 13;5008:109–118. SPIE. [Google Scholar]
- 27.Vernon-Parry K.D. Scanning electron microscopy: an introduction. III-Vs Rev. 2000;13(4):40–44. [Google Scholar]
- 28.Movasaghi Z., Rehman S., ur Rehman D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008 Feb 1;43(2):134–179. [Google Scholar]
- 29.McCarty L.S., Whitesides G.M. Electrostatic charging due to separation of ions at interfaces: contact electrification of ionic electrets. Angew. Chem. Int. Ed. 2008 Mar 7;47(12):2188–2207. doi: 10.1002/anie.200701812. [DOI] [PubMed] [Google Scholar]
- 30.Burkinshaw S.M., Howroyd J., Kumar N., Kabambe O. The wash-off of dyeings using interstitial water: Part 3. Disperse dyes on polyester. Dyes Pigments. 2011 Dec 1;91(3):340–349. [Google Scholar]
- 31.Ahmed H.B., Emam H.E., Mashaly H.M., Rehan M. Nanosilver leverage on reactive dyeing of cellulose fibers: color shading, color fastness and biocidal potentials. Carbohydrate polymers. 2018 Apr 15;186:310–320. doi: 10.1016/j.carbpol.2018.01.074. [DOI] [PubMed] [Google Scholar]
- 32.Bhuiyan M.R., Shaid A., Khan M.A. Cationization of cotton fiber by chitosan and its dyeing with reactive dye without salt. Chemical and materials engineering. 2014;2(4):96–100. [Google Scholar]
- 33.Viarengo A., Ponzano E., Dondero F., Fabbri R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar. Environ. Res. 1997 Jul 1;44(1):69–84. [Google Scholar]
- 34.Ertugrul S., Ucar N. Predicting bursting strength of cotton plain knitted fabrics using intelligent techniques. Textil. Res. J. 2000 Oct;70(10):845–851. [Google Scholar]
- 35.Tissera N.D., Wijesena R.N., de Silva K.N. Ultrasound energy to accelerate dye uptake and dye–fiber interaction of reactive dye on knitted cotton fabric at low temperatures. Ultrason. Sonochem. 2016 Mar 1;29:270–278. doi: 10.1016/j.ultsonch.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Hardinger S. sixth ed. Hayden-McNeil Publishing; Plymouth: 2013. Chemistry 14C: Organic Molecular Structures and Interactions; pp. 223–225. [Google Scholar]
- 37.Hessel C., Allegre C., Maisseu M., Charbit F., Moulin P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007 Apr 1;83(2):171–180. doi: 10.1016/j.jenvman.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Pei L., Luo Y., Saleem M.A., Wang J. Sustainable pilot scale reactive dyeing based on silicone oil for improving dye fixation and reducing discharges. J. Clean. Prod. 2021 Jan 10;279 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.