Abstract
The ribbon retting method has been developed as a remedy for the issues associated with the conventional water retting method. But this method has not yet gained popularity among jute growers due to the unavailability of catalyst, inadequate training and lack of interest of farmers. The study deals with the improvement of the existing ribbon retting process by using a concrete tank with or without fermented soybean as a natural catalyst in different proportions. For this purpose, 25 fibre samples were developed using different conditions such as concrete tank without natural catalyst, concrete tank with 2.5%, 5% and 7.5% natural catalyst and a micro pond without natural catalyst for various observational time periods. After that, samples collected under mentioned conditions which were measured to assess the fibre properties. The samples produced in a concrete tank with 7.5% natural catalyst demonstrated better fibre characteristics than the other conditions, including fibre fineness, fibre strength, improved fibre color, open surface structure and smooth surface etc. The best conditions for microbial growth were achieved using a concrete tank with more natural catalysts, which improved bacterial growth, fibre quality and reduced the retting time. The use of more natural catalysts increased microbial activity, which in turn affected total dissolved solids (TDS), Biochemical oxygen demand (BOD), Chemical oxygen demand (COD), and pH value of the retted water. In comparison to the existing ribbon retting method, this improved method is significantly faster and produces fibers with better properties. Farmers will gain more from the successful implementation of an improved ribbon retting method because it shortens retting time, conserves water, and uses a concrete tank during retting that can be used for multiple purposes.
Keywords: Ribbon retting, Concrete tank, Natural catalyst, Ribbon weight loss, Fibre strength
1. Introduction
Jute is the most versatile bast fibre produced in South Asia nations particularly in Bangladesh, India, Pakistan and Nepal [1]. Retting is a method of obtaining bast fibre in which bundles of stems are submerged in liquids such as water, chemicals, or enzymes to allow bacteria to break down non-cellulosic materials like lignin, pectin, and hemicellulose in order to release the fibre. Various methods such as traditional water retting, chemical or enzyme retting are practiced for jute fibre extraction [2]. Among the various methods, traditional water retting is very popular in our country. However, traditional water retting requires a lot of water and pollutes the environment. Due to climate change, ponds or rivers are drying up day by day. As a result, the scarcity of water has made it difficult for farmers to ret the fibre after harvesting specially in Bangladesh [3]. Alternatively, chemical and enzymatic retting are not widely used due to their high processing cost [4]. In order to address the aforementioned problems, many researchers have developed a new retting technique called ribbon retting. Ribbons are separated from the stem before being retted using a mechanical or manual process, and they are then retted in a tank made of cement or a micro pond covered in polythene. The process not only reduces the retting time but also saves water. However, this process takes around 12–15 days to complete the retting process with material and liquor ratio is 1:5 [5]. The ribbon retting method of jute fibre has been the subject of extensive research by many scientists. Md. Rostom Ali et al. provided information about different retting process along with their advantages and disadvantages. Finally, they concluded that ribbon retting technique is essential and farmers can be able to produce high quality jute fibre [3]. S. Sarkar and K. Sengupta explained that ribbon retting along with bacterial culture inoculation could be one of the most promising eco-friendly techniques for producing high quality jute fibre [6]. According to S. Banik et al. finding more effective pectinolytic bacteria is still needed to improve the fiber quality, but the ribbon retting process is a feasible and environmentally friendly alternative [7]. S. Sow et al. investigated ribbon retting with microbial formulations and found that it decreased the retting time as well as improved the fibre quality [8]. Rajnee Hasan et al. investigated the effect of pectinolytic bacterial consortia on retting time and fibre quality of jute and found that bacteria consortia reduced the retting time as well as improved the fibre quality [9]. The rapid growth of aerobic bacteria accelerates the existing ribbon retting method but depends on several factors such as temperature, moisture, oxygen and pH [10]. During ribbon retting, it is challenging to maintain a temperature that is conducive to bacterial development inside polythene-covered micro pond. Besides, the use of polythene will have a negative impact on the environment. Moreover, this method has not yet gained popularity among jute growers due to unavailability of catalyst, inadequate training and lack of interest of farmers. During retting, anaerobic microorganisms are found more prevalent during the later stage of retting while aerobic microorganisms are active on initial stage. Numerous anaerobic and aerobic species, including Clostridium species and Bacillus species, were isolated from retting water [11]. The ribbon retting process can be sped up by using one of two types of catalyst: natural or chemical. Natural catalyst such as enzymes and soil also help the growth and activity of retting microbes. However, numerous chemical catalysts, including ammonium sulphate, ammonium oxalate, and magnesium phosphate, were found to shorten the retting period. Due to their high cost and complicated requirements, enzymes and chemical catalysts aren't used very often. Presence of ferrous iron in soil content causes discoloration of jute fibre during retting [12]. The choice of a natural catalyst depends on the source of bacteria that is accessible and speeds up the retting process. One of the bacteria that quickens retting is Bacillus subtilis. The optimal growth temperature and pH range of 30–35 °C and 5.5–6.5 are capable of doubling time as fast as 20 min. It is typically found in soil, dried rice straw, pasteurized milk, dairy products, and fermented soybean food called natto [13]. Here, fermented soybean seed is used as a source of bacterial growth because it is widely available and simple to process.
Therefore, an approach has been taken to improve the existing ribbon retting method by using fermented soybean seed as a natural catalyst in a concrete tank to promote bacterial growth and shorten the retting time with better fiber quality.
2. Experimental
2.1. Materials
The details of the materials used in this experiment are provided in Table 1. Fig. 1, Fig. 2 illustrate the jute plant and the soybean seed.
Table 1.
Specification of raw materials.
| Raw materials | Specification |
|---|---|
| Jute plant (Tossa jute) | Height (meter): 1.83 Plant age: 120 days Scientific name: Corchorus olitorius Source: Nalia, Rajbari, Bangladesh. |
| Natural catalyst | Catalyst name: Fermented Soybean seed Scientific name: Glycine max Bacteria found in fermented soybean: Bacillus subtilis Source: Katabon, Romna, Dhaka. |
| Concrete tank | Construction Materials: Cement, sand, coarser aggregates Mixing ratio: 1:2:4 Dimension (meter): 0.91 × 0.61 × 0.46 |
| Water | Source: Pond water Total dissolved solid (milligram/liter): 240 Biological Oxygen Demand (milligram/liter): 9.58 Chemical Oxygen demand (milligram/liter): 75.72 pH: 7.6 |
| Rice straw | Source: Field Scientific name: Oryza sativa L |
Fig. 1.
Tossa jute plant.
Fig. 2.
Soybean seed.
2.2. Methods
2.2.1. Extraction of ribbon from jute plant
100 bundles (each bundle contained 10 plants) of defoliated jute plant were collected from the field. The green ribbons were then manually stripped from the stalks by hand-stripping and beating the plants base. The amount of green ribbon after stripping was 50 kg (Fig. 3).
Fig. 3.
Bundles of extracted ribbon.
2.2.2. Construction of micro pond for the existing method
A small pond was dug in the ground with the dimensions of 0.91 m (length) × 0.61 m (width) × 0.46 m (height). Polythene has been used to prevent water from falling inside the pond. Micro pond is shown in Fig. 4.
Fig. 4.
Polyethylene covered micro pond.
2.2.3. Ribbon retting in concrete tanks with or without a natural catalyst
In existing method, ribbon retting is done inside a polyethylene-covered micro pond (Fig. 5a) or a plastic or cemented tank. Microbial culture is used to speed up the retting process. It is not environmentally friendly to use a plastic tank or a micro pond covered with polyethylene, and microbe cultures are not readily available. This makes it necessary to improve the current ribbon retting system. An approach was used to solve the problem by taking into account the above-mentioned issue and bacteria growth factors. In this new approach, a concrete tank with natural catalyst or without natural catalyst were used to ret the ribbons (Fig. 5b). The use of natural catalysts accelerates the growth of bacteria.
Fig. 5.
Ribbon retting in (a) An existing micro pond, (b) Concrete tanks.
2.2.3.1. Construction of concrete tank
For this experiment, a concrete tank was chosen. Four concrete tanks were prepared by using the mix proportion of 1:2:4 of cement, sand, and coarse aggregates respectively. The dimensions of each concrete tank was 0.91 m (length) × 0.61 m (width) × 0.46 m (height). Consideration of some crucial characteristics of concrete, such as specific heat capacities and thermal conductivities, led to the decision to use a concrete tank [14]. These characteristics allow the concrete tanks to easily absorb heat from sunlight and transfer it to the water. Due to the tank's characteristics, bacterial growth is accelerated because the temperature is kept stable for a prolonged period of time inside the tank. The diagram of the concrete tank is shown in Fig. 6. Advantages of using concrete tank are given below
Fig. 6.
Concrete tank.
i. A prolonged life expectancy ii. This tank is reusable iii. It can be used as a water reservoir, except when retting occurs.
2.2.3.2. Preparation of natural catalyst from soybean seed
Soybean seed (glycine max) was selected as a natural catalyst. Soybean seeds weighing 1.5 kg were collected from Katabon, Romna Dhaka. Before being boiled, soybean seeds were first cleaned and soaked for the night. After being boiled for 30 min, the soybean seeds were covered with dried rice straw (Fig. 7a) and left at room temperature for 48 h. The fermentation of soybean seeds for bacterial growth took place as a result of the wrapping with dried rice straw (Fig. 7b) [15]. After that, the fermented soybean seeds were used as a catalyst in the concrete tank for retting.
Fig. 7.
Fermentation process (a) Boiled soybean seeds wrapped in rice straw, (b) Fermented soybean seed.
2.2.3.3. Using rice straw and bricks during retting
Due to its capacity to retain heat and encourage bacterial growth, rice straw is chosen as the covering material for ribbon retting in existing micro ponds and concrete tanks with or without a natural catalyst. On the other hand, regular bricks are employed to apply the proper pressure to the ribbon prior to submersion.
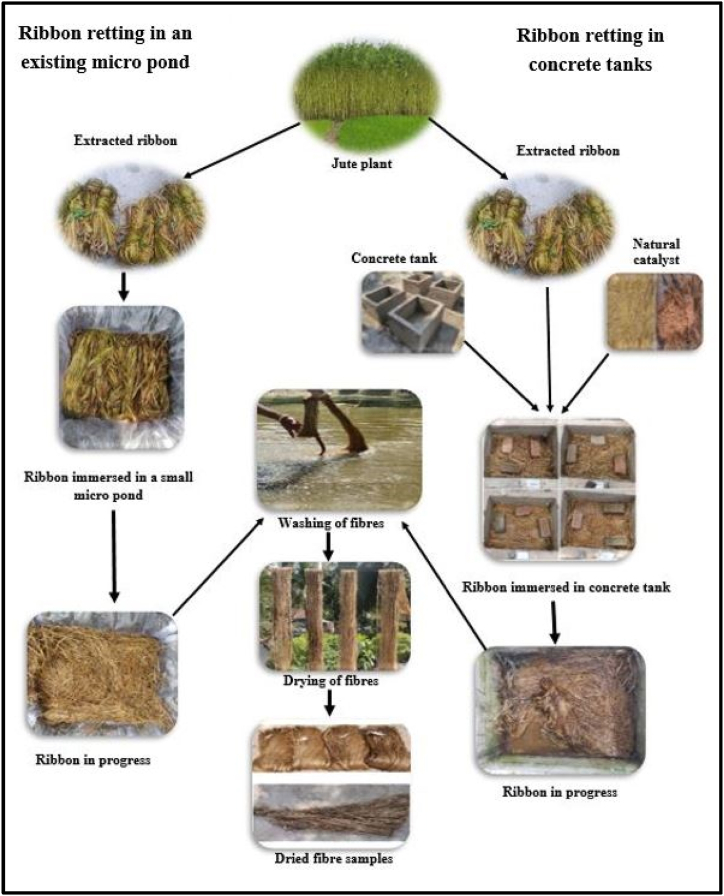
2.2.4. Schematic diagram of ribbon retting in an existing micro pond and concrete tanks with or without a natural catalyst
Fig. 8 depicts a schematic diagram of ribbon retting in concrete tanks and an existing micro pond, either with or without a natural catalyst.
Fig. 8.
Schematic diagram of ribbon retting in an existing micro pond and concrete tanks.
2.2.5. Research plan
In this study, samples were prepared under different ribbon retting conditions on the day of observations, including concrete tanks with or without natural catalyst and a micro pond without a natural catalyst. Different conditions were denoted by letters A, B, C, D, and E. The following were taken into consideration for the convenience of sample identification: A for concrete tank 01 without catalyst, B for concrete tank 02 with 2.5% natural catalyst, C for concrete tank 03 with 5% natural catalyst, D for concrete tank 04 with 7.5% natural catalyst, and E for a micro pond without catalyst. Twenty-five (25) samples were made according to sample matrix shown in Table 2 to identify the best ribbon retting conditions out of five, considering fiber quality parameters and the shortest retting time. Based on the first trial of retting, the observation period of five days was decided.
Table 2.
Sample matrix.
|
Different conditions |
Sample code | Observation days |
||||
|---|---|---|---|---|---|---|
| 09 | 12 | 15 | 18 | 21 | ||
| Tank 1 (Without natural catalyst) | A | Sample-A1 | Sample-A2 | Sample-A3 | Sample-A4 | Sample-A5 |
| Tank 2 (2.5% natural catalyst) | B | Sample-B1 | Sample-B2 | Sample-B3 | Sample-B4 | Sample-B5 |
| Tank 3 (5% natural catalyst) | C | Sample-C1 | Sample-C2 | Sample-C3 | Sample-C4 | Sample-C5 |
| Tank 4 (7.5% natural catalyst) | D | Sample-D1 | Sample-D2 | Sample-D3 | Sample-D4 | Sample-D5 |
| Existing method (Without catalyst) | E | Sample-E1 | Sample-E2 | Sample-E3 | Sample-E4 | Sample-E5 |
2.2.6. Recipe for different ribbon retting condition
50 kg of green ribbon was taken from the jute plant for this study. Following that, 50 kg of ribbons were evenly divided for different conditions. 10 kg of ribbons were present in each condition. The proportion of ribbon to water was 1:4. The volume of water of ribbon retting for each condition was 40 L. Table 3 illustrates the distribution of the natural catalyst according to the percentage of concentration. A total of 0.35 kg of rice straw was used for each condition as a covering material.
Table 3.
Natural catalyst amount for different ribbon retting conditions.
| Different conditions | Natural catalyst (Fermented soybean), Catalyst was taken on the weight of the material, kg |
|---|---|
|
Concrete Tank 1 (A) (Without natural catalyst) |
– |
|
Concrete Tank 2 (B) (2.5% natural catalyst) |
0.25 |
|
Concrete Tank 3 (C) (5% natural catalyst) |
0.50 |
|
Concrete Tank 4 (D) (7.5% natural catalyst) |
0.75 |
|
Existing method (E) (Polyethylene covered micro pond without catalyst) |
– |
2.2.7. Working procedure
-
i.
This study was conducted in the 2nd week of August. Tossa jute plant was collected and ribbons were extracted from the plant followed by manual beating and stripping.
-
ii.
The front yard of the house contained the concrete four tanks and a small micro pond that was covered in polyethylene.
-
iii.
Fermented soybean seed used a natural catalyst to promote the bacterial growth inside the concrete tank.
-
iv.
450 kg of green ribbon was distributed equally among the small pond and four concrete tanks. From 10 kg of fibre, five bundles were produced. The amount of fibre in each bundle was 2 kg. Twenty five fibre bundles were submerged in four concrete tanks and a tiny pond covered in polyethylene. The volume of water in each tank and pond was 40 L. Tanks 2, 3, and 4 of the four tanks each contained 0.25, 0.50, and 0.75 kg of fermented soybean seeds, while Tank 1 and the small pond did not have any catalyst. Concrete tanks and a small pond were covered with 0.35 kg of dried rice straw. The water-immersed ribbon was subjected to constant pressure using normal bricks.
-
v.
After that, fibre samples were observed after 5 days by test and feel method with fiber separation to avoid over or improper retting of fibre. Temperature, TDS and pH under different condition of concrete tanks and a small pond were recorded daily. After that, 25 (twenty five) samples were obtained under various retting conditions and time periods. Observation days of retting were finalized based on 1st trial. Fig. 9 presents the washing (Fig. 9a) and drying (Fig. 9b) procedures of the fiber samples, along with the dried fiber (Fig. 9c).
-
vi.
Quality parameters of fibres samples such as weight loss (%), fineness, strength, elongation and color were measured. Then the mesh structure and surface morphology of fibre samples were observed with a mobile phone fitted with a macro lens and optical microscope.
-
vii.
Finally, test results of twenty five samples were analyzed and compared those results between existing and concrete tanks method. Based on test results and retting periods, a suitable condition was found.
-
viii.
After completion the retting of concrete tanks and existing method, five sample of retted water were collected. Then, the tests were conducted to determine the effluent water quality on pH, TDS (Total Dissolved Solids), BOD (Biochemical Oxygen Demand) and COD (Chemical Oxygen Demand).
Fig. 9.
(a) Washing of fibre samples (b) Drying (c) Dried fibre samples.
2.2.8. Test method
Table 4 displays various physical and chemical tests conducted in accordance with standard procedures, which are relevant to this experiment.
Table 4.
Sample observation based on test method.
| Parameter | Test method | References |
|---|---|---|
| Observation of the end point of retting | Touch and feel method | [16] |
| Total aerobic plate count | Bacteriological Analytical Manual (BAM), Chapter 3 | [17] |
| Ribbon weight loss (%) | Weight loss (%) = | [18] |
| Ribbon mesh structure | Visual assessment using a smartphone equipped with a macro lens | – |
| Fibre fineness | ASTM D 1577 | [19] |
| Fibre strength and elongation | ASTM D1445-05 | [20] |
| Fibre color | ASTM E313 | [21] |
| Surface morphology | 100× digital optical microscope | – |
| Biochemical oxygen demand (BOD) | APHA Standard Methods (5210 B) | [22] |
| Chemical oxygen demand (COD) | APHA Standard Methods (5220 D, Closed Reflux Method) | |
| Total Dissolved Solids (TDS) | Conductivity measurement | [23] |
| Temperature | ASTM E2877-12, thermal expansion of the liquid | [[24], [25]] |
| pH | ASTM D1067 |
2.2.9. Testing procedure
2.2.9.1. Observation of the completion of retting
In this study, after five days of retting, the extracted ribbon was started to be observed using the “Touch and Feel” method. This technique manually examined the fiber separation [16]. 25 (Twenty five) samples were observed by using this technique.
2.2.9.2. Determination of the counting of total aerobic plate
In accordance with the testing method described in BAM chapter 03, the number of aerobic bacteria was measured under various conditions, including a micro pond without a natural catalyst, a concrete tank with a natural catalyst concentration of 7.5%, and a concrete tank without a natural catalyst. In the APC procedure outlined in the BAM, bacteria from a sample were grown under aerobic conditions on a suitable culture medium, typically tryptic soy agar (TSA) (in the presence of oxygen). The number of colonies that formed on the plate were counted after the bacteria were incubated for 24–48 h at the proper temperature, usually 30–35 °C [17].
2.2.9.3. Determination of ribbon weight loss (%)
The percentage of mass difference between non-retted and retted jute fibre was used to define the weight loss and calculate the yield of fibre [18]. Weight loss (%) can be calculated according to equation (1).
| (1) |
Where, green ribbon weight expressed in kilograms (kg).
2.2.9.4. Determination of fibre fineness
Linear density was measured according to the ASTM D 1577 standard through gravimetric method [19]. The fibre samples were cut and weighed in gram using a standard length (L = 10 cm). The formula is used to calculate the denier (D) of the fibre sample using equation (2). After that, the calculated vales are converted into tex.
| (2) |
Where N is the no of fibres, W is the cut weight of samples and L is the cut length of fibre samples.
2.2.9.5. Determination of fibre strength and elongation
According to ASTM D1445-05, the jute fibres breaking load (kg) and elongation were measured for zero gauge. Bundle fiber strength tester was used to take this measurement (Fibrostelo, India) [20]. Following that, the breaking load was converted to pounds and the weight of the broken sample was taken in milligrams to determine the bundle strength using equation (3). The middle portion of the fibre bundle was used to conduct the test.
| (3) |
2.2.9.6. Determination of fibre color
The yellowness index is a measure used to quantify the yellow color in a substance or material. It is calculated as the ratio of the intensities of the light absorbed at specific wavelengths to the total amount of light absorbed. Spectrophotometer was used to measure the yellowness index of fibre samples according to the ASTM E313. The sample is placed on a spectrophotometer, which measures the amount of light reflected at different wavelengths. The data collected is then used to calculate the yellowness index, which is expressed as a number on a scale that ranges from 0 to 100 [21].
2.2.9.7. Analysis of mesh structure and surface morphology of fibre samples
The mesh structure of 25 samples was observed using a smartphone equipped with a macro lens. After the five samples had been retted, the surface morphology was examined under a 100× digital optical microscope.
2.2.9.8. Determination of biochemical oxygen demand (BOD)
The test was performed according to the APHA Standard Methods (5210 B). The BOD test is also known as “BOD5” because it is based on an accurate measurement of DO (dissolved oxygen) at the start and end of a five-day period during which the sample is held in dark, incubated conditions (i.e., 20 °C or 68 °F). The unit of BOD is mg/L [22].
2.2.9.9. Determination of chemical oxygen demand (COD)
COD test was performed according to APHA Standard Methods (5220 D, Closed Reflux Method). The unit of COD is mg/L [22].
2.2.9.10. Determination of total dissolved solids (TDS)
Total dissolved solids (TDS) is measured as a volume of water with the unit milligrams per liter (mg/L). TDS meter (Model No. CTR-002) based on conductivity measurement principle was used in this study to measure TDS values of waste water [23].
2.2.9.11. Determination of temperature and pH (potential of hydrogen)
Temperature and pH levels of water in the concrete tank were continuously monitored using a thermometer and pH meter (Hanna pH-98107) in accordance with ASTM E2877-12, the liquid's thermal expansion, and ASTM D1067 [24,25].
3. Results and discussion
3.1. Observation of temperature during retting period
The use of a concrete tank allowed for the maintenance of the ideal temperature for bacterial growth. In several concrete tanks and a micro pond, average temperatures that were determined during the retting process are shown in Fig. 10. At various times throughout the day, the temperature was measured using a thermometer. Average temperatures were recorded for 21 days of retting periods under different conditions such as concrete tanks 01 (A), 02 (B), 03 (C), and 04 (D) and a micro pond (E). In comparison to the micro pond, the temperature in the concrete tanks was about 2–3° higher. This difference resulted from the concrete tank's unique thermal conductivities and heat capacities. Higher temperature favors the growth of bacteria inside concrete tanks, in contrast to micro ponds.
Fig. 10.
Temperature (0C) of concrete tanks and a micro pond.
3.2. Effect of natural catalyst on microbial growth
Aquatic microbes, primarily bacteria, are responsible for the decomposition of non-fibrous materials while the jute plants are submerged in water. In order to determine how the presence of a natural catalyst influenced bacterial growth, three conditions—a concrete tank without catalyst, a concrete tank with 7.5% catalyst, and a micro pond without catalyst (existing method)—were chosen for microbial growth tests. The difference in microbial population (aerobic bacteria) between the water samples taken before and after retting for different conditions is shown in Fig. 11. According to this number, the microbial population in post-retted water increased by 1.4–2.25 times. Because more natural catalyst encouraged the growth of microbes in the water samples, the microbial population (aerobic bacteria) in concrete tank 04 with 7.5% natural catalyst was the highest among the three conditions. However, hemicellulose was only partially removed and pectin was completely broken down during the retting of jute fiber, dissolving it in the water sample and encouraging the growth of microbes [26].
Fig. 11.
Observation of microbial growth under different conditions.
3.3. Effect of retting condition and observation day on ribbon weight loss (%)
The weight loss percentage of ribbon is plotted against various conditions and observation days in Fig. 12. The ribbon weight loss percentage was significantly increased by retting time and using more natural catalysts in the concrete tank. As shown in this graph, samples made from different concentration of natural catalyst (B, C and D) all showed greater weight loss percentage of ribbon than samples made under conditions A and E. A sample from condition D showed a higher ribbon weight loss percentage over a shorter retting period than the other 25 samples. Pectin and hemicellulose are successfully removed as bacterial growth increases. However, a high percentage of weight loss in the ribbon may be an indication of cellulosic material degradation, which ultimately lowers the quality of the fiber.
Fig. 12.
Ribbon weight loss for retting condition and observation day.
Contrarily, in existing method, the weight loss percentage of fiber gradually increased with retting period.
3.4. Effect of retting condition and observation day on mesh structure
Mesh structure are greatly affected by retting conditions and duration of retting. The successful completion of the retting method is required for proper mesh structure opening. Retting allows the structure to be more open by removing the cementing substances found in ultimate cells like pectin and hemicellulose. Fig. 13 displays the mesh structure of 25 (twenty-five) fiber samples under various retting conditions and the observation days. Due to their more open mesh structures, a few of the 25 samples are highlighted in yellow. Under the conditions of D, C, and B, the mesh structure of Samples D1 (Fig. 13d), C2 (Fig. 13c), and B3 (Fig. 13b) exhibited greater openness compared to the others. Higher amounts of natural catalyst prove to be effective in efficiently removing the cementing material within a shorter retting period shown in Fig. 13d (D1). On the other hand, samples made from a concrete tank 01 without a catalyst (A) (Fig. 13a) and existing method (E) (Fig. 13e) showed less opening of mesh structure. This can be attributed to the lack of a natural catalyst, resulting in incomplete removal of cementing materials. The amount of fiber entangled in the mesh structure visibly increased with longer retting periods, as evident in Fig. 13b (B4), 13c (C5), and 13 d (D5). On the other hand, shorter retting periods without a catalyst resulted in reduced opening of the mesh structure, observed in Fig. 13a (A1 to A3), 13 b (B1 and B2), 13c (C1), and 13e (E1 to E3).
Fig. 13.
The mesh structure for the five different retting conditions (a) A- Concrete Tank 1 without natural catalyst (b) B- Concrete Tank 2 with 2.5% natural catalyst (c) C- Concrete Tank 3 with 5% natural catalyst (d) D- Concrete Tank 4 with 7.5% natural catalyst (e) E- Micro Pond without natural catalyst, observed on the 09th, 12th, 15th, 18th, and 21st day.
3.5. Effect of retting condition and observation day on fibre fineness (tex)
Longer retting times resulted in a gradual decrease in the binding elements of fibre and an increase in fiber fineness [27]. Fig. 14 illustrates how various retting conditions and observation days affect fibre fineness. Samples prepared under conditions A and E have completely different patterns than samples prepared under conditions B, C, and D. The samples made under condition D, D1 had a higher fineness within the shortest retting times than the other samples made under conditions C and B. This happened as a result of sample D1's use of a concrete tank and a higher concentration of natural catalyst, which sped up the retting process and extracted the fibre binding materials and decreased the fibre diameter. More catalyst inside the concrete tank sped up the bacterial growth that enabled the removal of non-cellulosic materials in the shortest period of retting time. In comparison to other retting conditions, the existing method and the concrete tank without a catalyst showed lower fibre fineness.
Fig. 14.
Fibre fineness for retting condition and observation day.
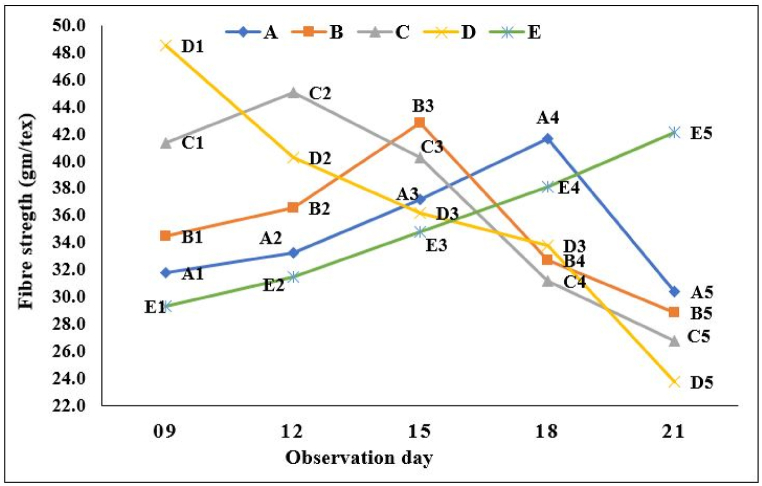
3.6. Effect of retting condition and observation day on fibre strength (gm/tex)
Jute fibre is considered as a natural composite of cellulose micro fibrils and a matrix of non-cellulosic components; the mechanical properties especially tenacity are controlled not only by the cellulose and crystallinity index, but also by the coherence between the cellulose and non-cellulosic components. The bundle fibre strength is displayed in Fig. 15 at various retting intervals and under various retting conditions. Retting revealed that the strength of fibre samples made under conditions A, B and C gradually increased, peaked, and then gradually decreased. In case of condition D, sample D1 had reached its maximum strength within the shortest retting periods. Then, as retting times increased, strength gradually decreased. The use of a natural catalyst enhanced bacterial growth and may increase the cellulose fraction and cellulose chain packing order, which results in better fibre strength. Longer retting periods degrade non-cellulosic materials to a greater extent, weakens the coherence between the cellulose micro fibrils and non-cellulosic matrix, and lowers the tensile properties as a result [28,29]. In the existing method, higher fibre strength was obtained on the 21st day of observation.
Fig. 15.
Fibre strength for retting condition and observation day.
3.7. Effect of retting condition and observation day on fibre elongation (%)
Throughout the retting periods, Fig. 16 displays the percentage of fibre elongation for various conditions and observation days. The chemical elements in the fibre, which were the main influencing factors, and hemicellulose, which gave the fibre its elasticity and toughness, had an impact on the elongation at break. When the amount of hemicelluloses in a fibre decreases, less flexible hemicelluloses-cellulose-hemicelluloses bonds form, which results in more rigid cellulose-cellulose bonds [29]. The fibre elongation at break is ultimately affected by that. The results of retting showed that the elongation of fibre samples produced under conditions A, B, C, D and E gradually decreased. Under various conditions and observation, the decreasing trend of the elongation of fibre samples was not more noticeable.
Fig. 16.
Fibre elongation (%) for retting condition and observation day.
3.8. Effect of retting condition and observation day on fibre color
The yellowness index of fibre samples under various conditions is shown in Fig. 17. In comparison to samples made from a concrete tank and a micro pond without a catalyst, fibre samples from the concrete tank with 2.5%, 5%, and 7.5% natural catalyst showed a higher yellowness index.
Fig. 17.
Fibre color for retting condition and observation day.
The use of a natural catalyst inside the concrete tank encouraged bacterial growth, which increased the yellowness index values and gave the fibre a more lustrous, reddish yellow color. The non-cellulosic materials were removed by the increased bacterial growth in the retted water, which also improved the color of the fibre samples. Samples collected in a micro pond using the existing method displayed more dark color while having a lower yellowness index.
3.9. Observation of the completion of retting
After examining the fibre properties of 25 (twenty five) samples, the retted samples are divided into three categories, including partially retted (A1, A2, A3, B1, B2, C1, and E1 to E4), completely retted (D1, C2, B3, A4 and E5), and over retted (A5, B4, B5, C3, C4, C5, and D2 to D5), as shown in Table 5. The retting procedure was completed in 18, 15, 12, 9, and 21 days for concrete tank 01, concrete tank 02, concrete tank 03, concrete tank 04 and existing method. The appropriate temperature for bacterial growth is maintained in a concrete tank while a larger concentration of natural catalyst accelerates bacterial development.
Table 5.
Completion of retting period for different conditions.
| Retting condition | Sample code | Status of retted samples for different observation day |
||||
|---|---|---|---|---|---|---|
| 09 | 12 | 15 | 18 | 21 | ||
| Concrete Tank 1 (Without natural catalyst) | A | A1 (Partial) | A2 (Partial) | A3 (Partial) | A4 (Completed) | A5 (Over) |
| Concrete Tank 2 (2.5% natural catalyst) | B | B1 (Partial) | B2 (Partial) | B3 (Completed) | B4 (Over) | B5 (Over) |
| Concrete Tank 3 (5% natural catalyst) | C | C1 (Partial) | C2 (Completed) | C3 (Over) | C4 (Over) | C5 (Over) |
| Concrete Tank 4 (7.5% natural catalyst) | D | D1 (Completed) | D2 (Over) | D3 (Over) | D4 (Over) | D5 (Over) |
| Existing method (Without catalyst) | E | E1 (Partial) | E2 (Partial) | E3 (Partial) | E4 (Partial) | E5 (Completed) |
3.10. Surface morphology of completed retted fibre samples
Using an optical digital microscope, the surface morphology of jute fibre can be examined in great detail. When viewed at higher magnification, the fibre are seen to be covered in a variety of structures despite initially appearing smooth and shiny. After the retting process was finished, five samples such as D1 (Fig. 18a), C2 (Fig. 18b), B3 (Fig. 18c), A4 (Fig. 18d) and E5 (Fig. 18e) were photographed using an electronic microscope. Samples D1, C2, and B3 displayed a significantly cleaner surface in comparison to samples A4 and E5. This noticeable difference in surface cleanliness can be attributed to the effective action of the natural catalyst during the retting process, which successfully eliminated the gummy substance from the fiber surface.
Fig. 18.
Surface morphology of completed retted fibre samples (a) D1- Concrete Tank 4 with 7.5% natural catalyst on the 9th day (b) C2- Concrete Tank 2 with 5% natural catalyst on the 12th day (c) B3- Concrete Tank 3 with 2.5% natural catalyst on the 15th day (d) A4- Concrete Tank 4 without natural catalyst on the 18th day (e) E5- Micro Pond without natural catalyst on the 21st day.
3.11. Completed retted fibre samples characteristics
The retting process was completed in 18, 15, 12, 9, and 21 days for samples A4, B3, C2, D1, and E5, respectively. These samples are regarded as fully completed retted samples. Table 6 lists the characteristics of fully completed fibre samples.
Table 6.
Retted fibre samples characteristics.
| Retting condition | Sample code | Fibre properties |
||||
|---|---|---|---|---|---|---|
| Weight loss (%) | Fineness (tex) | Bundle Strength (gm/tex) | Elongation (%) | Fibre color (Yellowness index) | ||
| Concrete Tank 1 (Without natural catalyst) | A4 | 73.5 | 1.58 | 41.7 | 1.79 | 72.7 |
| Concrete Tank 2 (2.5% natural catalyst) | B3 | 74.5 | 1.60 | 42.8 | 1.80 | 80.3 |
| Concrete Tank 3 (5% natural catalyst) | C2 | 75 | 1.56 | 45.1 | 1.76 | 82.7 |
| Concrete Tank 4 (7.5% natural catalyst) | D1 | 76 | 1.51 | 48.5 | 1.70 | 84.9 |
| Existing method (Without catalyst) | E5 | 73 | 1.70 | 42.1 | 1.77 | 53.4 |
In comparison to the four conditions (A, B, C, and D), sample D1 displayed better fibre properties and was obtained after 9th days of retting. Table 6 shows that the fibre qualities were improved by adding more natural catalyst to the concrete tank. When compared to condition D, the obtained fibre qualities in the case of the existing method were not very good, and this method required more time to ret the fibre. Fibre obtained from the existing method showed lower yellowness index compared to the samples obtained using the concrete tank and natural catalyst. The fibre developed a more lustrous, reddish-yellow color due to increased bacterial growth.
3.12. Effect of retting condition on BOD and COD of retted water
Before and after the retting, Table 7 shows the BOD and COD of water samples under three different conditions. Values for BOD and COD change quickly during retting.
Table 7.
Effect on BOD and COD of retted water for retting condition.
| Retting condition | Sample code | BOD (mg/l) |
COD (mg/l) |
||
|---|---|---|---|---|---|
| Before retting | After retting | Before retting | After retting | ||
| Concrete Tank 1 (Without natural catalyst) | A | 9.58 | 80.12 | 75.78 | 319.12 |
| Concrete Tank 4 (7.5% natural catalyst) | D | 84.34 | 344.45 | ||
| Existing method (Without catalyst) | E | 78.67 | 328.78 | ||
The initial BOD and COD values of pond water had 9.58 and 75.78. Concrete tank 4 which contained 7.5% fermented soybean seed showed higher BOD and COD values compared to others. Higher amount of natural catalyst enhanced the microbial growth in concrete tank-04 during the retting. Higher BOD levels during the retting process showed that more dissolved oxygen was being consumed by the bacterial community as it broke down organic matter, whereas higher COD levels in the post-retting water indicated the presence of more suspended and colloidal organic matter [30]. The BOD and COD values of retted water are not suitable for fish farming, but this retted water can be used for irrigation of land. The maximum BOD and COD values for water used to irrigate land, according to environmental conversation rules from 1997, are 100 and 400, respectively [31].
3.13. Effect of retting condition on TDS and pH of retted water
Table 8 displays the TDS (mg/l) and pH values of waste water before and after retting under various conditions.
Table 8.
Effect on TDS and pH of retted water for retting condition.
| Retting condition | Sample code | TDS (mg/l) |
pH |
||
|---|---|---|---|---|---|
| Before retting | After retting | Before retting | After retting | ||
| Concrete Tank 1 (Without natural catalyst) | A | 240 | 1690 | 7.4 | 5.8 |
| Concrete Tank 2 (2.5% natural catalyst) | B | 1735 | 5.7 | ||
| Concrete Tank 3 (5% natural catalyst) | C | 1770 | 5.8 | ||
| Concrete Tank 4 (7.5% natural catalyst) | D | 1800 | 5.7 | ||
| Existing method (Without catalyst) | E | 1730 | 6 | ||
The TDS value in concrete tank 4 was higher compared to others. Higher levels of natural catalyst increased the amount of dissolved materials in the waste water, including minerals, salts, anion or cations. The microbial movement that affects the TDS values of water was accelerated by the solid extraction from the jute fiber during retting [32]. The obtained values fall within the range of the maximum TDS value of 2100. As a result, this retted water is also used for land irrigation [31].
On the other hand, the initial pH of water was 7.4 and this values were changed during retting time. The breakdown of cementing materials like pectin, hemicellulose, etc. By microbes during retting results in the release of organic acids like galactouronic acid, which lowers pH values [7]. The pH levels in concrete tank 4 were lower than those in the other four conditions. This occurred as a result of the maximum release of organic acids during retting. The maximum pH value for water used for land irrigation is 6–9. The pH level of water obtained from different conditions was slightly acidic in nature and this pH value was around 6 which is not harmful [31].
4. Conclusion
The study investigated a novel strategy for enhancing the current ribbon retting method. Using a concrete tank containing 7.5% natural catalyst, the process completed in 9 days in this method as opposed to 21 days for the existing method. Using a concrete tank, the temperature of retting liquor increased by 2–3° which encouraged bacterial growth. Considering fibre qualities and retting period, the concrete tank with 7.5% natural catalyst is preferable to the other conditions. Through greater amount of natural catalyst improve fibre properties, the retting cost will also increase. On the other hand, BOD, COD and TDS values of retted water from the concrete tank with 7.5% natural catalyst revealed higher in comparison to the other conditions. Farmers will benefit from the improved ribbon retting process if it is successfully implemented.
Author contribution statement
Amit Chakrabortty: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Dr. Hosne Ara Begum: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
There were no specific grants provided for this study by government or private funding organizations.
Data availability
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Roy S. International Jute Study Group; Dhaka: 2010. Jute Basics; p. 250. [Google Scholar]
- 2.Lee C.H., Khalina A., Lee S., Liu M. A comprehensive review on bast fibre retting process for optimal performance in fibre-reinforced polymer composites. Adv. Mater. Sci. Eng. 2020;2020:1–27. doi: 10.1155/2020/6074063. [DOI] [Google Scholar]
- 3.Ali M.R., Kozan O., Rahman A., Islam K.T., Hossain M.I. Jute retting process: present practice and problems in Bangladesh. Agric. Engin. Int.: CIGR J. 2015;17(2) https://cigrjournal.org/index.php/Ejounral/article/view/3212/2120 [Google Scholar]
- 4.Tavares T.D., Antunes J.C., Ferreira F., Felgueiras H.P. Bio functionalization of natural fiber-reinforced bio composites for biomedical applications. Biomolecules. 2020;10(1):148. doi: 10.3390/biom10010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjan S., Sow S., Kumar A. Ribbon retting of jute: an eco-friendly method for quality jute fibre production. Food and Scie. Reports. 2021;2(2):44. [Google Scholar]
- 6.Sarkar S., Sengupta K. Comprehensive technique for jute fibre retting. Int. J. Bio-res. Stress Manag. 2015;6(1):170–175. [Google Scholar]
- 7.Banik S., Basak M.K., Paul D., Nayak P., Sardar D., Sil S.C., et al. Ribbon retting of jute—a prospective and eco-friendly method for improvement of fibre quality. Ind. Crop. Prod. 2003;17(3):183–190. doi: 10.1016/S0926-6690(02)00097-3. [DOI] [Google Scholar]
- 8.Sow S., Ranjan S., Kumar A., Kumar M. Improved retting methods of jute to enhance fibre quality and retting waste management. J. Pharmacogn. Phytochem. 2021;10(1):2614–2618. [Google Scholar]
- 9.Hasan R., Aktar N., Kabir S.M., Honi U., Halim A., Islam R., Sarker M.D., Haque M., Alam M., Islam M. Pectinolytic bacterial consortia reduce jute retting period and improve fibre quality. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-61898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jufri R.F. The effect of environmental factors on microbial growth. J. La Lifesci. 2020 Jan 30;1(1):12–17. [Google Scholar]
- 11.Das B., Chakrabarti K., Tripathi S., Chakraborty A. Review of some factors influencing jute fiber quality. J. Nat. Fibers. 2014 Jul 3;11(3):268–281. [Google Scholar]
- 12.Majumdar B., Das S., Saha A.R., Chowdhury H., Kundu D.K., Mahapatra B.S. vol. 32. Central Research Institute for Jute and Allied Fibres (ICAR); Barrackpore, Kolkata: 2013. (Improved Retting of Jute and Mesta with Microbial Formulation (Bulletin No. 04/2013)). [Google Scholar]
- 13.Errington J., van der Aart L.T. Microbe Profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse. Microbiology. 2020;166(5):425. doi: 10.1099/mic.0.000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GreenSpec. GreenSpec: Thermal Performance: Thermal Mass in Buildings. 2022. www.greenspec.co.ukhttps://www.greenspec.co.uk/building-design/thermal-mass/ [Google Scholar]
- 15.Endo A., Irisawa T., Dicks L., Tanasupawat S. Fermentations of east and southeast Asia. Encycl. Food Microbiol. 2014;1:767–773. doi: 10.1016/B978-0-12-384730-0.00119-1. [DOI] [Google Scholar]
- 16.Haque M.S., Ahmed Z., Asaduzzaman M., Quashem M.A., Akhter F. Distribution and activity of microbial population for jute retting and their impact on water of jute growing areas of Bangladesh. Pakistan J. Biol. Sci. 2002;5(6):704–706. doi: 10.3923/pjbs.2002.704.706. [DOI] [Google Scholar]
- 17.Nutrition C for FS and A. Bacteriological Analytical Manual (BAM). FDA. 2020. https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam May 6; Available from: [Google Scholar]
- 18.Saravanan D., Ramanathan V.A., Karthick P., Murugan S.V., Nalankilli G., Ramachandran T. Optimisation of multi-enzyme scouring process using Taguchi methods. Indian J. Fibre Text. Res. 2010;35:164–171. [Google Scholar]
- 19.Patel B.Y., Patel H.K. Retting of banana pseudostem fibre using Bacillus strains to get excellent mechanical properties as biomaterial in textile & fiber industry. Heliyon. 2022;8(9) doi: 10.1016/j.heliyon.2022.e10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begum H.A., Saha S.K., Siddique A.B., Stegmaier T. Investigation on the spinability of fine areca fiber. J. Textil. Inst. 2019;110(9):1241–1245. [Google Scholar]
- 21.Yellowness index (YI) ASTM E313. www.intertek.comhttps://www.intertek.com/polymers/testlopedia/yellowness-index-astm e313/#:∼:text=Yellowness%20Index%20is%20a%20number
- 22.Understanding laboratory wastewater tests: I. Organics (BOD, COD, toc, O&g) https://extension.uga.edu/publications/detail.html?number=C992#BOD [Internet]. extension.uga.edu. Available from:
- 23.User's Guide Conductivity and TDS Meter Pen Style Water Quality Meter [Internet]. Available from: https://www.fondriest.com/pdf/extech_ec150_manual.pdf.
- 24.ASTM E2877-12. 2019. www.techstreet.comhttps://www.techstreet.com/standards/astm-e2877-12-2019?product_id=2079204 [Google Scholar]
- 25.Standard Test Methods for Acidity or Alkalinity of Water [Internet]. www.astm.org. Available from: https://www.astm.org/d1067-16.html.
- 26.Majumdar B., Chattopadhyay L., Barai S., Saha A.R., Sarkar S., Sarkar S.K., Mazumdar S.P., Saha R., Jha S.K. Impact of conventional retting of jute (Corchorus spp.) on the environmental quality of water: a case study. Environ. Monit. Assess. 2019;191(7):1–3. doi: 10.1007/s10661-019-7589-7. [DOI] [PubMed] [Google Scholar]
- 27.Li Z., Zou Y., Li S., Guo Y., Zeng X., Zhu J., et al. Direct extraction of fibre from a ramie bark. J. Engin. Fibers and Fabrics. 2020;15 doi: 10.1177/1558925020940109. [DOI] [Google Scholar]
- 28.Mazian B., Bergeret A., Benezet J.C., Malhautier L. A comparative study of the effect of field retting time on the properties of hemp fibres harvested at different growth stages. Fibers. 2019;7(12):108. [Google Scholar]
- 29.Ishak M.R., Leman Z., Sapuan S.M., Rahman M.Z., Anwar U.M. Chemical composition and FT-IR spectra of sugar palm (Arenga pinnata) fibers obtained from different heights. J. Nat. Fibers. 2013;10(2):83–97. [Google Scholar]
- 30.Majumdar B., Sarkar S., Kar G. Jute retting water Jute retting water. A potential source of essential plant nutrients. Indian Farming. 2020;7(1):21–23. [Google Scholar]
- 31.The Environment Conservation Rules. 1997. https://www.mutualtrustbank.com/wp-content/uploads/2020/11/ecr.pdf CONTENTS [Internet]. Available from: [Google Scholar]
- 32.Ali M.R., Kabir M., Shawon M.T., Hussain M.M., Islam M.H., Alam M. Effect of conventional retting of jute on the quality of water and jute fiber. Progressive Agric. 2021;32(2):151–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.