Abstract
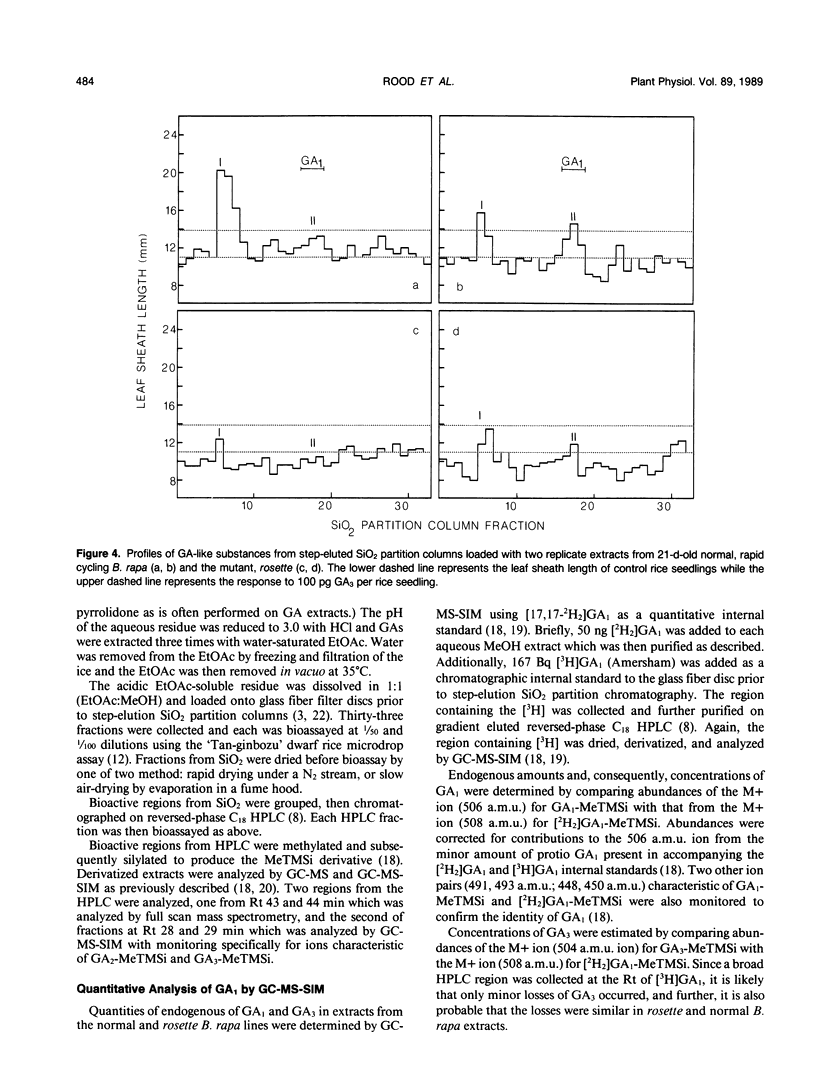
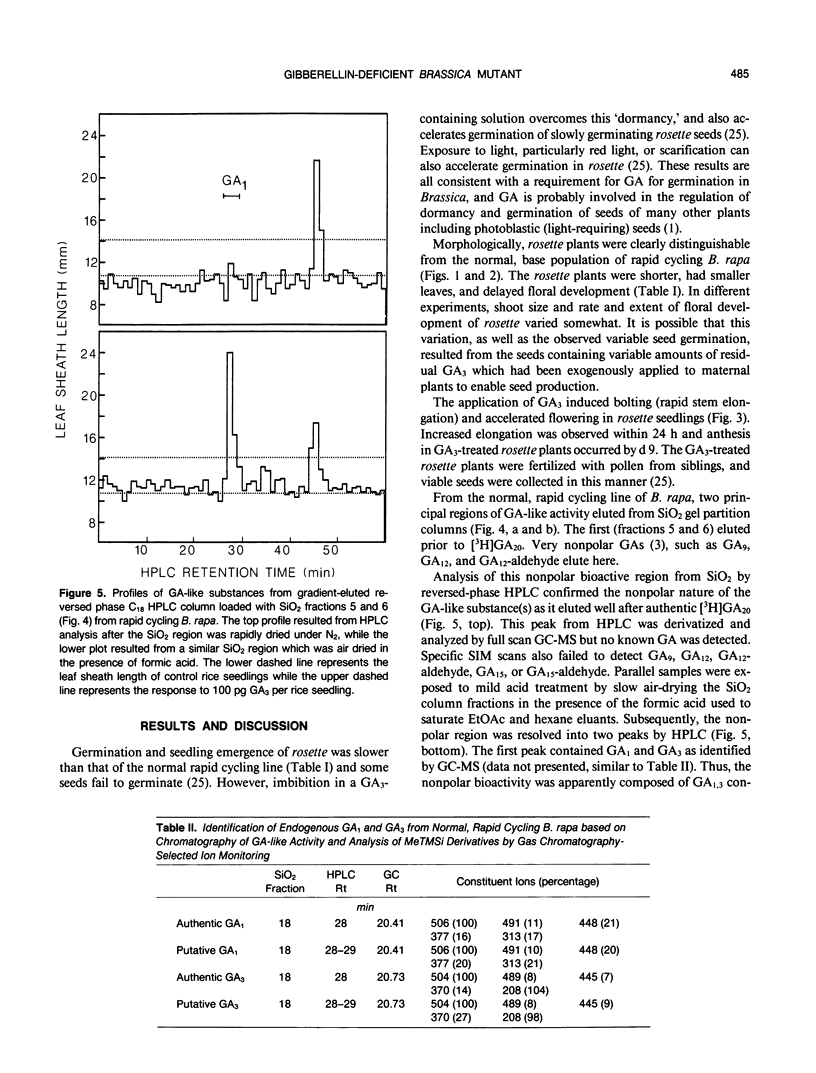
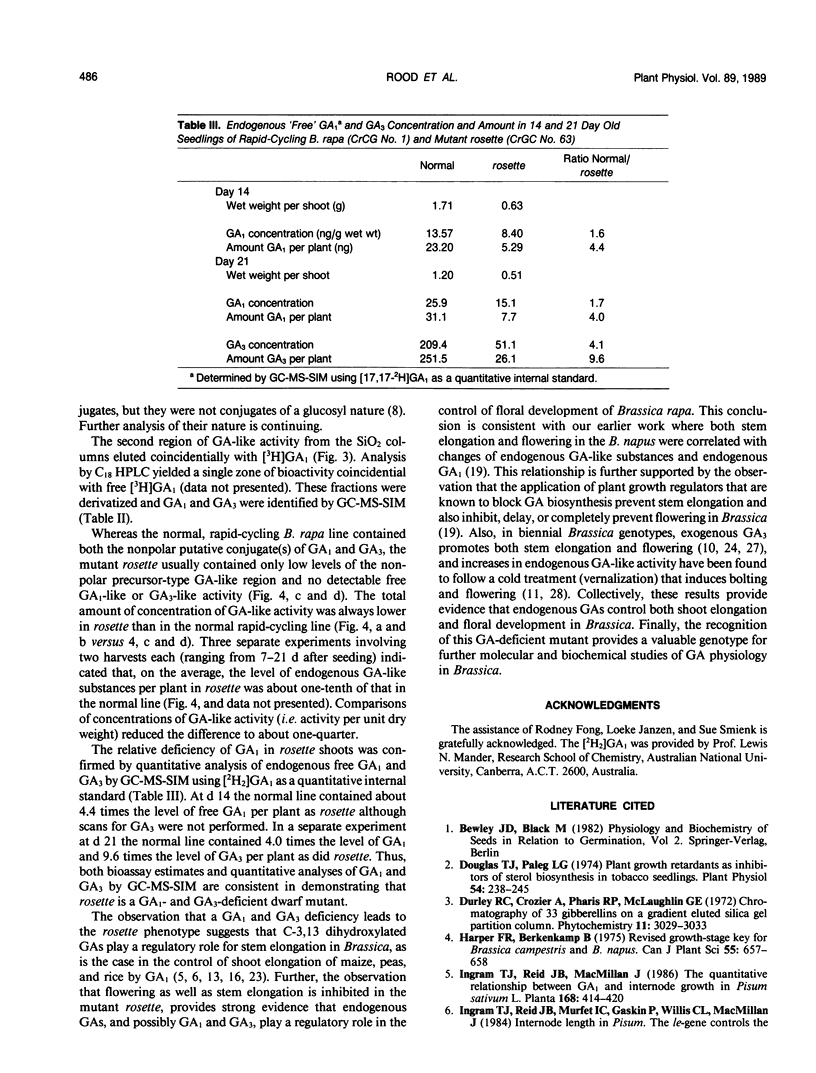
A single-gene mutant (rosette [ros/ros]) in which shoot growth and development are inhibited was identified from a rapid cycling line of Brassica rapa (syn campestris). Relative to normal plants, the mutant germinated slowly, had delayed or incomplete floral development, and reduced leaf, petiole, and internode growth. The exogenous application of GA3 by foliar spray or directly to the shoot tip of rosette resulted in rapid flowering, bolting (shoot elongation), and viable seed production. Shoots of rosette contained endogenous levels of total gibberellin (GA)-like substances (`Tan-ginbozu' dwarf rice assay) of about one-tenth of that of the normal rapid-cycling line of B. rapa which consisted almost entirely of a very nonpolar, GA-like substance which yielded GA1 and GA3 upon mild acid hydrolysis. In a normal rapid-cycling B. rapa line, the nonpolar putative GA1 and GA3 conjugates were present, but additionally, free GA1 and GA3 were abundant and identified by gas chromatography-mass spectrometry-selected ion monitoring. The quantities of free GA1 and GA3 in the normal line and in rosette were quantified by GC-MS-SIM using [2H2]GA1 as an internal standard. Fourteen-day-old rosette and normal seedlings contained 5.3 and 23.2 ng GA1 per plant, respectively. At day 21 the rosette plants contained 7.7 and 26.1 nanograms per plant of GA1 and GA3, while normal plants contained 31.1 and 251.5 nanograms per plant, respectively. Thus, normal plants contained from four to ten times higher levels of total GA-like substances, GA1, or GA3, than rosette. The ros allele results in reduced GA level, yielding the rosette phenotype whose delayed germination and flowering, and reduced shoot growth responses indicate a probable role for endogenous GA1 and GA3 in the regulation of these processes in Brassica.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Douglas T. J., Paleg L. G. Plant growth retardants as inhibitors of sterol biosynthesis in tobacco seedlings. Plant Physiol. 1974 Sep;54(3):238–245. doi: 10.1104/pp.54.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A. THE EFFECT OF GIBBERELLIN UPON FLOWER FORMATION. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):709–717. doi: 10.1073/pnas.43.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney B. O., West C. A., Ritzel M., Neely P. M. EVIDENCE FOR "GIBBERELLIN-LIKE" SUBSTANCES FROM FLOWERING PLANTS. Proc Natl Acad Sci U S A. 1957 May 15;43(5):398–404. doi: 10.1073/pnas.43.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Larsen K. M., Mander L. N., Abe H., Pharis R. P. Identification of endogenous gibberellins from sorghum. Plant Physiol. 1986 Sep;82(1):330–332. doi: 10.1104/pp.82.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Mandel R., Pharis R. P. Endogenous Gibberellins and Shoot Growth and Development in Brassica napus. Plant Physiol. 1989 Jan;89(1):269–273. doi: 10.1104/pp.89.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pearce D., Pharis R. P. Identification of endogenous gibberellins from oilseed rape. Plant Physiol. 1987 Nov;85(3):605–607. doi: 10.1104/pp.85.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pharis R. P. Changes of Endogenous Gibberellin-like Substances with Sex Reversal of the Apical Inflorescence of Corn. Plant Physiol. 1980 Nov;66(5):793–796. doi: 10.1104/pp.66.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pharis R. P., Koshioka M. Reversible conjugation of gibberellins in situ in maize. Plant Physiol. 1983 Oct;73(2):340–346. doi: 10.1104/pp.73.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Hill C. B. Rapid-cycling populations of brassica. Science. 1986 Jun 13;232(4756):1385–1389. doi: 10.1126/science.232.4756.1385. [DOI] [PubMed] [Google Scholar]
- Wittwer S. H., Bukovac M. J. Gibberellin Effects on Temperature and Photoperiodic Requirements for Flowering of Some Plants. Science. 1957 Jul 5;126(3262):30–31. doi: 10.1126/science.126.3262.30. [DOI] [PubMed] [Google Scholar]



