Graphical abstract
Keywords: Glucosamine, ROS production, Nrf2, Endothelium, O-GlcNAc, Scavenging activity
Highlights
-
•
Glucosamine (GlcN) is a weak direct antioxidant.
-
•
GlcN induces ROS production in a concentration-dependent manner in HMEC-1.
-
•
GlcN is an indirect antioxidant, it induces Nrf2 expression in HMEC-1.
-
•
O-GlcNAc plays a key role in ROS production and Nrf2 expression in HMEC-1.
Abstract
Glucosamine (GlcN) is the most used supplement for osteoarthritis treatment. In vitro studies have related GlcN to beneficial and detrimental effects on health. The aim of this study was to evaluate the effects of O-linked-N-acetylglucosaminylation (O-GlcNAc) on GlcN-induced ROS production and Nrf2 expression in human dermal microvascular endothelial cells-1 (HMEC-1) and to evaluate the antioxidant capacity of GlcN compared to well-known antioxidants. For this, we evaluate the antioxidant capacity by in vitro assays. Besides, the GlcN (5–20 mM) effects on cell viability, reactive oxygen species (ROS) production, O-GlcNAc, and nuclear factor erythroid-2-related factor 2 (Nrf2) expression with and without the O-GlcNAc inhibitor OSMI-1 (10 μM) in HMEC-1 were evaluated. GlcN showed high inhibitory concentration (low scavenging activity) against superoxide (O2•─, IC20 = 47.67 mM), 2,2-diphenyl-1-picrylhydrazyl (DPPH•, IC50 = 21.32 mM), and hydroxyl (HO•, IC50 = 14.04 mM) radicals without scavenging activity against hydrogen peroxide (H2O2) and low antioxidant capacity determined by oxygen radical absorbance capacity (ORAC, 0.001 mM Trolox equivalent) and ferric reducing antioxidant power (FRAP, 0.046 mM Trolox equivalent). In cell culture, GlcN (20 mM) reduced cell viability up to 26 % and induced an increase in ROS production (up to 70 %), O-GlcNAc (4-fold-higher vs. control), and Nrf2 expression (56 %), which were prevented by OSMI-1. These data suggest an association between O-GlcNAc, ROS production, and Nrf2 expression in HMEC-1 cells stimulated with GlcN.
1. Introduction
Glucosamine (GlcN) is an amino sugar widely distributed in nature. It has several biomedical applications in the food and cosmetic industry (Eshwaran et al. 2021). It is a precursor of glycosaminoglycans in articular cartilage and is employed for osteoarthritis treatment (Derwich et al. 2023). GlcN is easily accessible since it is found as a food supplement in pharmacies and shopping malls. It is the fourth most-used dietary supplement worldwide and the number one for patients with osteoarthritis (Eshwaran et al. 2021).
GlcN is incorporated into metabolism through the hexosamine biosynthetic pathway, increasing O-linked-N-acetylglucosaminylation (O-GlcNAc) in several proteins. It is a reversible and dynamic post-translational modification that binds to serine and threonine hydroxyl residues of cytoplasmic, nuclear, and mitochondrial proteins (Paneque et al. 2023). O-GlcNAc is carried out by the enzyme O-GlcNAc transferase (OGT), and it is removed by the enzyme β-N-acetylglucosaminidase (OGA) (Paneque et al. 2023). O-GlcNAc regulates the cellular response to hormones, stress response and modulates cell growth and division. In addition, it is essential for cell viability, activation of the immune system, apoptosis, stress response, embryonic development, cell transport, gene transcription, and it acts as a nutrient and stress sensor, among others (Chen et al., 2018, Paneque et al., 2023).
Reactive oxygen species (ROS) are partially reduced species of molecular oxygen that are continuously produced in cellular metabolism; examples are superoxide anion (O2•─), hydroxyl radicals (HO•), peroxyl radicals (ROO•), and hydrogen peroxide (H2O2), among others. These ROS can react with each other and with biomolecules like proteins, carbohydrates, lipids, and deoxyribonucleic acid affecting their structure and function. ROS production and oxidative stress increase are associated with the development of cancer, type 2 diabetes mellitus, atherosclerosis and cardiovascular diseases, among other diseases (Kaneto et al. 2010). Nuclear factor erythroid-2-related factor 2 (Nrf2) could be induced to prevent oxidative stress. This transcription factor coordinates the basal cytoprotective gene activation and inducible activation by oxidative and electrophilic stress (Qi and Dong 2021). Nrf2 promotes the transcription of cytoprotective genes, including antioxidant or antioxidant-generating enzymes, metabolizers of xenobiotics, and nicotinamide adenine dinucleotide phosphate (NADPH)-synthesizing enzymes (Yu and Xiao, 2021).
GlcN is considered a potent antioxidant against O2•-, HO•, DPPH• and others. However, it has not yet been compared to standard antioxidants (Yan et al., 2007, Xing et al., 2009, Jamialahmadi et al., 2014), so the magnitude of the antioxidant capacity of GlcN is still unknown. GlcN is also an indirect antioxidant since it activates Nrf2 in iodinated contrast media-damaged kidneys of rats (Hu et al. 2017). These antioxidant properties of GlcN could become important for endothelium, the cellular layer that covers the blood vessels and performs diverse biological effects, emphasizing the immunological and hemostatic ones. The direct antioxidant capacity and the expression of Nrf2 in endothelial cells may counter some of the mechanisms, though GlcN and other compounds could contribute to endothelial dysfunction. GlcN increases vascular contraction in the aorta rings in diffusion (Kim et al. 2011), induces vascular damage, and increases the risk of hypertension and atherosclerosis (Dela Justina et al. 2020). Besides, GlcN increases O2•─ levels and decreases tumor necrosis factor-α-induced nitric oxide production in human umbilical vein endothelial cells (HUVEC) (Rajapakse et al. 2009), induces apoptosis and inflammation in HUVEC cells (Cai et al. 2017), increases the O2•─ production of mitochondrial and the O-GlcNAc of endothelial nitric oxide synthase (eNOS), decreasing its activity by 67 % in bovine aortic endothelial cells (Du et al. 2001). Furthermore, increased O-GlcNAc induces endothelial dysfunction by activating the enzyme NADPH oxidase (Souza-Silva et al. 2018) and inactive eNOS, inducing endothelial dysfunction of mouse penile tissue (Musicki et al. 2005). By contrast, GlcN showed beneficial effects in preventing the expression of ICAM-1 and MCP-1 induced by TNF-α in HUVEC (Ju et al. 2008). Based on the above, the aim of the present study was to evaluate the effects of GlcN induced-O-GlcNAc on ROS production and Nrf2 expression in human dermal microvascular endothelial cells-1 (HMEC-1) to explore the possible association between the three biological processes. In addition, by first time the magnitude of the antioxidant capacity of GlcN was evaluated against to well-known antioxidant standards. Our research contributes to clarify whether GlcN is a potent antioxidant compound or not and establishes a link between ROS production and Nrf2 expression in GlcN-induced O-GlcNAc in HMEC-1.
2. Material and methods
2.1. Reagents
The HMEC-1 (CRL-3243TM ATCC) cell line was generously donated by Dr. Felipe Masso of the Instituto Nacional de Cardiología Ignacio Chávez (CDMX, Mexico). Bovine serum albumin (BSA), 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH), xanthine, xanthine oxidase, nitroblue tetrazolium (NBT), sodium fluorescein, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), terephthalic acid, ascorbic acid, ammonium ferrous sulfate, Amplex Red, butylated hydroxytoluene, dimethyl sulfoxide (DMSO), ferric tripyridyl triazine (Fe3+-TPTZ), quercetin hydrate (Q), D-(+)-glucosamine hydrochloride (GlcN, 99 %), 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT), dihydroethidium (DHE), triton X-100, Tris(2-pyridyl)-s-triazine, p-formaldehyde, αR)-α-[[(1,2-Dihydro-2-oxo-6-quinolinyl)sulfonyl]amino]-N-(2-furanylmethyl)-2-methoxy-N-(2-thienylmethyl)-benzeneacetamide (OSMI-1), xylenol orange, iron (III) chloride (FeCl3) and 2-propanol were from Sigma-Aldrich (St. Louis, MO, USA). Trolox was from EMD Millipore (Billerica, MA, USA, 98 %). 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was from Molecular Probes (Eugene, OR, USA). Fetal bovine serum (FBS) was from Invitrogen Life Technologies (Carlsbad, CA, USA). Alexa Fluor® 488 and Alexa Fluor® 594 (Jackson ImmunoResearch, PA, USA), 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, from Invitrogen, CA, USA), Nrf2 (SC-722) and O-GlcNAc (SC-59623) antibodies were from Santa Cruz Biotechnology (Dallas, TX, USA). Ethylenediaminetetraacetic acid disodium salt (Na2EDTA) and H2O2 (30 %) were from JT Baker (Xalostoc, Edo. de Mexico, Mexico). Endothelial basal medium (EBM)-2 medium and its supplements were purchased from Lonza (Walkersville, MD, USA). All other reagents were of analytical grade and commercially available.
2.2. Antioxidant activity of GlcN
GlcN (500 mM) or Trolox (10 μM) were dissolved in deionized water, while Q (250 mM) was prepared in DMSO. The compound concentration was based on a previous study (Gallegos-Velasco et al. 2023) and preliminary tests (data not shown). All dilutions were made in deionized water. The direct antioxidant activity tests were performed in a Synergy HT multi-mode microplate reader (Biotek Instruments, Winooski, VT, USA).
2.3. O2•─ assay
Trapping activity was evaluated by prevention of formazan production in the presence of GlcN, Q or water (H2O, 0 % scavenging). The radical was produced by the xanthine-xanthine oxidase system. This radical reduces NBT into formazan. The 295 nm and 560 nm optical densities were used to measure xanthine oxidase activity and formazan production, respectively (Fernández-Rojas et al. 2015). Data were expressed as a percent of control (H2O).
2.4. HO• test
This radical was synthesized by the Fenton reaction. The fluorescent 2-hydroxy-terephthalate was produced after HO• reaction with terephthalic acid. A decrease in the fluorescence evidenced the radical scavenging activity. GlcN, Q or H2O (0 % scavenging) were added to the reaction mixture containing 0.2 mM ascorbic acid, 0.2 mM FeCl3, 0.208 mM Na2EDTA, 1 mM H2O2, 1.4 mM terephthalic acid and 20 mM phosphate buffer, pH 7.4. The fluorescence was measured at λex 325 nm and λem 432 nm after 30 min (Trujillo et al. 2016). Data were expressed as a percent of control (H2O).
2.5. H2O2 assessment
Trapping activity against H2O2 was assessed after 20 min incubation of 75 µM H2O2 with GlcN, Q or H2O (0 % entrapment), then a solution of 3.96 mM butylated hydroxytoluene, 0.1 mM xylenol orange and 0.25 mM ferrous ammonium sulfate was added. After 10 min, the optical density at 560 nm was read against H2O2 standard curve (Pedraza-Chaverri et al. 2006). Data were expressed as a percent of control (H2O).
2.6. DPPH• assay
The procedure is based on the reduction of DPPH•, which is detected at 517 nm. GlcN, Q, trolox or H2O (0 % trapping) were mixed with 100 µM DPPH•. Optical density was measured at 517 nm (Fernández-Rojas et al. 2015). Data were expressed as a percent of control (H2O).
2.7. ORAC assay
This test is based on the Trolox equivalent capacity of compounds to delay the peroxyl radical-induced decrease fluorescein fluorescence at excitation and emission wavelengths of 485 and 520 nm, respectively. Results were expressed as Trolox equivalents. GlcN, Q, trolox standard (0–10 μM) were added to the reaction mixture containing 23 nM fluorescein and 19 mM AAPH. Fluorescence was measured for 1.5 h at excitation and emission wavelengths of 485 and 520 nm, respectively, using the Gen5 software (Biotek Instruments) (Fernández-Rojas et al. 2015).
2.8. FRAP assay
The principle of this analysis is based on the Trolox equivalent capacity to reduce Fe3+ to Fe2+, which forms a complex with 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ). This complex is detected at 593 nm. The antioxidant capacity of GlcN, trolox or Q were revealed after reacting with the colorless ferric tripyridyl triazine (Fe3+-TPTZ) complex and forming blue Fe2+-TPTZ. Briefly, samples were mixed with FRAP reagent (0.833 mM TPTZ, 1.66 mM FeCl3, in 250 mM acetate buffer, pH 3.6), and after 15 min, the absorbance at 593 nm was measured in a microplate reader (Trujillo et al. 2016). Results were expressed as Trolox equivalents.
2.9. Cell culture treatment
Assays were performed in the HMEC-1 cell line. Cells were used at passage number 8–12 and grown in EBM-2 medium supplemented with 10 % FBS, hydrocortisone, gentamicin, ascorbic acid, epidermal growth factor, and streptomycin sulfate according to the manufacturer's instructions and maintained at 37 °C. For experiments, 18,000 cells/cm2 were seeded and allowed to adhere for 24 h in EBM-2 medium with 10 % FBS. Cells were used at 80 % confluency in EBM-2 medium with 2 % FBS for experimental procedures overnight. The GlcN was dissolved in EBM-2 medium, the pH was adjusted to 7.2 and subsequently sterilized by filtration. Cells were treated with 0 (control group, C), 5, 10, 15 and 20 mM GlcN for 24 h (Gallegos-Velasco et al. 2023). The experimental conditions were carried out with EBM-2 medium with 2 % FBS for the following determinations.
2.10. Cell viability
The number of viable cells was estimated by the MTT assay. After treatment, 1 mM MTT was added to each well of 96-well plates and incubated for 3 h at 37 °C. Then, the medium was carefully removed, and the formazan crystals were dissolved with 150 μl of acid 2-propanol. Absorbance was measured at 570 nm using the Cytation 5 Cell Image Reader plate reader (Biotek Instruments, Inc.). Results were expressed as percent change from the control group (no treatment).
2.11. Time-course study of O-GlcNAc
GlcN-induced O-GlcNAc expression was assessed using 8-well slide cell culture with the Nunc Lab-Tek chamber system. Cells were seeded (30,000 cells/cm2) and allowed to adhere for 24 h. Subsequently, the cells were cultured with 2 % FBS and 5, 10 and 20 mM GlcN were placed for 2, 4, 6, 12 and 24 h (Cai et al. 2017). Then cells were fixed with 0.4 % p-formaldehyde for 15 min and permeabilized with 0.3 % triton X-100 for 5 min. Cells were blocked with 2 % BSA for 1 h and the anti-O-GlcNAc antibody (1:1000) was added for 2 h at room temperature. Later, the cells were washed with phosphate-buffered saline (PBS) and incubated with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:1000 + BSA 1 %) for 2 h at room temperature in the dark. The slides were mounted with cover slides and were sealed. Cells were observed with an AXIOSCOP 40 microscope with 10X (ZEISS, Oberkochen, Germany) and the images were analyzed with the ZEN program (ZEISS, Jena, Germany).
2.12. Measurement of ROS production
ROS production was quantified using two fluorescent dyes, H2DCFDA and DHE, as previously reported (Fernández-Rojas et al. 2020). Cells were seeded (28,000 to 37,500 cells/cm2) in 96-well fluorescence plates and allowed to adhere for 24 h. Subsequently, the cells were treated with GlcN for 6 h with or without the pre-treatment with 10 μM OSMI-1 for 24 h or treated with 1 mM H2O2 for 2 h, as a positive control. Then, the medium was removed, and the cells were washed with sterile PBS and co-incubated with 10 μM H2DCFDA or 40 μM DHE in EBM-2 medium with 2 % FBS for 30 min at 37 °C in the dark. The medium was then removed, the cells were washed with sterile PBS, and EBM-2 medium with 2 % FBS was added. Photographs and fluorescence were obtained with the multi-modal microplate reader with Cytation 5 cell culture imaging (imaging reader) at λx 485/20 and λem 528/20 for the fluorochrome H2DCFDA and λex 480/20 and λem 576/20 for DHE fluorochrome using 20X magnification. Results were expressed as percent change from the control group (no treatments).
2.13. Evaluation of O-GlcNAc and Nrf2 expression
HMEC-1 cells (250,000 cells/cm2) were seeded on sterile coverslips placed in a 12-well plate and allowed to adhere by maintaining them in EBM-2 medium with 2 % FBS for 24 h. Cells were treated for 6 h with 20 mM GlcN or 100 μM Q (Nrf2 positive control) with or without the pre-treatment with 10 μM OSMI-1 for 24 h. After treatment, the cells were fixed at room temperature with Karnosky's solution for 30 min under agitation. Subsequently, the cells were permeabilized with PBS 1X-triton 0.5 % for 15 min. Cells were blocked with 2 % BSA and washed three times. Antibodies against Nrf-2 (1:1,000), and O-GlcNAc (1:1000) were added and incubated overnight at 4 °C. Then the cells were incubated with Alexa Fluor® 488 and Alexa Fluor® 594-conjugated secondary antibodies for 2 h at room temperature in the dark with shaking. After 3 washes with PBS 1 μg/mL, DAPI was added to counterstain nuclei. Imaging and fluorescence intensity was obtained with Cytation 5 Cell Image Reader (Biotek Instruments, Inc.).
2.14. Statistical analysis
Results are expressed as mean value ± standard error of the mean (SEM). Results of scavenging activity were expressed as the ability of the sample to scavenge 50 % of each ROS (IC50). For this, values were determined by interpolation using the least-squares method calculated from at least three independent experiments. Cell culture data were analyzed by one-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparison test using GraphPad Prism for Windows version 8.02. (GraphPad Software, San Diego, CA, USA). At least three replications were performed for all biochemical and cell viability assays. A value of p ≤ 0.05 was considered statistically significant.
3. Results
3.1. GlcN has low antioxidant scavenging activity
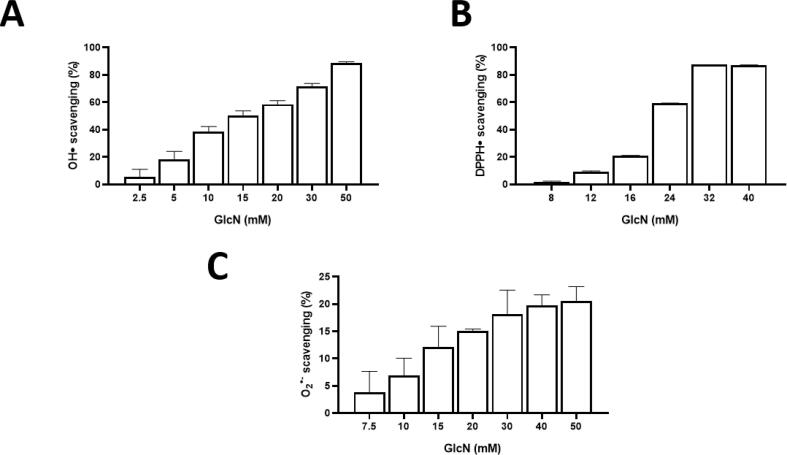
It was observed that GlcN scavenges ROS and possesses low total antioxidant capacity. As shown in Table 1 and Fig. 1, GlcN scavenges O2•─, HO•, and DPPH• at mM concentrations. At 50 mM (10.78 mg/mL), GlcN was able to scavenge 88.6 ± 0.9 % and 87.2 ± 0.22 % of HO• and DPPH•, respectively, but it was only able to scavenge 20.5 ± 4.6 % of O2•─ and does not scavenge H2O2. GlcN also showed antioxidant capacity, determined by ORAC and FRAP assays, although its values, expressed as Trolox equivalents, were low. Quercetin (Q), a well-recognized antioxidant, was tested in the same assays as the antioxidant standard. Unlike GlcN, it requires lower concentrations to scavenge DPPH• and O2•─ and has a higher total antioxidant capacity than GlcN (Table 1).
Table 1.
Scavenging and antioxidant activities of glucosamine (GlcN) and quercetin (Q).
| IC50 (mM) |
mM Trolox equivalents |
||||||
|---|---|---|---|---|---|---|---|
| O2•─ | HO• | DPPH• | H2O2 | ORAC | FRAP | ||
| GlcN | NR* | 14.69 ± 0.63 | 21.27 ± 0.01 | – | 1.1 ± 0.1 (1 0 −3) | 46 ± 0.2 (1 0 −3) | |
| Q | 10.45 ± 2.72 (1 0 −3) | ND | 7.69 ± 0.23 (1 0 −3) | – | 2.1 ± 0.1 | 22.06 ± 0.17 | |
GlcN: glucosamine, O2•─: Superoxide anion radical, HO•: Hydroxyl radical, DPPH•: 2,2-Diphenyl-1-picrylhydrazyl radical, H2O2: hydrogen peroxide, ORAC: Oxygen radical absorbance capacity, FRAP: Ferric reducing antioxidant power. Trolox: 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. IC50: concentration that neutralizes 50 % of each ROS, NR = Not reached (*GlcN at the maximum concentration of 50 mM scavenged 20.5 ± 4.6 %), -: Without scavenging activity. ND: Not detected. Data are shown as mean ± SEM. n ≥ 3.
Fig. 1.
Scavenging activity of glucosamine (GlcN) against A: hydroxyl (HO•), B: 2,2-Diphenyl-1-picrylhydrazyl (DPPH•) and C: superoxide (O2•-). Data are shown as mean ± SEM, n ≥ 3.
3.2. GlcN reduces cell viability in HMEC-1 cells
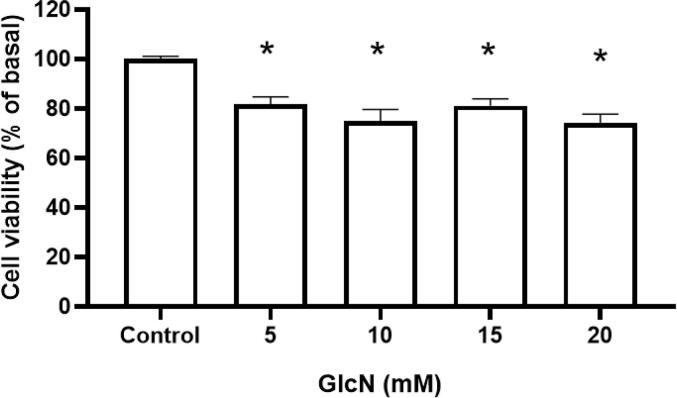
The MTT assay was performed to assess the GlcN effect on cell viability; the results showed that GlcN at 5–20 mM reduced cell viability up to 26 %, p < 0.001, (Fig. 2).
Fig. 2.
GlcN decreases cell viability in human dermal microvascular endothelial cells-1 (HMEC-1). GlcN (5–20 mM) treatment for 24 h reduced cell viability by the MTT assay. Data are shown as mean ± SD, * p ≤ 0.05 vs. control group (C), n = 5.
3.3. GlcN induces O-GlcNAc in a concentration- and time-dependent manner in HMEC-1 cells
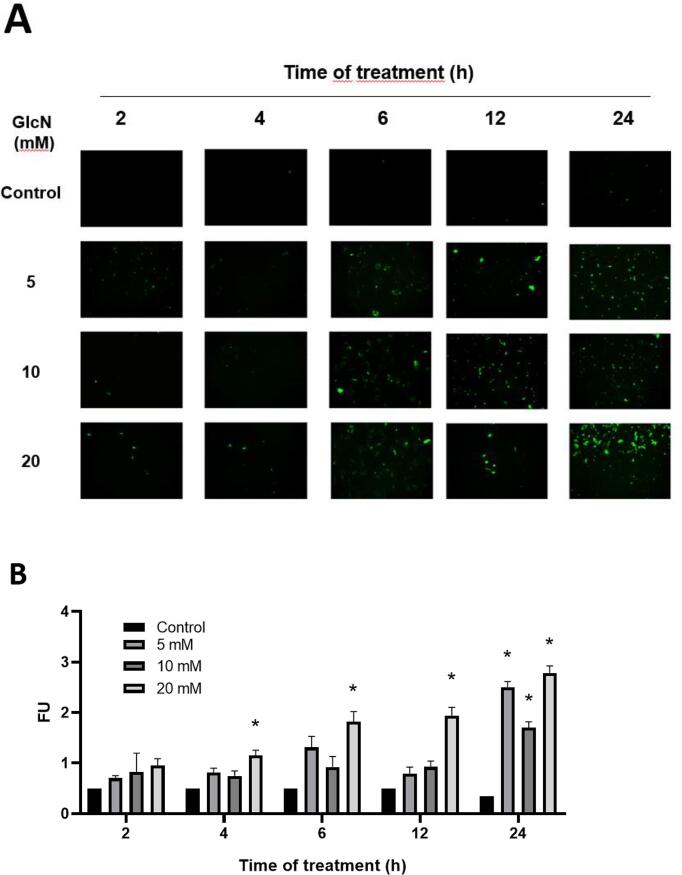
HMEC-1 cells were treated with 5, 10, and 20 mM GlcN in a time-course study (2, 4, 6, 12, and 24 h) to assess the induction of O-GlcNAc expression (Fig. 3A, B). After 2 h of treatment, an increase in O-GlcNAc was observed with all concentrations tested, although such increases were not statistically significant. After 4 and 6 h of treatment with 20 mM GlcN, a significant (p < 0.024) 2 and 4-fold increase in O-GlcNAc was observed (respectively), and it continues to increase up to 8-fold after 24 h (p = 0.0032). In addition, the treatments with 5 and 10 mM GlcN increase the O-GlcNAc up to 7 (p = 0.0026) and 5-fold (p = 0.0071) after 24 h, respectively (Fig. 3B). Due to a greater difference with the control group (4-fold increase) in few hours, it was decided to work at 6 h with 20 mM GlcN for further experiments.
Fig. 3.
Time-course of O-linked-N-acetylglucosaminylation (O-GlcNAcylation) induced by 5, 10, and 20 mM GlcN in HMEC-1 cells. A: Representative micrographs (10X) illustrating O-GlcNAcylation expression. B: Quantification of O-GlcNAcylation expression. Data are mean ± SEM, n = 3, *p ≤ 0.05 vs. control.
3.4. GlcN induced Nrf2
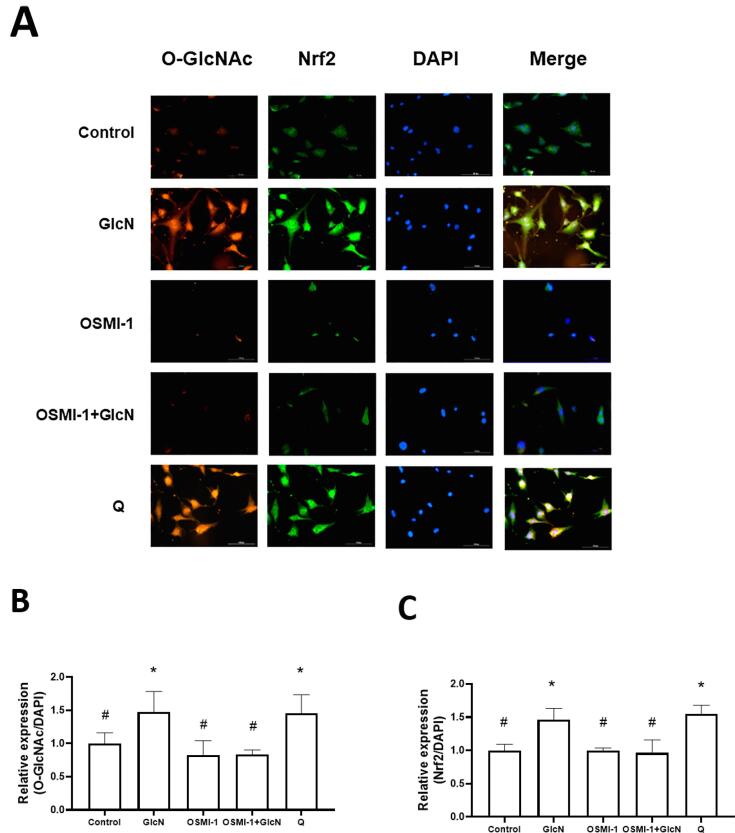
Based on the above results, HMEC-1 cells were treated with 20 mM GlcN for 6 h to analyze its effect on Nrf2 expression. As expected, GlcN increased (p = 0.0365 vs. control group) its expression (Fig. 4A and C). To test if the induction of Nrf2 expression was related to O-GlcNAc, GlcN-treated HMEC-1 cells were also treated for 24 h with 10 μM OSMI-1. It was observed that both O-GlcNAc (p = 0.004 vs. GlcN group) and Nrf2 (p = 0.0008 vs. GlcN group) expression did not increase (Fig. 4A and C), which suggest that these processes are related (no significant difference compared to the control group). The same was observed in the OSMI + GlcN group (p ≤ 0.004). Additionally, cells treated for 6 h with 100 μM quercetin (Q), a well-known Nrf2 inductor increased its expression (p = 0.002) (Fig. 4A and C).
Fig. 4.
GlcN-induced O-GlcNAc and nuclear factor erythroid-2-related factor 2 (Nrf2) expression in HMEC-1 is related to O-GlcNAcylation. A: Representative images (20X) of HMEC-1 cells treated with 20 mM GlcN (6 h), 10 μM OSMI-1 (24 h, O-GlcNAc transferase inhibitor), OSMI-1 + GlcN or 100 μM Q (6 h; as a Nrf2 inducer). The red, green, and blue signals represent the O-GlcNAcylation and Nrf2 expression, and nuclear stain, respectively. B: Quantification of O-GlcNAcylation expression. C: Quantification of Nrf2 expression. Data are mean ± SEM, n = 3 (independent experiments), *p ≤ 0.05 vs. control, #p ≤ 0.05 vs. GlcN.
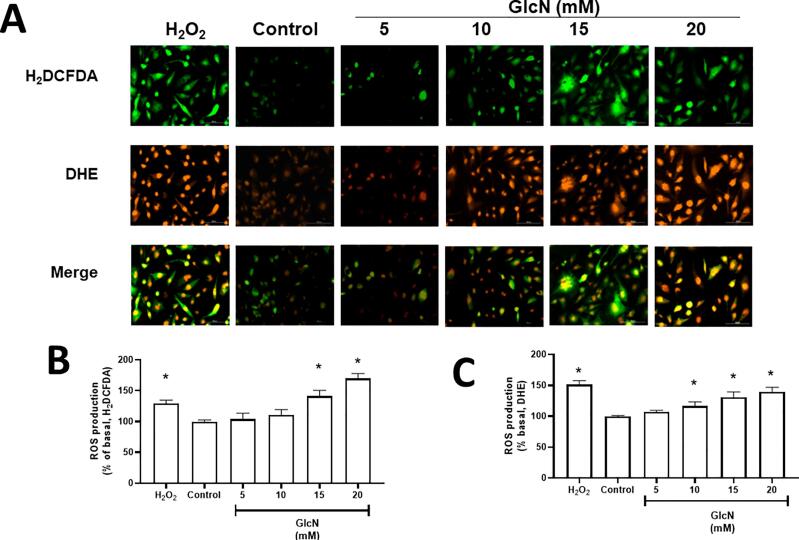
3.5. GlcN induces ROS production in a concentration-dependent manner in HMEC-1 cells
We investigated if ROS production is also related to O-GlcNAc. For this, HMEC-1 cells were treated with 5, 10, 15 and 20 mM GlcN for 6 h to evaluate ROS production using H2DCFDA and DHE. HMEC-1 cells treated with 15 mM GlcN showed an increase of ROS production both with H2DCFDA and DHE probes by 40 % (p = 0.0001) and 31 % (p = 0.0002), respectively, whereas treatment with 20 mM GlcN increased by 68 % (p < 0.0001) and 40 % (p < 0.0001), respectively (Fig. 5). In addition, cells exposed to 1 mM H2O2 for 2 h were used as a positive control for both probes.
Fig. 5.
GlcN induced reactive oxygen species (ROS) in a concentration-dependent manner in HMEC-1 cells. A: Representative images of intracellular ROS production (20X) by 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorochrome is observed in green, while the dihydroethidium (DHE) fluorochrome is shown in red. B: Quantification using H2DCFDA and C: DHE. Data are shown as mean ± SEM, n = 3. *p ≤ 0.05 vs. control. Treatment of 1 mM H2O2 for 2 h was used as the positive control.
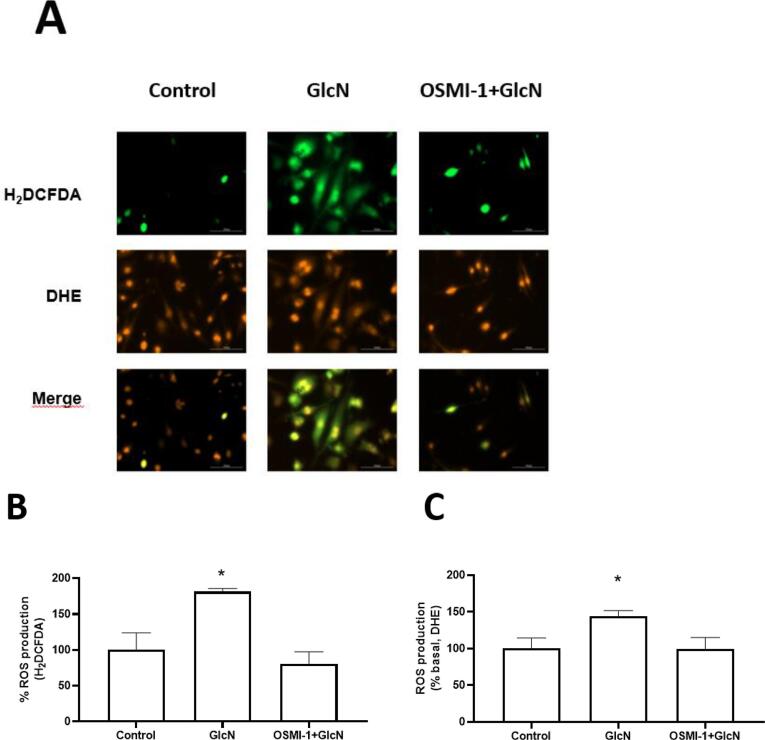
3.6. ROS production induced by GlcN is related to O-GlcNAc
OSMI- was used to establish the role of O-GlcNAc in ROS production in HMEC-1 cells. The results indicate that OSMI-1 reduces ROS production (with both fluorochromes) in HMEC-1 to similar levels to the control group (Fig. 6). In addition, ROS production induced by 20 mM GlcN started from 1 h and continued to increase until 6 h (data not shown), which suggests that GlcN-induced O-GlcNAc has a prooxidant activity since it enhances ROS production in HMEC-1 cells.
Fig. 6.
Inhibition of O-GlcNAcylation by OSMI-1 reduced GlcN-induced ROS production in HMEC-1 cells. A: representative images of intracellular ROS production (20X). Quantitative analysis of fluorescence intensity of B: H2DCFDA (green signal) and C: DHE (red signal). Each bar represents the mean ± SEM, n = 3. *p ≤ 0.05 vs. control.
4. Discussion
The present study evaluated the role of GlcN as a direct or indirect antioxidant in HMEC-1 to explore a possible beneficial effect on the vasculature and its association to O-GlcNAc. Nodaway, it is well-documented the clinical implication of endothelium dysfunction for the development of atherothrombosis disease (Beriault and Werstuck 2012). Oxidative stress is one of the leading causes of endothelial dysfunction (Beriault and Werstuck 2012), so it would be desirable that one of the most used dietary supplements worldwide have antioxidant activity. Investigating the antioxidant activity of GlcN, we realized that it had not been compared with compounds that are considered as antioxidant standards; first, we evaluated its antioxidant activity with Q or Trolox, some of the most used antioxidant standards. We found that GlcN scavenges HO• and DPPH• and to some extent O2•─ and shows activity both in FRAP and ORAC assays. However, the IC50 required to stabilize them are extremely high compared to Q (Table 1). We highlight that our study is the first to compare the magnitude of the antioxidant capacity of GlcN against standard antioxidants (Q or Trolox); however, GlcN did not show a homogeneous antioxidant capacity. Regarding O2•- scavenging activity, our study showed that GlcN 50 mM showed a O2•- scavenging activity of 20.5 ± 4.6 %. By contrast, Chen et al. (Chen et al. 2003) found that this compound scavenges 10.6 % at 100 μM; other authors have found that GlcN scavenges 20.6 % at 5.6 mM (Mendis et al. 2008), 40 % at 46.3 mM (Wang et al. 2012) and 84 % at 3.7 mM (Xing et al. 2006). For HO•, we observed that 50 mM GlcN scavenging HO• in 88.6 ± 0.9 % with an IC50 of 14.69 ± 1.3 mM, similar to (Xing et al. 2009). However, a previous study showed that GlcN 100 μM scavenges 37 % (Mendis et al. 2008). Other reports indicate a 10 % scavenging effect at 46.3 mM (Wang et al. 2012), 26.2 % at 5.6 mM (Mendis et al. 2008); 52 % at 15 mM (Xing et al. 2009), 56 % at 362 mM (Xing et al. 2006), and 72 % at 22.3 mM (Yan et al. 2007). Regarding DPPH•, we found a scavenging of 87.2 ± 0.22 % at 50 mM, while another study found a 50 % of scavenging at 40 mM. (Jamialahmadi et al. 2014). For the FRAP test, previous data showed that 1.11 mM has a chelating effect of 90 % (Yan et al. 2007); however, we found a similar result (89.6 ± 0.7 %) at a higher concentration (38.5 mM) and a low equivalent Trolox value (46 ± 0.2 μM).
The differences between study results may be a consequence of the diverse sources of GlcN and the different batches since some authors synthesize their own GlcN sample and even different methodologies used. For example, Xing et al. and Yan et al. induced HO• production by the Fenton reaction with H2O2 and iron, then the HO• reacts with deoxyribose. Although the deoxyribose degradation depends on HO• production, this method is usually employed to determine oxidative damage (Yan et al., 2007, Xing et al., 2009). By contrast, our results for H2O2 were similar to those reported by Chen et al., who indicated that GlcN did not scavenge H2O2 (Chen et al. 2003). However, a high amount of GlcN was required to neutralize O2•-, HO•, and DPPH• and the same for FRAP and ORAC assays. These results suggest that GlcN has low antioxidant activity for all assays tested. In addition, for FRAP and ORAC assays, the Trolox equivalents for GlcN were in the μM range. On the other hand, we also compare the antioxidant activity of GlcN vs. Q, a widely studied flavonoid with a potent antioxidant activity (Chittasupho et al. 2021). Our study showed that Q can scavenge O2•─ and DPPH• with a high capacity to reduce iron. These tests also showed that Q has low IC50 (in the μM range), and its equivalent Trolox values were in the mM range (Table 1), as other authors have found (Coballase-Urrutia et al., 2010, Chittasupho et al., 2021). These findings once again show that GlcN is a weak antioxidant compared to Q and Trolox. Besides, if we consider that GlcN is usually found in plasma at 2 to 20 μM after consumption (Rajapakse et al. 2009), so much of GlcN would be required to scavenge the ROS evaluated here. That is, the GlcN requirement to scavenge ROS is extremely high compared to the concentration in plasma; therefore, this amino sugar is not a powerful direct antioxidant as other authors suggested (Chen et al., 2003, Yan et al., 2007, Xing et al., 2009).
Regarding the toxicity of GlcN on HMEC-1 cells, this amino sugar at any concentration reduced cell viability at 24 h in a similar way to other authors found on HUVEC cells (1- ≥15 mM GlcN) (Reine et al., 2016, Cai et al., 2017) and in smooth muscle cells (SMC, 2.5 mM GlcN) (Duan et al. 2005). This reduction in cell viability is probably due to apoptosis, as determined by Cai et al. (2017), who observed apoptosis in HUVEC cells after incubation with 15 mM GlcN for 24 h. Although these data suggest that GlcN at high concentrations (5–20 mM) might affect endothelial cells, these concentrations were used in vitro assays and would hardly be reached in humans. Despite this, we highlight that high concentrations (5–20 mM) of GlcN are frequently used in other in vitro models to induce insulin resistance (Marshall et al., 1991, Sakai and Clemmons, 2003), mimic the early stages of vascular endothelial cell injury in diabetes (15 mM, 24 h) (Cai et al. 2017) and evaluate its anti-platelet aggregating effect (1–20 mM, human platelets) (Gallegos-Velasco et al. 2023). Whether or not the in vitro results found in HMEC-1 cells explain what could happen in vivo is an issue that should be addressed in other studies.
Considering the role of GlcN on the hexosamine biosynthetic pathway, we evaluated the expression of O-GlcNAc on HMEC-1 cells. In this sense, we observed that 20 mM GlcN (4, 6, and 12 h of treatment) induced an increase of O-GlcNAc in a time-dependent manner in HMEC-1 cells. The largest increase in O-GlcNAc expression on HMEC-1 cells was seen at 6 h with 20 mM GlcN, so further assays were performed at this experimental condition. The fact that O-GlcNAc regulates Keap1/Nrf2 axis (Chen et al. 2017, Xu et al. 2020) let us explore whether GlcN-induced O-GlcNAc could mediate Nrf2 activation in HMEC-1 cells. This mechanism was previously studied in rat renal cells (Hu et al. 2017); however, our research was the first to study it in HMEC-1 cells. As a result, we found that GlcN-induced O-GlcNAc increased Nrf2 expression in HMEC-1 cells, but this response decreased by the effect of OSMI-1 (an OGT enzyme inhibitor). Therefore, these data demonstrated the association between O-GlcNAc and Nrf2 expression in HMEC-1 cells as has been seen in other cells and tissues (Tan et al. 2017, Xu et al. 2020).
Finally, we evaluated whether O-GlcNAc induced by GlcN on HMEC-1 cells was also associated with ROS production. The probe DHE allowed us to monitor intracellular O2•─, H2O2, ONOO–, and HOCl production, while the H2DCFDA probe identified HO•, ROO•, and other ROS (Yazdani 2015). Our study demonstrated an increase in ROS production in HMEC-1 cells, agreeing with Du et al., who described that GlcN increases O2•─ production in bovine aortic endothelial cells (Du et al. 2001) and HUVEC cells (Chao et al. 2008, Rajapakse et al. 2009, Wu et al. 2012). Moreover, the addition of OSMI-1 to HMEC-1 cells reduced the production of ROS induced by GlcN with both fluorescent probes, suggesting an association between O-GlcNAc and ROS production, and likely, between ROS production and Nrf2 expression. Moreover, we assume that the decreased viability of HMEC-1 cells induced by GlcN could be mediated by the increased ROS, however, this hypothesis was not evaluated in this study. On the other hand, the enzymes eNOS and NADPH oxidase as well as the mitochondrial electron transport chain could be the sources of ROS production in the O-GlcNAc (Du et al. 2001; da Costa et al. 2018).
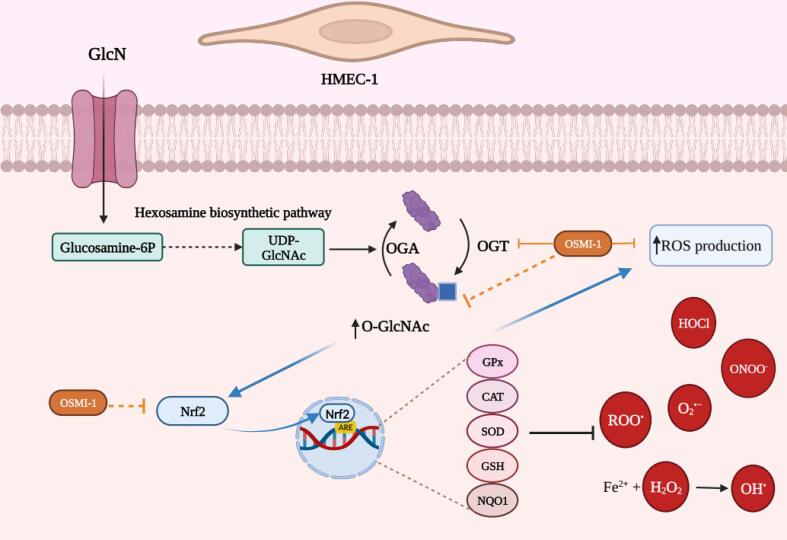
In vivo, our results may suggest that GlcN consumption might be beneficial by inducing Nrf2 expression and thus protecting the endothelium against oxidative damage induced by oxidizing agents or diseases associated with oxidative stress. In fact, GlcN consumption (3 g/day/4 weeks) increased glutathione levels (which can be modulated by Nrf2) in erythrocytes and improved endothelial function in 20 volunteers (Katoh et al. 2017). Therefore, GlcN consumption could be beneficial for health through the induction of Nrf2. However, this hypothesis should be handled with caution because the increased activation and expression of Nrf2 in HMEC-1 cells occurs at very high concentrations in vitro compared to those found in plasma, and further experiments should performed to verify in lower concentrations. In addition, the fact that O-GlcNAc plays a role in the indirect antioxidant activity of GlcN does not restrict the possibility that this glycosylation triggers other unfavorable responses in humans. It is well-known that increased O-GlcNAc is associated with the pathogenesis of some illnesses as type 2 diabetes mellitus, cancer, Alzheimer’s disease, hypertension, and atherosclerosis (Pezzella et al. 2022). Despite this, some in vivo models have described both the protective and adverse effects of GlcN on the endothelium. A clinical study showed that GlcN consumption (5 mg/kg, 8 weeks) was associated with atheroprotective effects in apoE null mice C57BL/6J and decreased mortality (Duan et al. 2005). By contrast, it was demonstrated that GlcN consumption could cause injury to the vascular endothelium in the initial stages of atherosclerosis through the stress of the endoplasmic reticulum (Cai et al. 2017). Besides, GlcN consumption in non-diabetic animals induced vascular damage in the retinas, similar to diabetic mice (Eshwaran et al. 2021). This damage may be associated with the development of atherosclerosis, cardiovascular risk, and mortality (Eshwaran et al. 2021). Given the opposing effects of GlcN in vivo studies and the significant variability of those in vitro, there is no doubt about the need for more research to clarify these inconsistencies. Meanwhile, Fig. 7 depicts a possible mechanism of the effect of GlcN on HMEC-1 cells; derived from our assumptions based on the main findings in this research and basic information about O-GlcNAc and Nrf2 induction.
Fig. 7.
Summary of the main findings on HMEC-1. GlcN is incorporated into the cell by glucose transporters (purple line), then is incorporated into the hexosamine biosynthetic pathway (HBP), where the uridine diphosphate (UDP)-GlcNAc substrate is used by the OGT enzyme for O-GlcNAc (blue box) several proteins, while the enzyme OGA removes it. GlcN treatment increases O-GlcNAcylation (blue lines), reactive oxygen species (ROS) production, and nuclear factor erythroid-2-related factor 2 (Nrf2) expression in human dermal microvascular endothelial cells-1 (HMEC-1). The inhibition of O-GlcNAcylation, with OSMI-1, prevents (orange lines) ROS production and Nrf2 expression, suggesting that these processes are related. In addition, GlcN also could scavenge some ROS. Biorender.com GPx = glutathione peroxidase, CAT = catalase, SOD = superoxide dismutase, GSH = glutathione, NQO1 = NAD(P)H quinone oxidoreductase 1, HOCl = hypochlorous acid, ONOO– = peroxynitrite anion, O2•- = superoxide anion, ROO• = peroxyl radicals, H2O2 = hydrogen peroxide, OH• = hydroxyl radicals, HMEC-1 = human dermal microvascular endothelial cells-1. Created with BioRender.com.
5. Conclusion
Our study demonstrated that GlcN has both low ROS scavenging activity and antioxidant capacity compared to well-known antioxidant standards. However, we demonstrated that GlcN is an indirect antioxidant in HMEC-1 cells because it induces the expression of Nrf2. We highlight that our study was the first to show an association between the GlcN-induced O-GlcNAc and Nrf2 expression in HMEC-1 cells. Due to the high concentrations of GlcN used in the research, our findings should be corroborated in vivo.
CRediT authorship contribution statement
B. Fernández-Rojas: Project administration, Supervision, Visualization, Resources, Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Validation, Methodology, Conceptualization. T. Gómez-Sierra: Supervision, Visualization, Writing – review & editing, Formal analysis, Validation, Methodology. O.N. Medina-Campos: Validation, Methodology. J. Hernández-Juárez: Writing – review & editing, Resources, Conceptualization. P.A. Hernández-Cruz: Resources, Conceptualization. I.B. Gallegos-Velasco: . Y. Pérez-Cervera: Writing – review & editing. J. Pedraza-Chaverri: Project administration, Writing – review & editing, Resources, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Dr. Masso for the donation of the HMEC-1 cells and the support provided to BFR as a fellow of the “Retención-CONACYT” program.
Data availability
Data will be made available on request.
References
- Yu C., Xiao J.-H. The keap1-nrf2 system: a mediator between oxidative stress and aging. Oxid. Med. Cell. Longev. 2021;2021:6635460. doi: 10.1155/2021/6635460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beriault, D.R. and Werstuck, G.H., 2012. The Role of Glucosamine-Induced ER Stress in Diabetic Atherogenesis. Experimental diabetes research, 2012, 187018. [DOI] [PMC free article] [PubMed]
- Cai X., Bao L., Ding Y., Dai X., Zhang Z., Li Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017;15(2):825–832. doi: 10.3892/mmr.2016.6054. [DOI] [PubMed] [Google Scholar]
- Chen P.H., Chi J.T., Boyce M. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology. 2018;28(8):556–564. doi: 10.1093/glycob/cwy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.-S., Taguchi T., Sakai K., Kikuchi K., Wang M.-W., Miwa I. Antioxidant activities of chitobiose and chitotriose. Biol. Pharm. Bull. 2003;26(9):1326–1330. doi: 10.1248/bpb.26.1326. [DOI] [PubMed] [Google Scholar]
- Chittasupho C., Manthaisong A., Okonogi S., Tadtong S., Samee W. Effects of quercetin and curcumin combination on antibacterial, antioxidant, in vitro wound healing and migration of human dermal fibroblast cells. Int. J. Mol. Sci. 2021;23(1):142. doi: 10.3390/ijms23010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coballase-Urrutia E., Pedraza-Chaverri J., Camacho-Carranza R., Cárdenas-Rodríguez N., Huerta-Gertrudis B., Medina-Campos O.N., Mendoza-Cruz M., Delgado-Lamas G., Espinosa-Aguirre J.J. Antioxidant activity of Heterotheca inuloides extracts and of some of its metabolites. Toxicology. 2010;276(1):41–48. doi: 10.1016/j.tox.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Dela Justina V., Priviero F., dos Passos R.R., Webb R.C., Lima V.V., Giachini F.R. O-GlcNAc impairs endothelial function in uterine arteries from virgin but not pregnant rats: The role of GSK3β. Eur. J. Pharmacol. 2020;880:173133. doi: 10.1016/j.ejphar.2020.173133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwich, M., Górski, B., Amm, E., and Pawłowska, E., 2023. Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review. International Journal of Molecular Sciences, 24 (5), 4925. [DOI] [PMC free article] [PubMed]
- Du X.L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Investig. 2001;108(9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Paka L., Pillarisetti S. Distinct effects of glucose and glucosamine on vascular endothelial and smooth muscle cells: evidence for a protective role for glucosamine in atherosclerosis. Cardiovasc. Diabetol. 2005;4:16. doi: 10.1186/1475-2840-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshwaran R., Kolibabka M., Poschet G., Jainta G., Zhao D.i., Teuma L., Murillo K., Hammes H.-P., Schmidt M., Wieland T., Feng Y. Glucosamine protects against neuronal but not vascular damage in experimental diabetic retinopathy. Molecular Metabolism. 2021;54:101333. doi: 10.1016/j.molmet.2021.101333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rojas B., Rodríguez-Rangel D.S., Granados-Castro L.F., Negrette-Guzmán M., León-Contreras J.C., Hernández-Pando R., Molina-Jijón E., Reyes J.L., Zazueta C., Pedraza-Chaverri J. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2015;406(1–2):183–197. doi: 10.1007/s11010-015-2436-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Rojas B., Vázquez-Cervantes G.I., Pedraza-Chaverri J., Gutiérrez-Venegas G. Lipoteichoic acid reduces antioxidant enzymes in H9c2 cells. Toxicol. Rep. 2020;7:101–108. doi: 10.1016/j.toxrep.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos-Velasco I.B., Pérez-Acevedo M.Á., Fernández-Rojas B., Hernández-Cruz P.A., Hernández-Juárez J. Glucosamine effects on platelet aggregation of type 2 diabetes mellitus patients: in vitro assays. Cell. Mol. Biol. 2023;69(4):46–52. doi: 10.14715/cmb/2023.69.4.7. [DOI] [PubMed] [Google Scholar]
- Hu J., Chen R., Jia P., Fang Y., Liu T., Song N., Xu X., Ji J., Ding X. Augmented O-GlcNAc signaling via glucosamine attenuates oxidative stress and apoptosis following contrast-induced acute kidney injury in rats. Free Radic. Biol. Med. 2017;103:121–132. doi: 10.1016/j.freeradbiomed.2016.12.032. [DOI] [PubMed] [Google Scholar]
- Jamialahmadi K., Arasteh O., Matbou Riahi M., Mehri S., Riahi-Zanjani B., Karimi G. Protective effects of glucosamine hydrochloride against free radical-induced erythrocytes damage. Environ. Toxicol. Pharmacol. 2014;38(1):212–219. doi: 10.1016/j.etap.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Ju Y., Hua J., Sakamoto K., Ogawa H., Nagaoka I. Modulation of TNF-alpha-induced endothelial cell activation by glucosamine, a naturally occurring amino monosaccharide. Int. J. Mol. Med. 2008;22(6):809–815. [PubMed] [Google Scholar]
- Kaneto, H., Katakami, N., Matsuhisa, M., and Matsuoka, T., 2010. Role of Reactive Oxygen Species in the Progression of Type 2 Diabetes and Atherosclerosis. Mediators of Inflammation, 2010, 453892. [DOI] [PMC free article] [PubMed]
- Katoh A., Kai H., Harada H., Niiyama H., Ikeda H. Oral administration of glucosamine improves vascular endothelial function by modulating intracellular redox state. Int. Heart J. 2017;58(6):926–932. doi: 10.1536/ihj.16-534. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Seok Y.M., Kim I.K., Lee I.K., Jeong S.Y., Jeoung N.H. Glucosamine increases vascular contraction through activation of RhoA/Rho kinase pathway in isolated rat aorta. BMB Rep. 2011;44(6):415–420. doi: 10.5483/BMBRep.2011.44.6.415. [DOI] [PubMed] [Google Scholar]
- Marshall S., Bacote V., Traxinger R.R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system: role of hexosamine in the induction of insulin resistance. J. Biol. Chem. 1991;266(8):4706–4712. [PubMed] [Google Scholar]
- Mendis E., Kim M.-M., Rajapakse N., Kim S.-K. Sulfated glucosamine inhibits oxidation of biomolecules in cells via a mechanism involving intracellular free radical scavenging. Eur. J. Pharmacol. 2008;579(1–3):74–85. doi: 10.1016/j.ejphar.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Musicki B., Kramer M.F., Becker R.E., Burnett A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O -GlcNAc in diabetes-associated erectile dysfunction. PNAS. 2005;102(33):11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneque, A., Fortus, H., Zheng, J., Werlen, G., and Jacinto, E., 2023. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes, 14 (4), 933. [DOI] [PMC free article] [PubMed]
- Pedraza-Chaverri J., Medina-Campos O.N., Ávila-Lombardo R., Berenice Zúñiga-Bustos A., Orozco-Ibarra M. Reactive oxygen species scavenging capacity of different cooked garlic preparations. Life Sci. 2006;78(7):761–770. doi: 10.1016/j.lfs.2005.05.075. [DOI] [PubMed] [Google Scholar]
- Qi J., Dong F. The relevant targets of anti-oxidative stress: a review. J. Drug Target. 2021;29(7):677–686. doi: 10.1080/1061186X.2020.1870987. [DOI] [PubMed] [Google Scholar]
- Rajapakse A.G., Ming X.-F., Carvas J.M., Yang Z. O -linked β-N-acetylglucosamine during hyperglycemia exerts both anti-inflammatory and pro-oxidative properties in the endothelial system. Oxid. Med. Cell. Longev. 2009;2(3):172–175. doi: 10.4161/oxim.2.3.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reine T.M., Jenssen T.G., Kolset S.O. Glucosamine exposure reduces proteoglycan synthesis in primary human endothelial cells in vitro. Food Nutr. Res. 2016;60(1):32615. doi: 10.3402/fnr.v60.32615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Clemmons D.R. Glucosamine induces resistance to insulin-like growth factor i (igf-i) and insulin in hep g2 cell cultures: biological significance of igf-i/insulin hybrid receptors. Endocrinology. 2003;144(6):2388–2395. doi: 10.1210/en.2002-221133. [DOI] [PubMed] [Google Scholar]
- Souza-Silva L., Alves-Lopes R., Silva Miguez J., Dela Justina V., Neves K.B., Mestriner F.L., Tostes R.d.C., Giachini F.R., Lima V.V. Glycosylation with O -linked β- N -acetylglucosamine induces vascular dysfunction via production of superoxide anion/reactive oxygen species. Can. J. Physiol. Pharmacol. 2018;96(3):232–240. doi: 10.1139/cjpp-2017-0225. [DOI] [PubMed] [Google Scholar]
- Trujillo J., Molina-Jijón E., Medina-Campos O.N., Rodríguez-Muñoz R., Reyes J.L., Loredo M.L., Barrera-Oviedo D., Pinzón E., Rodríguez-Rangel D.S., Pedraza-Chaverri J. Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: relation to oxidative stress. Food Funct. 2016;7(1):279–293. doi: 10.1039/c5fo00624d. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu B.o., Li X., Sun R. Novel glucosamine hydrochloride–rectorite nanocomposites with antioxidant and anti-ultraviolet activity. Nanotechnology. 2012;23(49):495706. doi: 10.1088/0957-4484/23/49/495706. [DOI] [PubMed] [Google Scholar]
- Xing R., Liu S., Guo Z., Yu H., Li C., Ji X., Feng J., Li P. The antioxidant activity of glucosamine hydrochloride in vitro. Bioorg. Med. Chem. 2006;14(6):1706–1709. doi: 10.1016/j.bmc.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Xing R., Liu S., Wang L., Cai S., Yu H., Feng J., Li P. The preparation and antioxidant activity of glucosamine sulfate. Chin. J. Oceanol. Limnol. 2009;27(2):283–287. [Google Scholar]
- Yan Y., Wanshun L., Baoqin H., Changhong W., Chenwei F., Bing L., Liehuan C. The antioxidative and immunostimulating properties of D-glucosamine. Int. Immunopharmacol. 2007;7(1):29–35. doi: 10.1016/j.intimp.2006.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.