Abstract
Cooking events can generate household air pollutants that deteriorate indoor air quality (IAQ), which poses a threat to human health and well-being. In this study, the emission characteristics and emission factors (EFs) of air pollutants of different meats (beef, lamb, chicken, pork, and fish) cooked by a novel oil-free process and common with-oil processes were investigated. Oil-free cooking tends to emit lower total volatile organic compound (TVOC) levels and fewer submicron smoke particles and can reduce the intake of fat and calories. However, TVOC emissions during oil-free cooking were significantly different, and the lamb EFs were nearly 8 times higher than those during with-oil cooking. The particle-bound polycyclic aromatic hydrocarbon (ƩPPAH) and benzo(a)pyrene-equivalent (ƩBaPeq) EFs during with-oil cooking ranged from 76.1 to 140.5 ng/g and 7.7–12.4 ng/g, respectively, while those during oil-free cooking ranged from 41.0 to 176.6 ng/g and 5.4–47.6 ng/g, respectively. The ƩPPAH EFs of chicken, pork, and fish were lower during oil-free cooking than during cooking with oil. Furthermore, the ƩBaPeq EFs of beef, chicken, pork, and fish were lower during oil-free cooking than during cooking with oil. Therefore, it is recommended to use the oil-free method to cook chicken, pork, and fish to reduce ƩPPAH and ƩBaPeq emissions, but not recommended to cook lamb due to the increase of ƩBaPeq emissions. The with-oil uncovered cooking EFs of aldehydes ranged from 3.77 to 22.09 μg/g, and those of oil-free cooking ranged from 4.88 to 19.96 μg/g. The aldehyde EFs were lower during oil-free covered cooking than with-oil uncovered cooking for beef, chicken, and fish. This study provides a better realizing of new cooking approaches for the reduction of cooking-induced emission, but further research on the effects of food composition (moisture and fat) and characteristics is needed.
Keywords: Cooking-induced emissions, Particle-bound polycyclic aromatic hydrocarbons (PPAHs), Particulate matter, Volatile organic compounds, Emission factors
1. Introduction
In recent years, cooking oil smoke (COS) has drawn substantial attention because indoor air pollution has a more significant effect on human health than outdoor air pollution. The emission of hazardous air pollutants from cooking activities depends on various factors, including cooking method, cooking temperature, additives, food materials, and cooking oil type [[1], [2], [3], [4]]. Cooking-induced emissions consist of toxic, harmful, and hazardous species such as particulate matter (PM), volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), and aldehydes, which might pose serious adverse health effects [[5], [6], [7]]. Epidemiological studies have linked long-term exposure to cooking-induced smoke to oxidative stress in lung cells [8,9], oxidative DNA damage [10,11], cardiovascular diseases [12,13], kidney damage [7], and carcinogenic effects [14,15]. Previous studies on household air pollution and reports by the World Health Organization (WHO, 2021a, 2021b) have highlighted that nearly 3.2 million premature deaths occur every year owing to household indoor air pollution, especially cooking-induced emissions from incomplete combustion of kerosene. Kerosene is commonly used as a cooking fuel in many parts of the world and is a significant source of household air pollution. According to the WHO 2021a, 2021b report, the causes of premature deaths in terms of percentages are as follows: cardiac ischemia heart disease (33%), stroke (23%), lower respiratory infection (19%), chronic obstructive pulmonary disease (19%) and lung cancer (6%), which is due to inappropriate use of kerosene and solid fuel during cooking activities [8,16,17]. Household cooking with solid fuels is positively correlated with health risks and accounts for approximately 12% of global PM [18]. COS pollutants are released into the ambient air through chimneys and building openings during the cooking process. This polluted ambient air circulates back into the home through windows, doors, and holes, further reducing indoor air quality (IAQ) [2]. Therefore, range hoods, chimneys, and natural ventilation methods can provide limited improvements. Currently, there are various technologies for removing harmful cooking-induced emissions, such as activated carbon filter adsorption, high-efficiency particulate air (HEPA) filters and electrostatic precipitators (ESPs) [[19], [20], [21], [22], [23]]. The best way to improve IAQ is by reducing source emissions by using better fuel sources, cooking methods, and cooking oils.
“Oil-Free cooking” aims to create a healthier diet and lifestyle, and its benefits include reducing fat and calories and improving weight loss. The “Oil-free cooking process” investigated in this study is to use the fat of the meat itself for cooking so that no oil is added during the cooking process. During cooking, the pot is covered; thus, the high-temperature steam generated during heating is effectively used to steam and cook the food thoroughly and quickly (cooking time is shorter). The covering can reduce the reaction of oil and air due to pyrolysis and oxidation at high temperatures and decrease the production of harmful indoor air pollutants.
The objective of the present study was to investigate the emission characteristics [PM, VOCs, and particle-phase PAHs (PPAHs)] of different meats (beef, lamb, chicken, pork, and fish) prepared using oil-free and oil-based cooking methods. The changes in COS concentrations and emission factors (EFs) of harmful substances from different cooking methods were also investigated. Finally, the statistical correlations of hazardous oil smoke pollutants under different cooking methods and conditions were analyzed. This study provides deep insights into PM, PPAHs, and TVOCs (the term TVOCs is used as the sum of VOCs in the air) emissions during “oil-free cooking” events. The significance of the study for improving IAQ and reducing the adverse health effects associated with cooking-induced emissions. The study can help policymakers and researchers design effective management protocols to mitigate household air pollution.
2. Materials and methods
2.1. Experimental settings
In situ experiments are conducted in the kitchen laboratory with a stainless-steel pan containing food ingredients heated by an electrical stove (halogen cooking plate, PHILIPS HD-4943, 1500 W power). The stainless-steel pan and halogen cooking plate were enclosed by a stainless-steel chamber (45 cm (H) x 45 (W) cm) equipped with a venting duct with a diameter of 15 cm (Fig. S1). The food ingredients comprised beef, lamb, chicken, pork, and fish. These food ingredients were weighed at room temperature using a precision balance (Ohaus PX523 - PIONEER™ Precision Balance, USA). Cooking-induced pollutants were sampled from a small hole (sampling port) in the air duct with a diameter of 15 cm above the ventilation hood.
2.2. Oil-free and with-oil cooking methods
The significance of the two cooking strategies is to investigate the emission of household air pollutants during cooking events. During the oil-free cooking method, the pot was covered and heated for approximately 2 min with a halogen cooking plate (PHILIPS HD4943, electric stove) with 1500 W power. Then, the power output was turned to 750 W, and the meats were placed on the greasy side of the pot, which was covered; in this stage, a timer was used (Table S1). After the cooked meat was removed from the pot, the pot and lid were cleaned with detergent and rinsed with clean water. The oil-free cooking method was repeated 10 times to cook different meats. The same procedure was followed in the oil cooking method except for the addition of 5 mL of soybean oil [24,25]. The halogen cooking plate temperature was monitored during four scenarios: (1) meat turn over, (2) meat when removed from the pot, (3) pot turn over, and (4) pot when meat was removed. An infrared thermometer was used to measure the temperature of the tested meats and three points of the pot. In the kitchen laboratory, we used 1.5–3 cm thick boneless beef short ribs, 1–2 cm thick lamb chops for mutton, 1.5–2.5 cm thick boneless chicken thighs, 1.5–2 cm thick plum pork pieces, and 2–2.5 cm thick salmon fillet (Fish). The mean weight of the tested meats is shown in Table S8.
2.3. Oil smoke monitoring system
2.3.1. Real-time monitoring of PMs
In this study, an optical particle counter (OPC, Lighthouse Handheld 3016-IAQ) equipped with an isokinetic probe operated at a sampling flow rate of 2.83 L per minute (l/min) and a scanning mobility particle sizer (SMPS, Model 3936L76, TSI Inc., St. Paul, MN) with a range of 11 nm–504 nm were used to measure the particle size distribution and concentration in real-time monitoring. The OPC had six size channels (0.3, 0.5, 1, 2.5, 5, and 10 μm). The calibration method of the optical particle counter is NIST traceable and has been accomplished in ISO 21501-4 2018: Light scattering airborne counter for clean spaces. The detection limit was 18 particles/m3, and the counting efficiencies for 0.3-μm and 0.5-μm particles were 51.5 ± 3.4% and 102.1 ± 4.8%, respectively.
2.3.2. Real-time monitoring of TVOCs
The total volatile organic compound (TVOC) concentration in the oil smoke was measured in real time by a handheld VOC monitor (ppbRAE-3000, RAE Systems by Honeywell, USA). The VOC monitor used a photoionization detector (PID, equipped with a 10.6 eV lamp) with built-in correction factors for >200 compounds comprising aromatics, ketones, aldehydes, alcohols, amines, amino compounds, halogens (containing Cl), and sulfide hydrocarbons. The calibration method of ppbRAE-3000 is two-point field calibration of zero and standard reference gas (isobutylene) and stores up to 8 sets of calibration data. The accuracy is ±3% at the calibration point. The measurement range is from 1 ppb to 10,000 ppm.
2.3.3. Analysis of aldehydes
The sampling and analysis process of gaseous aldehydes in COS was based on the reference method of the Environmental Protection Administration of Taiwan (NIEA R502.11C). A small suction pump (SKC-PCXR8 Universal Sample Pump) connected to 2,4-dinitrophenylhydrazine (2,4-DNPH) cartridges (LpDNPH S10, Supelco® Merck KGaA, Darmstadt, Germany) was used to sample the gaseous aldehydes in the oil smoke at a flow rate of 2.0 ± 0.1 L/min. The adsorption columns in the 2,4-DNPH cartridges were filled with silica spheres, and 2,4-DNPH was coated on the sphere surfaces, whereby 2,4-DNPH reacted with aldehydes to produce 2,4-DNPH derivatives of aldehydes. Acetonitrile (ACN) was used to wash the 2,4-DNPH cartridges and extract the 2,4-DNPH derivatives of the aldehyde compounds adsorbed on the silica. After filtering the samples to remove impurities, high-performance liquid chromatography (HPLC 1260, Agilent, USA) and a capillary column (XDB-C18, 4.6 × 250 mm, 5 μm) were utilized for separations of the target aldehyde pollutants. The aldehyde derivative was detected by a UV detector at 360 nm. The retention times (RT) and peak areas were used for qualitative and quantitative analysis, respectively. The aldehyde calibration curves were prepared with a standard mixed solution (T011A/IP-6A Aldehyde -DNPH Mix, Supelco® Merck KGaA, Darmstadt, Germany). The calibration curve included points at 15, 1.5, 0.75, 0.375, 0.15, 0.075, 0.015, and 0.0015 μg/L, and an R2 value of >0.995 was considered acceptable. The limit of detection (LOD) and limit of quantification (LOQ) of the analytical method are displayed in Table S6. Since the minimum concentration signal produced by the instrument was five times greater than the background noise value, the detection limit of aldehydes was set to 1.5 ng/L. This method detection limit (MDL) is estimated according to the “Definition and Procedure for the Determination of the Method Detection Limit, Revision 2, USEPA” that MDL is “the concentration value that corresponds to the instrument signal-to-noise ratio in the range of 3–5” [26].
2.3.4. Analysis of PPAHs and BaPeq
In this study, 30 PPAHs were analyzed. Particle samples from the COS were collected on 47-mm quartz filter paper (WHA1851047-Whatman® QM-A quartz filters) and measured with a micro-orifice uniform deposit impactor (MOUDI, 112R Nano Moudi-II™, MSP US). The increases in mass of the quartz filter papers as a result of the collected oil smoke particles were determined by a 6-digital microbalance (Precisa 40SM-200A, USA). The filter papers were ultrasonically treated with 20 mL of n-hexane and dichloromethane (DCM) for 30 min (ultrasonic unit: Elmasonic P60, Elma, Germany) to extract the PPAHs after weighing. The PPAH extract was qualitatively and quantitatively analyzed by gas chromatography‒mass spectrometry (GC‒MS, Agilent 7890B/5977A MSD) with an Agilent HP-5MS column (30 m × 0.25 mm i.d., DF = 0.25 μm). The retention times of the PAH standards were used as the parameters in qualitative analysis. The recovery of PPAH standards ranged from 80.9% to 102.3%. The recovery of surrogate compounds in each sample ranged from 75.1 to 110.1%. The method detection limits of the analyzed PPAHs were as follows: naphthalene (Nap): 0.062 ng; 2-methylnaphthalene (2-MeNAP): 0.058 ng; 1-methylnaphthalene (1-MeNAP): 0.067 ng; acenaphthylene (ACPy): 0.359 ng; acenaphthene (ACP): 0.581 ng; fluorene (FLU): 0.124 ng; 1-methylfluorene (1-MeFLU): 0.559 ng; phenanthrene (PHE): 0.100 ng; anthracene (ANTHR): 0.159 ng; 3-methylphenanthrene (3-MePHE): 0.717 ng; 3,6-dimethylphenanthrene (3,6-MePHE): 0.198 ng; fluoranthene (FLT): 0.117 ng; pyrene (PYR): 0.075 ng; benzo[c]phenanthrene (BcPH): 0.640 ng; benzo[b]naphtho(2,1-d)thiophene (BNT): 0.112 ng; cyclopenta(c,d)pyrene (CPP): 0.305 ng; benzo[a]anthracene (BaA): 0.178 ng; chrysene (CHR): 0.182 ng; benzo(b)fluoranthene (BbF): 0.290 ng; benzo(k)fluoranthene (BkF): 0.419 ng; benzo(e)pyrene (BeP): 0.153 ng; benzo(a)pyrene (BaP): 0.552 ng; perylene (PYL): 1.938 ng; indeno(1,2,3-cd)pyrene (IND): 0.237 ng; dibenzo(a,h)anthracene (DBA): 1.056 ng; benzo[ghi]perylene (BghiP): 0.157 ng; anthanthrene (ANTHN): 0.284 ng. These MDLs were “the concentration values that correspond to an instrument signal-to-noise ratio in the range of 3”, according to the “Definition and Procedure for the Determination of the Method Detection Limit, Revision 2” USEPA [26].
The toxic equivalency factor (TEF) for each PPAH relative to BaP was used to determine the potency of each PPAH in terms of BaP equivalent concentration (BaPeq). The sum of BaPeq values (Σ BaPeq) was used to determine the total health risk of PPAHs:
| (1) |
where [PPAHs]i is the concentration of PAH congener i and TEFi is the TEF of PPAH congener i (Table S4) [27].
However, our previous study [28] and other studies [29,30] showed that particle-phase PAHs contributed over 95% of the total PAH toxic potency (ƩBaPeq), and gas-phase PAHs contributed less than 5% of ƩBaPeq. In addition, the sampling and extraction of gas-phase PAHs are environmentally unfriendly (require many toxic organic solvents, including dichloromethane and n-hexane) and time-consuming. Therefore, gas-phase PAHs were not measured in this study.
2.4. Evaluation of pollutant EFs
All the meats were weighed before cooking to assess the emission factors (EFs) in mg/kg of pollutants under different cooking conditions by using Eq. (2):
| (2) |
where Ci is the concentration of air pollutants observed during the sampling period (μg/Nm3); Qi is the flow rate of the smoke exhaust fan during the sampling period (Nm3/min); ti is the time interval between measurements (minutes); i is the sampling time of the ith sample collected; n is the total number of samples in the whole test; and mo is the total weight of the ingredients (meat and oil) before cooking (g).
2.5. Data analyses
Spearman's rank correlation coefficient was used to assess the correlations between the EFs of all the measured indoor air pollutants in the COS. A linear regression model [generalized linear model (GLM)] was utilized to determine the influences of different cooking processes, meats, and temperatures on the EFs of indoor air pollutants in COS. (The “Oil-free” and “Lamb” serve as the reference groups for the variables of “Cooking” and “Meat,” respectively.) The p value was set to <0.05 as the threshold for variable selection, and a p value < 0.1 was set as the removal cutoff. The results of the GLM analyses were described with the model adjusted-R2 and unstandardized beta coefficient. The accepted levels of statistical significance were p values < 0.05* and 0.01** (two-sided test). Statistical analysis was executed by using IBM® SPSS® Statistics 20.
3. Results and discussion
3.1. EFs of PM
Table 1 shows that PM with a size of 2.5–5 μm (PM2.5-5) had the highest EF (μg/g) during the cooking of beef, lamb, chicken, and fish with oil, while the size fraction of 5–10 μm (PM5-10) had the highest EF (μg/g) during indoor cooking of pork, chicken, and fish. In addition, the PM EFs (μg/g) of beef and chicken cooked with oil were higher than the EFs of air pollutants generated from Chinese residential cooking; a previous study found that the emission rates of PM2.5 from panfried beef (480 g), fish (360 g), chicken (120 g), pork (240 g), and mutton (600 g) were 2.461 ± 0.606, 5.416 ± 2.861, 10.018 ± 0.466, 3.942 ± 1.414, and 0.923 ± 0.397 mg/min, respectively [31]. Similarly, the PM2.5 emissions from panfried chicken were also the highest in this study.
Table 1.
Emission factor (EF, μg/g) of particulate matter (PM).
| PM |
With oil-based |
|||||
|---|---|---|---|---|---|---|
| Particle size (μm) | Beef | Lamb | Chicken | Pork | Fish | |
| < 0.1 (PM0.1) | 0.458 | 0.049 | 0.382 | 0.505 | 0.061 | |
| 0.1-0.3 (PM0.1-0.3) | 7.012 | 0.365 | 3.124 | 2.099 | 1.326 | |
| 0.3-0.5 (PM0.3-0.5) | 7.49 | 1.12 | 3.12 | 1.28 | 2.52 | |
| 0.5-1 (PM0.5-1) | 5.51 | 0.99 | 2.36 | 0.74 | 1.33 | |
| 1.0–2.5 (PM1-2.5) | 10.92 | 2.93 | 10.20 | 3.85 | 3.31 | |
| 2.5–5 (PM2.5-5) | 47.06 | 10.96 | 45.19 | 18.02 | 13.11 | |
| 5–10 (PM5-10) | 46.22 | 8.56 | 35.15 | 19.13 | 9.32 | |
| 10–18 (PM10-18) | 2.84 | 0.34 | 1.90 | 1.33 | 0.36 | |
|
Total PM |
127.51 |
25.31 |
101.43 |
46.95 |
31.34 |
|
| Particle size (μm) | Oil-free process | |||||
|
< 0.1 (PM0.1) |
0.142 |
0.019 |
0.201 |
0.006 |
0.026 |
|
| 0.1-0.3 (PM0.1-0.3) | 4.016 | 0.261 | 1.717 | 0.114 | 0.481 | |
| 0.3-0.5 (PM0.3-0.5) | 2.24 | 0.70 | 3.43 | 0.69 | 0.95 | |
| 0.5-1 (PM0.5-1) | 6.24 | 2.42 | 5.81 | 1.60 | 1.76 | |
| 1.0–2.5 (PM1-2.5) | 39.87 | 20.03 | 26.46 | 9.68 | 5.89 | |
| 2.5–5 (PM2.5-5) | 212.76 | 110.42 | 136.16 | 42.19 | 24.54 | |
| 5–10 (PM5-10) | 310.95 | 158.98 | 165.66 | 34.92 | 26.58 | |
| 10–18 (PM10-18) | 47.02 | 210.60 | 22.12 | 1.65 | 6.87 | |
| Total PM | 623.24 | 503.43 | 361.56 | 90.85 | 67.10 | |
Particle emissions for fish (salmon) were lower than those for beef, lamb, chicken, and pork for most particle-size fractions during oil-free cooking with a lid due to several factors.
-
1.
Moisture retention: Salmon has a higher moisture content (68.1%) than beef (53.1% moisture) and lamb (58% moisture). The lid during oil-free cooking helped retain the natural moisture in the salmon, preventing it from evaporating and reducing the formation of particles. Moisture retention can contribute to lower particle emissions [32,33].
-
2.
Cooking time and temperature: Oil-free coking salmon with a lid required shorter cooking times than beef and chicken (Table S1), and the cooking temperature of salmon was lower than that of pork and beef (Fig. 4). The shorter cooking time and lower temperature can contribute to potentially lower particle emissions.
Fig. 4.
Temperature measured in different cooking processes.
Additionally, the composition and characteristics of different cuts of meat and salmon might also impact particle emissions. Therefore, it is essential to consider all the variables involved in cooking to obtain a comprehensive understanding of particle emissions, which will be our future work.
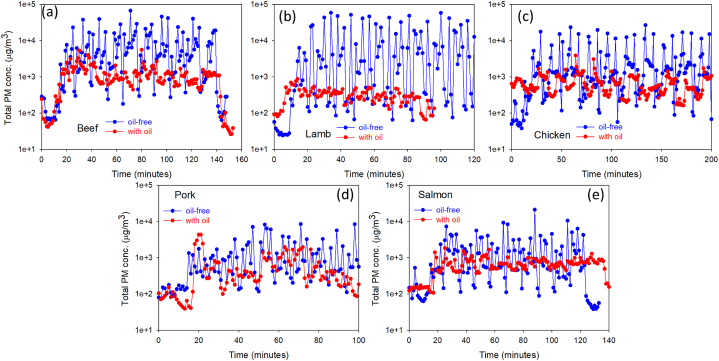
The measured time-dependent total particulate matter (TPM, μg/m3) concentrations are shown in Fig. 1(a–j). In the oil-free cooking process, PM5-10 had the highest EFs (μg/g) during the cooking of beef, chicken, and fish, while PM with a size larger than 10 μm had the highest EF (μg/g) during cooking of lamb. For pork, PM2.5-5 had the highest EF (μg/g). The EFs of particles in the oil-free cooking process were higher than those in the with-oil-based cooking process, and the highest EFs were found for beef. Particle emissions during cooking could be influenced by various factors, including the cooking method, temperature and duration, and fat and moisture contents of the meats. Steppeler et al. (2016) measured the fat contents and relative number of double bonds per gram of fat (unsaturated fatty acids, UFAs) of beef, pork, chicken, and salmon, which were 10% and 50.6, 9% and 72.8, 9.5% and 113.9, and 14% and 126.6, respectively [34]. Zhao et al. (2007) analyzed the particle organic matter emitted from frying. They found that saturated fatty acids (SFAs) and UFAs were predominant (78%) within the quantified compounds, and the average concentration of total SFAs was three times greater than that of total UFAs [35]. Therefore, the higher content of SFAs in beef might be one of the factors contributing to the higher particle EF. Similar results have also been reported in previous studies, in which foods with a higher fat content produced higher emission rates of PM [36,37]. In addition, among the five test meats, beef took the second longest time to be cooked (Table S1), and particle emission from meat could be higher when the cooking time was longer. Furthermore, a lid-covered pot would increase the cooking temperature (Fig. 4) and potentially trap fat and water vapor within the cooking vessel, leading to different cooking outcomes. When the lid was removed, the fat and water vapor were converted to particles owing to the rapid decrease in temperature. Thus, the EFs of particles in the oil-free cooking process were higher than those in the oil-based uncovered cooking process. However, in the cooking process with oil, particles with sizes <0.5 μm had higher EFs than those in the oil-free cooking process, as displayed in Table 1 and Fig. 2(a–e). This is because the large particles could be changed into smaller particles by the polymerization process during oil-based cooking activities [38]. When soybean oil was used for cooking, oil vapor was generated, and grease from the meats also evaporated. The oil vapor slowly condensed into particles smaller than 0.5 μm in the surrounding low-temperature environment. As previous studies have demonstrated, the emission rates of ultrafine particles (UFPs) from panfried beef (480 g), fish (360 g), chicken (120 g), pork (240 g), and mutton (600 g) were 17.370 ± 0.214, 16.507 ± 1.082, 20.211 ± 0.010, 14.086 ± 0.707, and 10.314 ± 0.268 ( × 1012 #/min), respectively [31]. Similarly, the emissions of UFPs from panfried beef, chicken and pork were very substantial in this study.
Fig. 1.
Submicron particulate matter (SPM, μg/m3) concentration of (a) oil-free beef, (b) beef with oil, (c) oil-free lamb, (d) lamb with oil, (e) oil-free chicken, (f) chicken with oil, (g) oil-free pork, (h) pork with oil, (i) oil-free fish, and (j) fish with oil.
Fig. 2.
Concentration of total particulate matter (TPM, μg/m3) emitted from cooking (a) beef, (b) lamb, (c) chicken, (d) pork, (e) salmon.
3.2. EFs of PPAHs and BaPeq
Table 1 shows that pork cooked with oil yielded the highest EF of ƩPPAHs (140.55 ng/g). The EF of ƩPPAHs during beef cooking was 114.41 ng/g, mainly distributed in the particle size range between 0.056 and 0.32 μm, which was similar to the result with a previously reported article [39]. In addition, the EF of ƩPPAHs when cooking lamb reached 76.11 ng/g, mainly distributed in the particle size range of 0.32–1.8 μm; the EF of ƩPPAHs for fish was 90.1 ng/g, distributed primarily in the particle size range of 0.056–0.32 μm. High concentrations of PPAHs (653.81–683.39 μg/g smoke) were also found in smoke from the oil-based frying of chicken legs [40]. Particle-bound polycyclic aromatic hydrocarbon emissions of over 4000 μg per kg of meat cooked were reported [41]. In a domestic kitchen, ƩPPAH concentrations of 33.5, 37.9, 38.2, and 44.2 μg/g−PM10 were discovered in PM10 samples from fried horse mackerel, stuffed chicken, grilled boneless pork strips, and fried boneless pork strips, respectively [37]. Temperatures of 160–200 °C can be reached while frying foods, triggering the formation of various organic compounds, such as PAHs and carbonyl compounds [11,42,43]. Unsaturated hydrocarbons in oils and fat-rich food samples undergo aromatization and dehydro cyclization during thermal cooking, favoring the formation of PPAHs [39,42]. Sixteen PPAHs were found in fried meats, including sardine, tuna, veal, hake, chicken, pork, and lamb, with higher concentrations (13.30–35.42 μg/kg) than those in the same samples cooked by other methods (3.15–27.93 μg/kg) [42].
The PPAH EF for chicken was 92.39 ng/g, and the particle size distribution was average. When pork and fish were cooked without oil, the ƩPPAHs EFs dropped to 51.25 and 41.04 ng/g, respectively, indicating that this method can significantly inhibit the release of PAHs from pork and fish. The ƩPPAHs EF of lamb cooked without oil increased to 176.65 ng/g. However, the two different cooking methods had little effect on beef and chicken ƩPPAH EFs. Table 2 shows that the ƩBaPeq EFs for cooking various meats with soybean oil were between 7.17 and 12.41 ng/g. Cooking chicken, pork, and fish without oil yielded lower ƩBaPeq EFs than those obtained when cooking with soybean oil. However, the ƩBaPeq EF of lamb cooked without oil and with a lid increased to 47.56 ng/g. Suleman et al. (2020) reviewed the effects of different cooking methods, including frying, smoking, steaming, boiling, roasting, grilling, and liquid smoke flavoring, on the formation of PAHs in lamb meat. The significant factors that influence the formation of PAHs are the temperature and duration of cooking and oil types. PAHs are produced as a result of pyrolysis of major components such as fat when heated at a temperature higher than 200 °C [44]. In our study, the temperature of oil-free cooking with a lid was higher than that of cooking with soybean oil, and thus, the ƩBaPeq EF of lamb cooked with the former method was higher than that of lamb cooked with the latter method. The addition of antioxidants from spices, marinades, ascorbic acid, tocophenol, and wrap flour to lamb helps to reduce PAHs [44].
Table 2.
Emission factor (EF, ng/g) of particle-bound PAHs and BaPeq[calculated byEquation (1)] and total volatile organic compounds (TVOCs, μg/m3).
| Cooking process |
With oil-based |
|||||
|---|---|---|---|---|---|---|
| Meats | Beef | Lamb | Chicken | Pork | Fish | |
| 0.056–0.32 μm | Ʃ PAHs | 47.90 | 11.33 | 35.78 | 54.31 | 42.55 |
| Ʃ BaPeq | 4.81 | 1.55 | 3.70 | 4.02 | 2.46 | |
| 0.32–3.2 μm | Ʃ PAHs | 39.43 | 47.51 | 33.83 | 43.60 | 20.68 |
| Ʃ BaPeq | 4.25 | 2.84 | 3.98 | 2.84 | 2.29 | |
| 3.2–18 μm | Ʃ PAHs | 27.08 | 17.27 | 22.78 | 42.64 | 26.78 |
| Ʃ BaPeq | 3.35 | 2.78 | 2.65 | 4.58 | 3.49 | |
| Total PPAHs | 114.41 | 76.11 | 92.39 | 140.55 | 90.01 | |
| Total BaPeq | 12.41 | 7.17 | 10.33 | 11.44 | 8.24 | |
|
TVOCs |
303.79 |
15.91 |
216.01 |
92.02 |
95.06 |
|
| Cooking process | Oil-free process | |||||
|
0.056–0.32 μm |
Ʃ PAHs |
33.29 |
63.86 |
27.66 |
24.77 |
13.69 |
| Ʃ BaPeq | 3.34 | 15.59 | 3.07 | 3.52 | 1.64 | |
| 0.32–3.2 μm | Ʃ PAHs | 44.61 | 60.80 | 30.97 | 16.93 | 15.72 |
| Ʃ BaPeq | 3.17 | 16.73 | 3.67 | 2.94 | 1.86 | |
| 3.2–18 μm | Ʃ PAHs | 41.77 | 51.99 | 28.06 | 9.55 | 11.63 |
| Ʃ BaPeq | 4.82 | 15.24 | 2.38 | 3.53 | 1.86 | |
| Total PPAHs | 119.67 | 176.65 | 86.69 | 51.25 | 41.04 | |
| Total BaPeq | 11.33 | 47.56 | 9.12 | 9.99 | 5.36 | |
| TVOCs | 187.45 | 131.01 | 161.03 | 129.02 | 45.92 | |
**PAHs: Polycyclic aromatic hydrocarbons; BaPeq: Benzo [a] pyrene equivalent concentration.
3.3. EFs of TVOCs and aldehydes
Table 2 shows that the TVOC EFs of beef were the highest among all meats tested. The reason for this might be the content of fat, which releases high VOCs during the high-temperature cooking process. Among the tested meats, beef and chicken produced 2 times higher TVOC emissions. A high cooking temperature induces the degradation of meat ingredients, such as fatty acids (lipolysis), resulting in VOC formation [[45], [46], [47]]. These results are similar to those of particulate emissions.
The measured time-dependent TVOC concentrations are displayed in Fig. 3(a–e). There was a significant difference in TVOC emissions generated during with-oil and oil-free cooking methods. In the oil-cooking method, the TVOCs emitted by beef, chicken, and fish were 1.5–2 times larger than those emitted in the oil-free cooking method. The TVOC EFs of lamb increased by nearly 8 times when oil-free (with cover) cooking was used. According to a previous report, the total fat content of lamb was 8.9–13.3% and 55.7–56.4% of the fats identified in lamb were unsaturated fatty acids (boiling points are lower than saturated fatty acids), which is different from other meats [48]. In addition, the temperatures during oil-free cooking were higher than those during oil cooking (Fig. 4). Therefore, the TVOC emission factor for lamb was higher in oil-free cooking (131 μg/g) than in with-oil cooking (15.6 μg/g), while the TVOC emission factors for other meat types were lower for oil-free cooking.
Fig. 3.
Total volatile organic compound (TVOC, μg/m3) concentration emitted from cooking (a) beef (b) lamb (c) chicken, (d) pork (e) salmon.
Fig. 5 depicts the aldehyde EFs during the cooking of various kinds of meats. Among them, the aldehyde EFs of chicken cooked with soybean oil were the highest (22.08 μg/g). The main aldehyde species emitted from chicken were dimethyl benzaldehyde, acrolein, acetaldehyde, and valeraldehyde (pentanal). The aldehyde EFs of beef were the second highest (14.6 μg/g), and the main aldehyde species were acrolein, acetaldehyde, and dimethylbenzaldehyde. The aldehyde EFs of lamb, pork, and fish cooked with oil ranged from 3.77 to 8.27 μg/g. According to previous reports, high concentrations of mutagenic aldehydes (the concentrations of alkanals and alkenals) were detected from 20.83 to 127.2 μg/m3 during pan frying of beef steak with soybean oil using gas and electric stoves [49]. Reportedly, oxidation and reactive oxygen species (ROS) are involved in the possible pathways related to the production of nonanal, hexanal (hexaldehyde), nonenal, pentanal, and butanal from the degradation of unsaturated fatty acids, including oleic acid, linoleic acid and linolenic acid [14,50].
Fig. 5.
Emission factors of aldehydes in different cooking processes.
During oil-free cooking, the aldehyde EFs of pork were the highest (19.96 μg/g), and the main aldehyde species were acrolein and dimethyl benzaldehyde; the aldehyde EFs of chicken were the second highest (17.33 μg/g), and the main species were acrolein, acetaldehyde, and dimethyl benzaldehyde. The aldehyde EFs of beef, lamb, and fish cooked without oil and with a cover were 4.88–8.29 μg/g. When beef, chicken, and fish were cooked without oil, the aldehyde EFs decreased, indicating that this method can significantly improve the release of aldehydes from beef, chicken, and fish. Giuffr et al. [51] investigated the emission of VOCs produced during the heat treatment of pomace olive oil, palm oil, and soybean oil, and 25 compounds were recognized, and alkanals, alkenals, and alkadienals were the most signified classes. Sjaastad and Svendsen detected 128 ± 53 μg/m3 alkanals and 4.0 ± 2.7 μg/m3 alkenals in the breathing zone during panfrying of beefsteak with soybean oil [52]. Fan et al. [53] found that fortifying soybean oil with docosahexaenoic acid, which is abundant in salmon (our test sample), increased toxic aldehyde emissions. Peng et al. [54] found significant aldehyde emission in COSs generated using soybean oil and sunflower oil in stir frying, pan frying, and deep frying in a typical kitchen, indicating that linoleic acid-rich oils (soybean oil contains 53.64% linoleic acid 18:2 and 6.34% linolenic acid 18:3 [14]) are more vulnerable to oxidation owing to the presence of multiple double bonds. Takhar et al. [55] et al. showed that aldehydes are significant chemical compounds formed during the thermal breakdown of edible oils, and antioxidants have double effects on aldehyde emissions dependent on the radical propagation reaction rates. When oil-free (with cover) cooking was used, the oxygen supply was much lower than that without cover. Thus, radical propagation reaction rates would be slowed and aldehyde emissions would be reduced. However, the aldehyde EFs of lamb and pork cooked without oil and with a cover increased significantly. This might be because the temperature when lamb and pork were oil-free cooked was higher than when they were cooked with oil. Among all the compounds in cooking-induced emissions, aldehydes are one of the most common species. Aldehydes are generated from the thermal degradation of fatty acids. Thus, the aldehyde EFs were related to the content of fatty acids in the meats [2,56]. As previous studies have shown, aldehydes are degraded during the cooking process from polyunsaturated fatty acids [14,[45], [46], [47], [48]].
3.4. Correlation between COS pollutants
Table S3 shows the correlation analysis between the EFs of hazardous pollutants in COS. According to the analysis results, air pollutants with highly correlated EFs had similar emission trends. For instance, TVOCs were highly correlated with PM smaller than 5 μm, acetaldehyde, propion-aldehyde, butyraldehyde, isovaleraldehyde, and hexaldehyde; PM-bound ∑BaPeq 56–320 nm was greatly correlated with PM-bound ∑BaPeq 0.32–3.2 and 3.2–18 μm; acrolein was significantly correlated with PM-bound ∑BaPeq 0.32–3.2 and 3.2–18 μm, formaldehyde and acetaldehyde. Consequently, all the cooking-induced pollutants mentioned above showed similar tendencies. Thus, household indoor air pollutants were significantly correlated with EFs of different tested meats.
3.5. Effect of temperature on cooking-induced emissions
According to Table 3, Table 4, the cooking temperature was an important factor affecting the EFs of cooking-induced hazardous pollutants. The temperatures measured at different cooking processes are presented in Fig. 4. The emissions of cooking-induced hazardous pollutants, including TVOCs and particles with diameters of 0.3–0.5, 0.5–1, 1–5, and 5–10 μm, were significantly correlated (p < 0.05) with cooking temperature, which was consistent with previous studies [25,57,58]. Previous studies clearly revealed that the particle size distribution of aerosols was lognormal and that the diameter of aerosols increased as the cooking temperature increased, especially in the size range of 0.3–1.0 μm [59].
Table 3.
Factors influencing the emission factor of particulate matter (PM) in oil cooking smoke (COS).
| Particle size |
<0.1 μm |
0.1–0.3 μm |
0.3–0.5 μm |
0.5–1 μm |
1–5 μm |
5–10 μm |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | p value | 95%CI |
B | p value | 95%CI |
B | p value | 95%CI |
B | p value | 95%CI |
B | p value | 95%CI |
B | p value | 95%CI |
||||||
| lower | upper | lower | upper | lower | upper | lower | upper | lower | upper | lower | upper | |||||||||||||
| Cooking | ||||||||||||||||||||||||
| With oil | 15.63** | >0.001 | 7.03 | 24.24 | 130.49** | >0.001 | 60.74 | 200.23 | 141.98 | 0.106 | −30.21 | 314.18 | 1.62* | 0.016 | 0.31 | 2.94 | −5.34 | 0.781 | −42.95 | 32.28 | 26.66 | 0.605 | −74.32 | 127.63 |
| Oil-free | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . |
| Meats | ||||||||||||||||||||||||
| Beef | 25.00** | >0.001 | 12.07 | 37.93 | 520.15** | >0.001 | 415.39 | 624.92 | 542.82** | >0.001 | 284.17 | 801.48 | 8.95** | >0.001 | 6.98 | 10.93 | 107.44** | >0.001 | 50.94 | 163.94 | −5.98 | 0.938 | −157.67 | 145.70 |
| Fish | −0.42 | 0.954 | −14.54 | 13.71 | 108.33 | 0.064 | −6.14 | 222.81 | 281.29 | 0.051 | −1.32 | 563.90 | 3.73** | 0.001 | 1.57 | 5.89 | 45.14 | 0.152 | −16.60 | 106.88 | −7.14 | 0.933 | −172.87 | 158.60 |
| Chicken | 16.81** | 0.001 | 6.52 | 27.09 | 117.68** | 0.006 | 34.36 | 200.99 | 51.24 | 0.625 | −154.45 | 256.93 | 3.55** | >0.001 | 1.98 | 5.12 | 11.49 | 0.616 | −33.45 | 56.42 | −158.46* | 0.010 | −279.08 | −37.84 |
| Pork | 17.70** | 0.001 | 7.43 | 27.97 | 142.18** | 0.001 | 58.98 | 225.38 | 297.07** | 0.005 | 91.67 | 502.47 | 2.87** | >0.001 | 1.30 | 4.44 | 50.10* | 0.029 | 5.23 | 94.97 | −45.82 | 0.456 | −166.27 | 74.64 |
| Lamb | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . |
| Temperature | −0.12 | 0.640 | −0.60 | 0.37 | 3.53 | 0.076 | −0.38 | 7.44 | 12.35* | 0.012 | 2.70 | 22.00 | 0.15** | >0.001 | 0.08 | 0.22 | 4.63** | >0.001 | 2.52 | 6.74 | 10.97** | >0.001 | 5.31 | 16.63 |
*p value < 0.05; **p value < 0.01.
Table 4.
Factors influencing the emission factors of TVOCs and particle-bound ∑BaPeq in COS.
|
Variable |
TVOCs |
Particle-bound ∑BaPeq |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.2–18 μm |
0.32–3.2 μm |
0.056–0.32 μm |
||||||||||||||||
| B | p value |
95%CI |
B | p value |
95%CI |
B | p value |
95%CI |
B | p value |
95%CI |
|||||||
| lower | upper | lower | upper | lower | upper | lower | upper | |||||||||||
| cooking rowhead rowhead | ||||||||||||||||||
| With oil | 29.11 | 0.263 | −21.84 | 80.07 | −5.29 | 0.189 | −13.18 | 2.61 | −4.31 | 0.319 | −12.80 | 4.17 | −4.24 | 0.378 | −13.67 | 5.19 | ||
| Oil-free | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . | ||
| Meats rowhead rowhead | ||||||||||||||||||
| Beef | 174.55** | >0.001 | 98.01 | 251.09 | −3.38 | 0.576 | −15.24 | 8.48 | −4.76 | 0.464 | −17.51 | 7.98 | −2.99 | 0.679 | −17.16 | 11.18 | ||
| Fish | 54.82 | 0.199 | −28.81 | 138.44 | −16.61* | 0.012 | −29.56 | −3.65 | −15.94* | 0.025 | −29.87 | −2.02 | −15.71* | 0.047 | −31.19 | −0.23 | ||
| Chicken | 104.49** | 0.001 | 43.62 | 165.35 | −5.80 | 0.228 | −15.23 | 3.63 | −3.42 | 0.508 | −13.55 | 6.72 | −5.52 | 0.337 | −16.79 | 5.74 | ||
| Pork | 70.45 | 0.023 | 9.67 | 131.23 | −8.30 | 0.084 | −17.72 | 1.11 | −10.37* | 0.045 | −20.49 | −0.24 | −9.87 | 0.086 | −21.12 | 1.38 | ||
| Lamb | – | . | . | . | – | . | . | . | – | . | . | . | – | . | . | . | ||
| Temperature | 2.91* | 0.046 | 0.05 | 5.76 | −0.36 | 0.113 | −0.80 | 0.08 | −0.28 | 0.245 | −0.76 | 0.19 | −0.30 | 0.259 | −0.83 | 0.22 | ||
*p value < 0.05; **p value < 0.01.
In addition, adding cooking oil extremely significantly increased the EFs of ultrafine particles (<0.1 μm), 0.1-0.3-μm particles, propionaldehyde, and dimethyl benzaldehyde and significantly enhanced the EFs of 0.5-1-μm particles and crotonaldehyde. Compared to lamb, the EFs of acetaldehyde, butyraldehyde, and meta- and para-tolu aldehydes for beef, propionaldehyde for beef and chicken, and hexaldehyde for chicken were significantly higher (Table S2). Numerous previous studies have reported that high concentrations of UFPs and submicron particles are emitted from frying and cooking fatty food with oil [15,36,57,59]. Higher cooking temperatures led to the formation of more aerosols in the nuclei and accumulation modes, and bimodal distributions were detected [59]. Additionally, aldehyde formation is relevant to the degradation of fatty acids, especially polyunsaturated fatty acids, during the cooking process [45,46,48].
Generally, the EFs of particle-bound ∑BaPeq of beef, chicken, pork, and fish were lower than those of lamb. However, only those of pork (0.32–3.2 μm) and fish were statistically significant (Table 3). Previous studies have shown the concentrations of PPAHs in various foodstuffs before and after the cooking process. In general, the highest concentrations were found after frying; the carcinogenic PPAHs in lamb after frying were higher than those in fried pork, and the total PPAHs in lamb after frying were higher than those in fried chicken [60]. In addition, the cooking times for different cooking processes and meats were different (Table S1). Longer cooking times would lead to more significant PAH formation. This factor could apply to both pork and fish when fried or cooked using the oil-free method because the cooking time for pork and fish was the shortest among the meats tested.
3.6. Strengths and limitations
First, although cooking emissions have been investigated by several prior studies, our study could be the first to use an enclosed chamber to measure the emission factors (EFs) of cooking-induced pollutants. Therefore, our experimental design could avoid measurement bias due to the influencing factors in an open space, which occurred in prior studies. Therefore, our measurement should be more accurate than prior studies. This dataset of cooking-induced air pollutant EFs could be applied for modeling [61] and the design of controlling strategies [62]. Second, to our knowledge, this study provides the first dataset of air pollutant emission factors for “oil-free cooking with cover” to date. This study proved that “oil-free cooking with cover” could reduce some air pollutant EFs in certain scenarios.
However, the findings of this work may be helpful for investigating the emission characteristics of household air pollutants in the real-condition kitchen environment. Some of the limitations to this work are that only the meats were cooked in the investigation of emissions characteristics and EFs in the kitchen laboratory environment. However, in real cooking scenarios, other food ingredients, such as vegetables, spices and seasonings, would be added and cooked with meats. These food ingredients could affect the emission characteristics and EFs of air pollutants.
4. Conclusions
Two cooking methods were investigated in this study: oil-free and with-oil cooking approaches. TVOC concentrations during oil-based cooking tended to be higher than those during oil-free cooking for all tested meats except lamb. The ƩPPAH EFs of chicken, pork, and fish during oil-free covered cooking were lower than those during uncovered cooking activities. In addition, the ƩBaPeq EFs of beef, chicken, pork, and fish during uncovered cooking with oil were lower than those during the oil-free uncovered cooking process. Similarly, the aldehyde EFs of beef, chicken, and fish during oil-free uncovered cooking were lower than those during with-oil uncovered cooking. Cooked beef, lamb, and chicken generated more hazardous oil smoke pollutants than pork and fish, which might be due to the fat contents of the meats. Theoretically, the blocking of air by the lid can reduce the high-temperature cracking and oxidation reactions of oil and fat, which may reduce the production of harmful pollutants, including aldehydes and PAHs. The experimental results showed that the ƩPPAHs EFs of chicken, pork, and fish during oil-free cooking were lower than those during with-oil cooking, while the ƩBaPeq EFs of beef, chicken, pork, and fish during oil-free cooking were lower than those during with-oil cooking. The aldehyde EFs of beef, chicken, and fish during oil-free uncovered cooking were lower than those during with-oil uncovered cooking. Nevertheless, the ƩPPAHs, ƩBaPeq, and aldehyde EFs of lambs during oil-free uncovered cooking were higher than those during with-oil uncovered cooking. This study highlights the need for alternative cooking strategies to minimize the generation of household air pollutants in the indoor environment.
Author contribution statement
Wei-Wen Huang: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper; Rasham Sallah-Ud-Din, Wonder Nathi Dlamini, Abiyu Kerebo Berekute: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper; Mastewal Endeshaw Getnet: Analyzed and interpreted the data; Wrote the paper; Kuo-Pin Yu: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to appreciate the financial support from the Ministry of Science and Technology and Environmental Protection Administration of Taiwan (Grant No: MOST 107-EPA-F-001-002).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19531.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhao Y., Zhao B. Emissions of air pollutants from Chinese cooking: a literature review. Build. Simulat. 2018;11:977–995. doi: 10.1007/s12273-018-0456-6. [DOI] [Google Scholar]
- 2.Yin Y., Pei J., Liu J. The effectiveness of kitchen ventilation for organic gaseous compound control in Chinese residential buildings. Build. Environ. 2022;226 doi: 10.1016/j.buildenv.2022.109764. [DOI] [Google Scholar]
- 3.Lin P., Gao J., He W., Nie L., Schauer J.J., Yang S., Xu Y., Zhang Y. Estimation of commercial cooking emissions in real-world operation: particulate and gaseous emission factors, activity influencing and modelling. Environ. Pollut. 2021;289 doi: 10.1016/j.envpol.2021.117847. [DOI] [PubMed] [Google Scholar]
- 4.Yu K.P., Chen Y.C., Miao Y.J., Siregar S., Tsai Y.W., Lee W.M.G. Effects of oil drops and the charcoal's proximate composition on the air pollution emitted from charcoal barbecues. Aerosol Air Qual. Res. 2020;20:1480–1494. doi: 10.4209/aaqr.2019.01.0042. [DOI] [Google Scholar]
- 5.Loftus C.T., Hazlehurst M.F., Szpiro A.A., Ni Y., Tylavsky F.A., Bush N.R., Sathyanarayana S., Carroll K.N., Karr C.J., LeWinn K.Z. Prenatal air pollution and childhood IQ: preliminary evidence of effect modification by folate. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Zheng X., Stevanovic S., Wu X., Xiang Z., Yu M., Liu J. Characterization particulate matter from several Chinese cooking dishes and implications in health effects. J. Environ. Sci. (China) 2018;72:98–106. doi: 10.1016/j.jes.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Ari A., Erturk Ari P., Yeni̇soy-Karakas S., Gaga E.O. Source characterization and risk assessment of occupational exposure to volatile organic compounds (VOCs) in a barbecue restaurant. Build. Environ. 2020;174 doi: 10.1016/j.buildenv.2020.106791. [DOI] [Google Scholar]
- 8.Zhao Y., Liu L., Tao P., Zhang B., Huan C., Zhang X., Wang M. Review of effluents and health effects of cooking and the performance of kitchen ventilation. Aerosol Air Qual. Res. 2019;19:1937–1959. doi: 10.4209/aaqr.2019.04.0198. [DOI] [Google Scholar]
- 9.Kang K., Kim H., Kim D.D., Lee Y.G., Kim T. Characteristics of cooking-generated PM 10 and PM 2.5 in residential buildings with different cooking and ventilation types. Sci. Total Environ. 2019;668:56–66. doi: 10.1016/j.scitotenv.2019.02.316. [DOI] [PubMed] [Google Scholar]
- 10.Zhai S.R., Albritton D. Airborne particles from cooking oils: emission test and analysis on chemical and health implications. Sustain. Cities Soc. 2020;52 doi: 10.1016/j.scs.2019.101845. [DOI] [Google Scholar]
- 11.Bandowe B.A.M., Lui K.H., Jones T., BéruBé K., Adams R., Niu X., Wei C., Cao J.J., Lee S.C., Chuang H.C., Ho K.F. The chemical composition and toxicological effects of fine particulate matter (PM2.5) emitted from different cooking styles. Environ. Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117754. [DOI] [PubMed] [Google Scholar]
- 12.Pope D.P., Mishra V., Thompson L., Siddiqui A.R., Rehfuess E.A., Weber M., Bruce N.G. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol. Rev. 2010;32:70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- 13.Pardo M., Shafer M.M., Rudich A., Schauer J.J., Rudich Y. Single exposure to near roadway particulate matter leads to confined inflammatory and defense responses: possible role of metals. Environ. Sci. Technol. 2015;49:8777–8785. doi: 10.1021/acs.est.5b01449. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D.C., Liu J.J., Jia L.Z., Wang P., Han X. Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments. Atmos. Environ. 2019;211:6–17. doi: 10.1016/j.atmosenv.2019.04.043. [DOI] [Google Scholar]
- 15.Lin C., Huang R.J., Duan J., Zhong H., Xu W. Polycyclic aromatic hydrocarbons from cooking emissions. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.151700. [DOI] [PubMed] [Google Scholar]
- 16.Balmes J.R. Household air pollution from domestic combustion of solid fuels and health. J. Allergy Clin. Immunol. 2019;143:1979–1987. doi: 10.1016/j.jaci.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P., Singh A.B., Arora T., Singh S., Singh R. Critical review on emerging health effects associated with the indoor air quality and its sustainable management. Sci. Total Environ. 2023;872 doi: 10.1016/j.scitotenv.2023.162163. [DOI] [PubMed] [Google Scholar]
- 18.Chafe Z.A., Brauer M., Klimont Z., VanDingenen R., Mehta S., Rao S., Riahi K., Dentener F., Smith K.R. Household cooking with solid fuels contributes to ambient PM2.5air pollution and the burden of disease. Environ. Health Perspect. 2015;122:1314–1320. doi: 10.1289/ehp.1206340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M., Zhu Y., Lin C.J., Wang S., Xing J., Jang C., Huang J., Huang J., Jin J., Yu L. Effects of air pollution control measures on air quality improvement in Guangzhou, China. J. Environ. Manag. 2019;244:127–137. doi: 10.1016/j.jenvman.2019.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obada D.O., Peter M., Kulla D.M., Omisanya N.O., Atta A.Y., Dodoo-Arhin D. Catalytic abatement of CO species from incomplete combustion of solid fuels used in domestic cooking. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma R., Balasubramanian R. Evaluation of the effectiveness of a portable air cleaner in mitigating indoor human exposure to cooking-derived airborne particles. Environ. Res. 2020;183 doi: 10.1016/j.envres.2020.109192. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y.C., Yang X.E., Lin K.Y., Huang W.W., Lin C.C., Yu K.P. Feasibility of using bed filters packed with rice-straw-based activated carbon and selected biomass waste for the control of frying fume exhaust. Environ. Sci. Pollut. Res. 2020;27:38321–38333. doi: 10.1007/s11356-020-09929-0. [DOI] [PubMed] [Google Scholar]
- 23.Wei H., Kerebo A., Siregar S., Yu K. High-efficiency carbon-coated steel wool filter for controlling cooking-induced oil smoke ☆. Environ. Pollut. 2023;334 doi: 10.1016/j.envpol.2023.122144. [DOI] [PubMed] [Google Scholar]
- 24.Gao J., Cao C., Xiao Q., Xu B., Zhou X., Zhang X. Determination of dynamic intake fraction of cooking-generated particles in the kitchen. Build. Environ. 2013;65:146–153. doi: 10.1016/j.buildenv.2013.04.006. [DOI] [Google Scholar]
- 25.Gao J., Cao C., Zhang X., Luo Z. Volume-based size distribution of accumulation and coarse particles (PM0.1-10) from cooking fume during oil heating. Build. Environ. 2013;59:575–580. doi: 10.1016/j.buildenv.2012.10.009. [DOI] [Google Scholar]
- 26.U.S. EPA . 2016. Definition and Procedure for the Determination of the Method Detection Limit—Revision 1.https://www.law.cornell.edu/cfr/text/40/part-136/appendix-B%5Cn 11. Epa 821-R-16-006. [Google Scholar]
- 27.Nisbet I.C.T., LaGoy P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs) Regul. Toxicol. Pharmacol. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-X. [DOI] [PubMed] [Google Scholar]
- 28.Yu K.P., Yang K.R., Chen Y.C., Gong J.Y., Chen Y.P., Shih H.C., Candice Lung S.C. Indoor air pollution from gas cooking in five Taiwanese families. Build. Environ. 2015;93:258–266. doi: 10.1016/j.buildenv.2015.06.024. [DOI] [Google Scholar]
- 29.Li C.T., Lin Y.C., Lee W.J., Tsai P.J. Emission of polycyclic aromatic hydrocarbons and their carcinogenic potencies from cooking sources to the urban atmosphere. Environ. Health Perspect. 2003;111:483–487. doi: 10.1289/ehp.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao P., Yu K.P., Lin C.C. Risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons in Taiwanese workers at night markets. Int. Arch. Occup. Environ. Health. 2011;84:231–237. doi: 10.1007/s00420-010-0551-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen C., Zhao Y., Zhao B. Emission rates of multiple air pollutants generated from Chinese residential cooking. Environ. Sci. Technol. 2018;52:1081–1087. doi: 10.1021/acs.est.7b05600. [DOI] [PubMed] [Google Scholar]
- 32.Kamruzzaman M., Makino Y., Oshita S. Parsimonious model development for real-time monitoring of moisture in red meat using hyperspectral imaging. Food Chem. 2016;196:1084–1091. doi: 10.1016/j.foodchem.2015.10.051. [DOI] [PubMed] [Google Scholar]
- 33.Tan D.X., Zanghi B.M., Manchester L.C., Reiter R.J. Melatonin identified in meats and other food stuffs: potentially nutritional impact. J. Pineal Res. 2014;57:213–218. doi: 10.1111/jpi.12152. [DOI] [PubMed] [Google Scholar]
- 34.Steppeler C., Haugen J.E., Rødbotten R., Kirkhus B. Formation of malondialdehyde, 4-hydroxynonenal, and 4-hydroxyhexenal during in vitro digestion of cooked beef, pork, chicken, and salmon. J. Agric. Food Chem. 2016;64:487–496. doi: 10.1021/acs.jafc.5b04201. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y., Hu M., Slanina S., Zhang Y. The molecular distribution of fine particulate organic matter emitted from Western-style fast food cooking. Atmos. Environ. 2007;41:8163–8171. doi: 10.1016/j.atmosenv.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Buonanno G., Morawska L., Stabile L. Particle emission factors during cooking activities. Atmos. Environ. 2009;43:3235–3242. doi: 10.1016/j.atmosenv.2009.03.044. [DOI] [Google Scholar]
- 37.Alves C.A., Vicente E.D., Evtyugina M., Vicente A.M.P., Sainnokhoi T.A., Kováts N. Cooking activities in a domestic kitchen: chemical and toxicological profiling of emissions. Sci. Total Environ. 2021;772 doi: 10.1016/j.scitotenv.2021.145412. [DOI] [PubMed] [Google Scholar]
- 38.Li Y.C., Qiu J.Q., Shu M., Ho S.S.H., Cao J.J., Wang G.H., Wang X.X., Zhao X.Q. Characteristics of polycyclic aromatic hydrocarbons in PM2.5 emitted from different cooking activities in China. Environ. Sci. Pollut. Res. 2018;25:4750–4760. doi: 10.1007/s11356-017-0603-0. [DOI] [PubMed] [Google Scholar]
- 39.Saito E., Tanaka N., Miyazaki A., Tsuzaki M. Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chem. 2014;153:285–291. doi: 10.1016/j.foodchem.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y.C., Chen B.H. Determination of polycyclic aromatic hydrocarbons in fumes from fried chicken legs. J. Agric. Food Chem. 2003;51:4162–4167. doi: 10.1021/jf020856i. [DOI] [PubMed] [Google Scholar]
- 41.Gysel N., Dixit P., Schmitz D.A., Engling G., Cho A.K., Cocker D.R., Karavalakis G. Chemical speciation, including polycyclic aromatic hydrocarbons (PAHs), and toxicity of particles emitted from meat cooking operations. Sci. Total Environ. 2018;633:1429–1436. doi: 10.1016/j.scitotenv.2018.03.318. [DOI] [PubMed] [Google Scholar]
- 42.Singh L., Varshney J.G., Agarwal T. Polycyclic aromatic hydrocarbons' formation and occurrence in processed food. Food Chem. 2016;199:768–781. doi: 10.1016/j.foodchem.2015.12.074. [DOI] [PubMed] [Google Scholar]
- 43.Yao Z., Li J., Wu B., Hao X., Yin Y., Jiang X. Characteristics of PAHs from deep-frying and frying cooking fumes. Environ. Sci. Pollut. Res. 2015;22:16110–16120. doi: 10.1007/s11356-015-4837-4. [DOI] [PubMed] [Google Scholar]
- 44.Suleman R., Wang Z., Aadil R.M., Hui T., Hopkins D.L., Zhang D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: current challenges and future prospects. Meat Sci. 2020;167 doi: 10.1016/j.meatsci.2020.108172. [DOI] [PubMed] [Google Scholar]
- 45.Serra A., Buccioni A., Rodriguez-Estrada M.T., Conte G., Cappucci A., Mele M. Fatty acid composition, oxidation status and volatile organic compounds in “Colonnata” lard from Large White or Cinta Senese pigs as affected by curing time. Meat Sci. 2014;97:504–512. doi: 10.1016/j.meatsci.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Cartoni Mancinelli A., Silletti E., Mattioli S., Dal Bosco A., Sebastiani B., Menchetti L., Koot A., vanRuth S., Castellini C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poultry Sci. 2021;100:1273–1282. doi: 10.1016/j.psj.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C., Chen J., Zhang X., Cai K., Chen C., Xu B. Emission characteristics and quantitative assessment of the health risks of cooking fumes during outdoor barbecuing. Environ. Pollut. 2023;323 doi: 10.1016/j.envpol.2023.121319. [DOI] [PubMed] [Google Scholar]
- 48.Ortuño J., Mateo L., Rodríguez-Estrada M.T., Bañón S. Effects of sous vide vs grilling methods on lamb meat colour and lipid stability during cooking and heated display. Meat Sci. 2021;171 doi: 10.1016/j.meatsci.2020.108287. [DOI] [PubMed] [Google Scholar]
- 49.Sjaastad A.K., Jørgensen R.B., Svendsen K. Exposure to polycyclic aromatic hydrocarbons (PAHs), mutagenic aldehydes and particulate matter during pan frying of beefsteak. Occup. Environ. Med. 2010;67:228–232. doi: 10.1136/oem.2009.046144. [DOI] [PubMed] [Google Scholar]
- 50.Goicoechea E., Guillén M.D. Volatile compounds generated in corn oil stored at room temperature. Presence of toxic compounds. Eur. J. Lipid Sci. Technol. 2014;116:395–406. doi: 10.1002/ejlt.201300244. [DOI] [Google Scholar]
- 51.Giuffrè A.M., Capocasale M., Macrì R., Caracciolo M., Zappia C., Poiana M. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. Lwt. 2020;117 doi: 10.1016/j.lwt.2019.108631. [DOI] [Google Scholar]
- 52.Sjaastad A.K., Svendsen K. Exposure to mutagenic aldehydes and particulate matter during panfrying of beefsteak with margarine, rapeseed oil, olive oil or soybean oil. Ann. Occup. Hyg. 2008;52:739–745. doi: 10.1093/annhyg/men060. [DOI] [PubMed] [Google Scholar]
- 53.Fan Z., Wang L., Jiang Q., Fan D., Xiao J., Wang M., Zhao Y. Effects of quercetin on emissions of aldehydes from heated docosahexaenoic acid (DHA)-fortified soybean oil. J. Hazard Mater. 2023;442 doi: 10.1016/j.jhazmat.2022.130134. [DOI] [PubMed] [Google Scholar]
- 54.Peng C.Y., Lan C.H., Lin P.C., Kuo Y.C. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J. Hazard Mater. 2017;324:160–167. doi: 10.1016/j.jhazmat.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 55.Takhar M., Li Y., Ditto J.C., Chan A.W.H. Formation pathways of aldehydes from heated cooking oils. Environ. Sci. Process. Impacts. 2022;25:165–175. doi: 10.1039/d1em00532d. [DOI] [PubMed] [Google Scholar]
- 56.Fullana A., Carbonell-Barrachina A.A., Sidhu S. Comparison of volatile aldehydes present in the cooking fumes of extra virgin olive, olive, and canola oils. J. Agric. Food Chem. 2004;52:5207–5214. doi: 10.1021/jf035241f. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q., Gangupomu R.H., Ramirez D., Zhu Y. Measurement of ultrafine particles and other air pollutants emitted by cooking activities. Int. J. Environ. Res. Publ. Health. 2010;7:1744–1759. doi: 10.3390/ijerph7041744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin J.S., Chuang K.T., Huang M.S., Wei K.M. Emission of ethylene oxide during frying of foods in soybean oil. Food Chem. Toxicol. 2007;45:568–574. doi: 10.1016/j.fct.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Yeung L.L., To W.M. Size distributions of the aerosols emitted from commercial cooking processes. Indoor Built Environ. 2008;17:220–229. doi: 10.1177/1420326X08092043. [DOI] [Google Scholar]
- 60.Perelló G., Martí-Cid R., Castell V., Llobet J.M., Domingo J.L. Concentrations of polybrominated diphenyl ethers, hexachlorobenzene and polycyclic aromatic hydrocarbons in various foodstuffs before and after cooking. Food Chem. Toxicol. 2009;47:709–715. doi: 10.1016/j.fct.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 61.Lai A.C.K., Chen F.Z. Modeling of cooking-emitted particle dispersion and deposition in a residential flat: a real room application. Build. Environ. 2007;42:3253–3260. doi: 10.1016/j.buildenv.2006.08.015. [DOI] [Google Scholar]
- 62.Lai A.C.K. Modeling of airborne particle exposure and effectiveness of engineering control strategies. Build. Environ. 2004;39:599–610. doi: 10.1016/j.buildenv.2003.12.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.