Abstract
Background
Ibrutinib is an effective and well-tolerated treatment for B-cell lymphomas but is associated with an increased risk of atrial fibrillation (AF) by altering the structure of the atrium. 5-Methoxytryptophan (5-MTP) inhibits inflammatory and fibrotic processes. This study aimed to determine the effects and mechanisms of 5-MTP on the underlying mechanisms of AF caused by ibrutinib.
Methods
The effect of 5-MTP on ibrutinib-related AF was investigated in male Sprague Dawley rats using echocardiographic, electrophysiological, immunofluorescent, Masson staining, and molecular analyses.
Rusults
The ibrutinib+5-MTP group showed (1) a lower incidence and shorter duration of AF and accelerated atrial conduction; (2) a decreased left atrial mass and left atrial diameter; (3) decreased myocardial fibrosis in the left atrium; (4) lower atrial inflammation; (5) increased sarcoplasmic reticulum Ca2+-ATPase 2a protein expression, decreased phosphorylation of phospholamban at Thr17, and decreased sodium/calcium exchanger 1 protein expression and phosphorylation of ryanodine receptor 2 at S2814; and (6) decreased phosphorylation of CaMKII expression. 5-MTP treatment markedly activated the PI3K-Akt signaling. Inhibiting PI3K-Akt signaling significantly reversed the protective effect of 5-MTP on ibrutinib-related AF.
Conclusions
These findings suggest that 5-MTP administration decreases the vulnerability of ibrutinib-related AF mainly caused by ameliorated maladaptive left atrial remodeling and dysregulation of calcium handling proteins. Mechanistically, 5-MTP treatment markedly enhanced the activation of cardiac PI3K-Akt signaling.
Keywords: 5-Methoxytryptophan, Ibrutinib, Atrial fibrillation, Structural remodeling, PI3K-Akt signaling
1. Introduction
Research has demonstrated that ibrutinib exhibits remarkable effectiveness as an initial therapy for individuals diagnosed with chronic lymphocytic leukemia (CLL) [1]. However, many studies have found that ibrutinib increases the incidence of atrial fibrillation (AF) by up to 10-fold compared to the control arm [2,3], among patients who have undergone a median follow-up duration of 28 months while taking ibrutinib, AF rates have reached as high as 16% [2]. Notably, AF frequently serves as a primary reason for the discontinuation of ibrutinib therapy [4]; and can result in considerable morbidity. Despite these facts, managing AF induced by ibrutinib poses a challenge due to limited understanding of its underlying pathophysiology. Hence, it is imperative to identify effective strategies for the prevention and treatment of AF induced by ibrutinib.
The pathophysiology of ibrutinib-induced AF promotion is complex, involving multiple cellular and molecular interactions. A recent study demonstrated that the phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt) pathway plays a significant role in cardioprotective mechanisms [5]. Ibrutinib has been found to potentially elevate the likelihood of AF through its inhibition of PI3K-Akt signaling in cardiac tissue [6]. Additionally, Jiang et al. have documented that ibrutinib can induce atrial structural remodeling and disrupt calcium regulation, thereby contributing to the promotion of AF [7]. Therefore, regulating PI3K-Akt signaling and improving atrial structural remodeling represent potential therapeutic strategies.
5-Methoxytryptophan (5-MTP) is a metabolite of tryptophan produced by human fibroblasts and released into the body [8]. Previous research has shown through in vitro and in vivo studies that 5-MTP possesses anti-inflammatory properties [9]. Furthermore, the impact of 5-MTP on cardiac injury following myocardial infarction encompasses various beneficial effects, such as mitochondrial stabilization, decreased infarct size, mitigated fibrosis, and enhanced myocardial function [10]. A recent study has shown that 5-MTP inhibits pulmonary fibrosis by regulating PI3K-Akt signaling pathways [11]. Hence, we postulated that treatment with 5-MTP could mitigate atrial remodeling in ibrutinib-associated AF by modulating the PI3K-Akt pathway. The objective of this study was to examine the involvement of 5-MTP in ibrutinib-associated AF and elucidate the underlying mechanisms.
2. Methods
2.1. Experimental animals
Experimental animals employed in this investigation comprised adult male Sprague-Dawley (SD) rats, aged 8 weeks and weighing between 180 and 220 g. The study received approval from the Animal Care and Use Committee of Renmin Hospital of Wuhan University and adhered to the guidelines outlined in the eighth edition of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (National Resource Council, 2011). Briefly, the experimental animals were randomized into three groups: the first group received saline as the vehicle control (Control group, n = 18); the second group received ibrutinib (25 mg/kg/d) orally until day 28 (Ibrutinib group, n = 20); the third group received ibrutinib (25 mg/kg/d) orally as well as 5-MTP (17 mg/kg) intraperitoneally at 0.5 h, 24 h, 3 days, and 7 days, and then twice weekly from day 7 until day 28 (Ibrutinib + 5-MTP group, n = 18) (Fig. 1A). To investigate the role of the PI3K/AKT signaling pathway, the PI3K inhibitor LY294002 (Selleck Chemicals, S1105) was applied. Briefly, the ibrutinib+5-MTP group received LY294002 (Selleck Chemicals, 1.2 mg/kg/d, i.p., n = 16), or not (n = 16), for 1 week beginning on day 28 (Fig. 1A). The administration protocol for 5-MTP is primarily based on a previously published study, which investigated its adverse effects and pharmacokinetic parameters following intravenous and intraperitoneal administration [10]. The dosage of ibrutinib was selected based on a previously published study [7]. In a preliminary study conducted on mice, it was determined that ibrutinib at a dosage of 25 mg/kg/d exhibited optimal tumor suppression, and mice demonstrated good tolerance following chronic treatment [12]. The dosage of LY294002 was selected based on previous published studies [13,14]. All pretreatment was blinded to the researchers during all experiments and data analysis.
Fig. 1.
Statistical analysis of surface electrocardiography in three groups. (A) The study protocol. (B) A representative trace of the surface electrocardiography. The arrows indicate the RR interval, P wave duration, PR interval, QRS duration, and QTc interval. (C–G) Result of surface electrocardiographic measurements (n = 8 per group). Data are expressed as mean ± SEM. NS: no statistical differences.
2.2. The measurement of echocardiography
Transthoracic echocardiography was performed using a Mylab30CV (ESAOTE) ultrasound system with a 15Mz probe. As part of the cardiovascular evaluation, the left atrial diameter (LAD), left ventricular end-diastolic dimension (LVEDd), left ventricular end-systolic diameter (LVESd), left ventricular fractional shortening (LVFS), and left ventricular ejection fraction (LVEF) were measured. LVFS and LVEF were calculated as previously described [15]. The echocardiograms were performed and interpreted by an ultrasound technician who was blinded to the treatment allocation.
2.3. Electrocardiograph analysis
Rats were anesthetized with inhaled isoflurane (1.5–2% isoflurane). Electrodes were positioned subcutaneously to simulate surface-lead ECG (lead II). The following parameters were recorded in the four groups: heart rate (RR interval), P wave period, PR, QRS, and QTc interval (in ms). The data were analyzed using Lab-Chart 7 Pro (AD Instruments). To account for differences in heart rate, the QTc interval was calculated using Fridericia's formula: QTc = QT/ .
2.4. Electrophysiological studies
Langendorff-perfused hearts were prepared using established methods, and the experiments were conducted after a period of four weeks [16]. Electrophysiological studies were carried out on isolated perfused hearts using a specific solution known as HEPES-buffered Tyrode's solution. This solution consisted of various components including NaCl, KCl, CaCl2, MgCl2, Na2HPO4, HEPES, glucose, and NaOH for pH adjustment. The Langendorff apparatus was utilized to facilitate these studies. The hearts were placed in a controlled environment consisting of a mixture of 95% O2 and 5% CO2 at a temperature of 37 °C and a constant pressure of 60 mmHg. Electrodes were positioned on the right atrium of the heart to measure the induction of AF and the interatrial conduction time (IACT). In order to achieve stabilization, all isolated hearts were perfused continuously for 20 min prior to stimulation. Any hearts that did not return to regular rhythm or experienced irreversible myocardial ischemia were excluded from the study.
Silver bipolar electrodes, covered in Teflon except for the tips, were positioned on the appendages of the right atrium (RA), left atrium (LA), and left ventricle (LV). T To measure IACT, RA pacing was employed at different cycle lengths (200 ms, 150 ms, 120 ms, and 100 ms) with a 5 mm interelectrode distance separating the RA and LA. AF inducibility was assessed using burst pacing. Three sets of 2-s burst pacing, administered by the automated stimulator, were used to test AF induction. In the initial 2-s burst, the cycle length (CL) was set to 40 ms (pulse duration = 5 ms). After a stabilization period of 3 min, the second 2-s burst had a CL of 20 ms (pulse duration = 5 ms). Lastly, the final 2-s burst had a CL of 20 ms and a pulse duration of 10 ms. AF was defined as a rapid and irregular atrial rhythm with irregular RR intervals lasting at least 1 s. The duration of AF was measured from the end of burst pacing until the first detected P wave following the rapid irregular atrial rhythm.
2.5. Histology
To assess interstitial fibrosis, the isolated LAs were preserved in 4% paraformaldehyde, enclosed in paraffin, and sectioned into sections of 5 mm in thickness. The extent of fibrosis was determined using Masson trichrome staining technique. The fibrosis percentage was computed by dividing the fibrotic tissue area by the area of the normal myocardial tissue.
2.6. Immunofluorescence
Three atrium sections from each rat's left atrium were incubated with primary antibodies to CD68 (GB11067, Servicebio Technology) and myeloperoxidase (MPO) (ab208670; 1/500; Abcam). A second antibody conjugated with fluorescence was used to incubate sections after the primary antibody. The fluorescence signals of five randomly chosen fields were captured using a fluorescence microscope (Nikon Eclipse C1; Nikon, Tokyo, Japan). All images were analyzed using Image-Pro Plus 6.0 software. The area positively stained in the perforation was quantified as a percentage of the total area at a magnification of × 400.
2.7. Quantitative real-time PCR (qRT-PCR)
In this experiment, RNA extraction from the LA samples was carried out using TRIzol reagent (Invitrogen, 15596-026). Subsequently, the purified RNA was transcribed into complementary DNA employing the TaKaRa PrimeScript RT reagent Kit (#RR047A). Following cDNA synthesis, qRT-PCR was conducted using cDNA, forward primers, reverse primers, and SYBR Premix Ex Taq (TaKaRa, #RR420A). To assess the relative expression of target genes, normalization was performed against the internal reference gene GAPDH. The primer sequences utilized for RT-PCR in this study can be found in Supplementary Table S1.
2.8. Western blot analysis
Protein extraction from the LA heart tissue was conducted, and the BCA method was utilized for determining protein concentrations. To denature the extracted protein samples, they were placed in SDS sample buffer (125 mmol/l of Tris–HCl at pH 6.8, 50% glycerol, 2% SDS, 5% mercaptoethanol, and 0.01% bromophenol blue). Subsequently, SDS-PAGE was performed, followed by blotting the proteins onto a polyvinylidene fluoride membrane (PVDF). To block the blotted membranes, they were treated with a solution containing 5% skim milk in Tris-buffered saline with 0.1% Tween 20 for a duration of 2 h. The membranes were then incubated overnight at 4 °C with primary antibodies (Supplementary Table S2). After three washes with Tris-buffered saline solution containing 0.1% Tween 20, the membranes were further incubated for 1 h with either anti-mouse or anti-rabbit IgG. To visualize the antibody binding, chemiluminescence (ECL) was employed, and the resulting images were collected and analyzed using a Chemidoc-It Imager from Ultra-Violet Products in Cambridge, UK.
2.9. Statistical analysis
The continuous variables were expressed as means ± standard errors of the means (SEM). Multiple group comparisons were performed using a one-way analysis of variance and Tukey's post hoc test. The incidence of AF between groups was compared using a Fisher exact test. Statistical analysis was conducted using GraphPad Prism Version 7.0 (GraphPad, San Diego, CA). A significance level of P < 0.05 was used to determine statistical significance.
3. Results
3.1. 5-MTP reduces the susceptibility to AF caused by ibrutinib
A total of 88 rats were included in the study, and none of the rat exhibited signs of morbidity or mortality during the course of the study. Electrocardiograms and Langendorff-perfused procedures were performed in all three groups to evaluate the potential of 5-MTP in reducing susceptibility to ibrutinib-associated AF. There were no significant differences in RR intervals, P waves, PR intervals, and QRS duration between the groups (Fig. 1B–G).
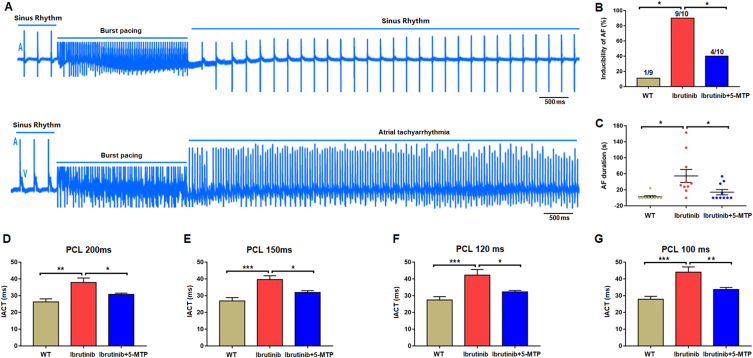
Furthermore, we assessed changes in electrophysiological parameters in Langendorff-perfused hearts, including AF induction rate, AF duration, and IACT (Fig. 2A). Ibrutinib treatment significantly increased the AF induction rate in the ibrutinib group compared to the control group (P < 0.05). However, the increase in AF induction rate observed in the ibrutinib group (9/10) was significantly decreased in the ibrutinib+5-MTP group (4/10) (Fig. 2B). Additionally, the ibrutinib treatment significantly prolonged AF duration in the ibrutinib group compared to the control group (P < 0.05). However, this prolongation was significantly attenuated in the ibrutinib+5-MTP group (Fig. 2C). Finally, the IACT of 100, 120, 150, and 200 ms cycles was significantly prolonged in the ibrutinib group compared to the control group (P < 0.05). Nevertheless, the increase in IACT was significantly attenuated in the ibrutinib+5-MTP group (Fig. 2D–G). Overall, our study results demonstrated that 5-MTP can reduce susceptibility to ibrutinib-associated AF.
Fig. 2.
Electrophysiological properties of the isolated perfusion atrium. (A) Representative electrograms of atrial fibrillation (AF) induction in the isolated perfused heart after atrial burst pacing. (B,C) Analysis of AF induction and duration (n = 9–10 per group). (D–G) Analysis of intra-atrial conduction time (IACT) of the left atrium (n = 8 per group). Data are expressed as mean ± SEM. * <0.05, **<0.01, ***<0.001. Scale bar in A, 500 mm.
3.2. 5-MTP restrains left atrial mass and left atrial diameter
As atrial structure remodeling is one of the critical mechanisms of AF, all animals were initially screened by echocardiography. The indicated group did not show any differences in LVEF, LVFS, LVEDD, or LVESD (Fig. 3A–D). However, a significant difference was observed between the ibrutinib-treated and saline-treated rats in terms of the LAD and the ratio of left atrium weight (LAW) to tibial length (TL). This difference was somewhat reduced in 5-MTP-treated rats (Fig. 3E–F). Additionally, WGA staining demonstrated that 5-MTP suppressed the ibrutinib-induced hypertrophy of atrial myocytes (Fig. 3G and H). The results showed that 5-MTP reduced the left atrial mass and left atrial diameter.
Fig. 3.
5-MTP restrained left atrial mass and left atrial diameter. (A–D) Statistical results for LVEF, LVFS, LVEDD, and LVESD in the indicated groups (n = 6 per group). Statistical results for left atrial diameter (E) and left atrial weight/tibia length (F) in the indicated groups (n = 6 per group). (G) Cardiomyocytes are stained with wheat germ agglutinin to reveal their structure and size (magnification, ×400 and n = 3 per group). (H) Areas of cardiomyocyte cross-sections quantified (n = 100+ cardiomyocytes in 4 samples). Data are expressed as mean ± SEM. * <0.05, **<0.01, ***<0.001. NS: no statistical differences.
3.3. 5-MTP reduces myocardial fibrosis in the left atrium
The process of atrial fibrosis results in the heterogeneity of conduction within the atria, which serves as a substrate for re-entry. Therefore, we assessed markers for atrial fibrosis. Rats treated with Ibrutinib exhibited a significant increase in the area of myocardial fibrosis compared to control rats, whereas 5-MTP treatment resulted in a significant decrease (Fig. 4 A, B). Consistent with these findings, the protein expression of molecular markers associated with atrial fibrosis, including collagen I, collagen III, and connective tissue growth factor (CTGF), was increased in the LA of ibrutinib-treated rats compared to control rats. However, the ibrutinib+5-MTP group demonstrated a significant reduction in these markers compared to the Ibrutinib group (Fig. 4C–F). These findings suggest that 5-MTP reduces myocardial fibrosis in the left atrium.
Fig. 4.
5-MTP reduced myocardial fibrosis in the left atrium. (A) Representative images of Masson's trichrome staining. Original magnification ×400. (B) Statistical results for myocardial fibrosis area (n = 4 per group). (C–F) Representative Western blots and statistical analysis of the levels of fibrosis-related proteins (n = 4 per group). Data are presented as means ± SEM. **<0.01, ***<0.001.
3.4. 5-MTP can blunt excessive inflammatory cell infiltration
The detrimental impact of ibrutinib on cardiac function is attributed to the activation of inflammatory responses. In order to evaluate the efficacy of 5-MTP in mitigating these responses, we assessed the degree of inflammatory cell infiltration within the atrium. Isoforms of myeloid markers, such as CD68 (Fig. 5A and B) and MPO (Fig. 5C and D), exhibited increased expression in the ibrutinib group, while these markers decreased in the ibrutinib+5-MTP group. Furthermore, we analyzed the gene expression of inflammatory cytokines in the myocardium. The levels of IL-1β, IL-6, and TNF-a were elevated in the ibrutinib group but decreased in the ibrutinib+5-MTP group (Fig. 5 E-G).
Fig. 5.
5-MTP blunted excessive inflammatory cell infiltration. (A) Representative double staining of CD68 (green) and nucleus (blue). Scale bar = 50 μm. (B) Quantifying CD68+ in cardiomyocytes in indicated group (n = 4 per group). (C) Representative double staining of MPO (green) and nucleus (blue). Scale bar = 50 μm. (D) Quantification of MPO in cardiomyocytes in indicated group (n = 4 per group). (E–G) The Statistical results for mRNA expression of IL-1β, IL-6, and TNF-α (n = 4 per group). Data are expressed as mean ± SEM. **<0.01, ***<0.001.
3.5. 5-MTP restored the Ca2+ handling protein disorders in the left atrium
There is compelling evidence indicating that modifications in calcium handling proteins, specifically ryanodine receptor 2 (RyR2), SR Ca2+-ATPase 2a (SERCA2a), phospholamban (PLB), and sodium/calcium exchanger 1 (NCX1), have an impact on intracellular calcium transients and diastolic SR Ca2+ release. Consequently, these modifications are known to contribute to the initiation of Ca2+-triggered arrhythmogenesis. With this in mind, we conducted an investigation to determine if the expression of the proteins RyR2, SERCA2a, PLB, and NCX1 is affected by 5-MTP.
Initially, our investigation revealed a conspicuous increase in the phosphorylation of RyR2 at Ser2814 within the ibrutinib group in comparison to the control group. Nevertheless, this adverse effect was alleviated to a great extent within the ibrutinib+5-MTP group when compared to the ibrutinib group (Fig. 6 A-C). Despite this, no variations were observed in RyR2 protein expression and the phosphorylation of RyR2 at Ser2814 among each group. Moreover, the data demonstrated that the administration of ibrutinib resulted in a significant augmentation in the phosphorylation of PLB (P-PLB) at Thr17, a site predominantly associated with the activation of calcium/calmodulin-dependent protein kinase II (CaMKII), as well as a reduction in SERCA protein expression compared to the control (p < 0.05). In contrast, the treatment with 5-MTP exhibited a noteworthy elevation in the expression of SERCA2a and a decrease in the expression of P-PLB at Thr17 when compared to the ibrutinib group (Fig. 6 D-F). Nonetheless, there were no substantial differences in the expression levels of P-PLB at Ser16 (primarily activated by PKA) and PLB protein (Fig. 6 G).
Fig. 6.
The expression of calcium handling proteins in three distinct groups. (A–C) Representative western blots and statistical analysis pertaining to the phosphorylation of RyR2 at Ser2814 and Ser2808, as well as the levels of RyR2 and GAPDH (n = 3 per group). (D–H) Representative western blots and statistical analysis regarding the phosphorylation of SERCA2a and PLB at Thr17 or Ser16, as well as the levels of PLB, NCX1, and GAPDH (n = 3 per group). (I–K) Representative western blots and statistical analysis concerning the phosphorylation of CaMKII, as well as the levels of CaMKII, PKA, and GAPDH (n = 3 per group). The data is presented as mean ± SEM.* <0.05, **<0.01, and ***<0.001. NS indicates no statistical difference.
Additionally, we examined the expression of NCX1 protein in the three groups of rats. As shown in Fig. 6H, the levels of NCX1 protein in the ibrutinib group were significantly higher than those in the control group (p < 0.05). Treatment with 5-MTP attenuated the increase in NCX expression observed in rats treated with ibrutinib. Finally, we measured the levels of PKA and the phosphorylation of CaMKII. The phosphorylation level of CaMKII and CaMKII expression in the ibrutinib group was significantly higher than those in the control group. However, treatment with 5-MTP significantly decreased the phosphorylation level of CaMKII and CaMKII expression in the ibrutinib-treated rats (Fig. 6I and J). In contrast, there was no significant difference in PKA expression between the ibrutinib+5-MTP group and the ibrutinib group (Fig. 6K). Therefore, these results support the conclusion that 5-MTP treatment may restore Ca2+ handling disorders in atrial myocytes.
3.6. 5-MTP treatment markedly enhances the activation of cardiac PI3K-Akt signaling
Based on the results above, 5-MTP may potentially mitigate vulnerability to AF through its effects on fibrosis, inflammation, and calcium-handling proteins. However, the precise mechanism by which 5-MTP reduces arrhythmia remains incompletely understood. Previous research has demonstrated the significance of the PI3K-Akt signaling pathway in atrial arrhythmia, as it influences atrial fibrosis, inflammation, and calcium-handling proteins [17]. Additionally, 5-MTP has been found to modulate PI3K-Akt signaling. Consequently, we conducted an investigation into the activation of PI3K-Akt signaling to validate the role of 5-MTP in ibrutinib-associated AF. The levels of PI3K and phosphorylated Akt were substantially decreased in the ibrutinib group. However, this decrease was significantly attenuated in the ibrutinib+5-MTP group, indicating that the activation of the PI3K-Akt signaling pathway was recovered by 5-MTP (Fig. 7A–C).
Fig. 7.
5-MTP regulates PI3K/Akt signalling pathway in ibrutinib-related atrial fibrillation. (A–C) Representative western blots and statistical analysis of PI3K, Akt phosphorylation, and Akt (n = 3 per group). *<0.05, **<0.01.
To investigate the involvement of the PI3K/Akt signaling pathway in the modulation of ibrutinib-related AF by 5-MTP, we conducted additional experiments using PI3K inhibitors (LY294002) to assess their potential to counteract the protective effect of 5-MTP. Our results showed that LY294002 significantly decreased the expression level of atrial PI3K and P-Akt in 5-MTP rats (Fig. 8A–C). Moreover, burst pacing rarely induced AF in 5-MTP rats. However, the AF inducibility and duration were increased by LY294002 in 5-MTP rats (Fig. 8D and E). Furthermore, we observed that rats in the LY294002 group exhibited a significant increase in atrial collagen deposition, higher collagen volume fraction (Fig. 8F and G), and higher expression levels of Collagen I, Collagen III, and CTGF (Fig. 8H–K). Taken together, these findings indicate that 5-MTP modulates atrial fibrosis and calcium-handling proteins by activating the PI3K-Akt signaling pathway.
Fig. 8.
Inhibiting PI3K-Akt signaling significantly reversed the protective effect of 5-MTP on ibrutinib-related atrial fibrillation. (A–C) Representative western blotting and statistical analysis of PI3K, Akt phosphorylation, and Akt in the left atria of the indicated groups (n = 3/per group). (D, E) Inducibility and duration of AF in the indicated groups (n = 8–9/per group). (F) Masson's Trichrome staining; (G) Myocardial fibrosis area (n = 3/per group); (H–K) Representative western blots and statistical analysis of fibrosis-related proteins (n = 3/per group). Data are presented as the mean ± SEM, * <0.05.
4. Discussion
In this investigation, the susceptibility of oral ibrutinib administration on rats was examined to determine the impact of 5-MTP treatment. The outcomes of our study exhibited a significant decrease in AF risk, along with the reduction of atrial fibrotic remodeling and inflammation after subjecting the rats to 5-MTP treatment. Additionally, the dysregulation of calcium-handling proteins in the atrium was restored. To elucidate the underlying mechanisms, the activation of cardiac PI3K-Akt signaling was found to be notably enhanced by 5-MTP treatment. This signaling pathway is recognized for its critical role in the regulation of cardiac function. Furthermore, it was observed that inhibiting PI3K-Akt signaling counteracted the protective influence of 5-MTP on ibrutinib-induced AF. These results suggest that the involvement of the PI3K-Akt signaling pathway is crucial in the mechanism of action of 5-MTP. Consequently, our findings propose that 5-MTP holds promise as a potential therapeutic intervention for the mitigation of ibrutinib-related AF.
Based on emerging evidence, ibrutinib has been identified as an independent risk factor for the development of AF in patients receiving this medication [18]. Previous research has shown that ibrutinib can significantly increase the risk of AF [7], and our experiments have yielded similar findings. Atrial fibrosis, inflammation, and imbalance of calcium homeostasis have been identified as arrhythmogenic mechanisms. These pathological changes have been confirmed by our study, suggesting that interventions targeting these processes may be key to preventing and treating AF in patients taking ibrutinib. 5-MTP was first discovered as a tryptophan metabolite produced and released by human fibroblasts [8]. In vitro and in vivo studies have reported that 5-MTP has anti-inflammatory properties [9]. Emerging evidence indicates that the decrease in 5-MTP levels in ischemic heart tissue may be associated with the inhibition of tryptophan hydroxylase-1 by proinflammatory mediators [19]. Additionally, 5-MTP has been shown to effectively prevent injury-induced liver, kidney, heart, and lungs, by inhibiting macrophage activation and preventing fibroblast differentiation [20]. Our study confirmed that 5-MTP significantly inhibited inflammation, decreased LA fibrosis, and reduced AF vulnerability in rats treated with ibrutinib-treated.
Numerous studies have demonstrated that alterations in calcium handling proteins lead to changes in intracellular Ca2+ transients and diastolic SR Ca2+ release, which may cause Ca2+-triggered AF. Calcium-handling disorders have been identified as the arrhythmogenic mechanisms underlying the initiation of AF [21]. Tuomi et al. suggested that ibrutinib-induced AF is caused by calcium dysregulation in atrial myocytes; however, no evidence was provided to support this theory [22]. Interestingly, Le et al. found that Ibrutinib increased phosphorylation at S2814 of RyR2, phosphorylation at Thr17 of PLB, decreased the amplitude of the Ca2+, increased the decay time of Ca2+ release, increased Ca2+ leak, and decreased Ca2+ content [7]. Consistent with these findings, we found that 5-MTP increased SERCA2a protein expression, decreased P-PLB at Thr17, and downregulated NCX1 and phosphorylation at S2814 of RyR2, but did not alter PLB or RyR2 protein expression. Several phosphorylation sites on RyR2 have been reported, including PKA phosphorylation sites S2808, and CaMKII site S2814 [23,24]. Most studies have shown that increased phosphorylation of S2808 and S2814 can enhance the RyR2 activity [25]. In this study, we found that the CaMKII site S2814 was significantly activated, while PKA phosphorylation sites S2808 were not activated, and the protein expression of PKA was not altered, indicating that PKA may not be involved in the regulation of RyR2 activity by ibrutinib. In line with Jiang et al.'s findings, no significant differences were observed in PKA levels and phosphorylation of RyR2-Ser2808 in a mouse model of AF following a 4-week oral administration of ibrutinib [7]. Phosphorylation of PLB can relieve its inhibition on SERCA2a and enhance calcium uptake by the SR. Decreased SERCA2a expression may reduce calcium uptake. The impact of ibrutinib on the overall phenotype, specifically intracellular calcium transients, and the potential for normalization through 5-MTP are yet to be fully elucidated. In summary, the evidence strongly suggests that 5-MTP can improve calcium handling and decrease AF vulnerability in rats treated with ibrutinib.
The underlying mechanisms through which 5-MTP regulates ibrutinib-induced AF are thought to be associated with its downstream PI3K-Akt signaling pathway. This pathway plays a crucial role in protecting the heart during stress [26]. Pretorius et al. suggested that decreased cardiac PI3K-Akt activity increases AF susceptibility [27]. Recent studies have found that ibrutinib increases the risk of AF, possibly by inhibiting PI3K-Akt signaling in the heart [6]. There is mounting evidence that increased CaMKII activity promotes cardiac remodeling, inhibits the PI3K-Akt signaling pathway, and adversely impacts cardiac function [7]. In our study, we found that ibrutinib significantly decreased the activity of PI3K-Akt signaling in the atrium and that 5-MTP decreased CaMKII activity and restored PI3K-Akt activity. Consistent with our observations, activation of the PI3K-AKT signaling pathway reduced atrial remodeling and therefore prevented AF [17]. However, a study showed that inhibition of PI3K is linked to the prolongation of cardiac repolarization, which is typically anti-not pro-AF [28]. Similarly, Greenwell et al. found that PI3K inhibitors are not associated with AF [29]. Therefore, the validity of the “PI3K hypothesis” need to be investigated. Our research results provide relevant evidence for further support of “PI3K involvement in ibrutinib-induced AF”. In addition, several studies have demonstrated that 5-MTP can prevent pulmonary fibrosis by inhibiting the PI3K-Akt signaling pathway. The observed discrepancies in the results could potentially be attributed to variations in the experimental models utilized across different studies. As a result, additional investigations are warranted to validate these findings and elucidate the underlying mechanisms involved.
5. Perspectives
Ibrutinib has shown efficacy as a frontline treatment for patients with chronic lymphocytic leukemia; however, AF often necessitates discontinuation of ibrutinib therapy. The underlying mechanisms of ibrutinib-associated AF remain incompletely understood, and effective strategies to mitigate cardiotoxicity are of significant clinical importance. In our study, we demonstrated that 5-MTP effectively protects against the development of ibrutinib-related AF in a rat model. Nonetheless, further investigations are required to confirm that the anticancer effects of ibrutinib remain unaffected by 5- MTP prior to clinical translation.
5.1. Limitations
The study has several limitations. First, we recommend including a group receiving daily 5-MTP injections before drawing conclusions about the long-term administration of 5-MTP and its cardioprotective benefits. Second, it is important to note that our findings do not establish a direct, causal relationship between 5-MTP and ibrutinib-related AF, which warrants exploration in future studies. Third, we did not investigate how 5-MTP affects anti-inflammatory function.
Fourth, this study involved a small sample size, which is a common challenge in animal-based experimental studies. This limitation is attributed to both cost constraints and ethical considerations, as it is crucial to prioritize the welfare of the animals involved. Therefore, the number of animals used in the study was minimized to meet the necessary requirements for addressing the predefined scientific inquiries. Finally, previous studies suggest that ibrutinib-induced AF may involve inhibition of the BTK/PI3K/Akt pathway. It is conceivable that 5-MTP could mitigate the antitumor effect of ibrutinib if used in combination to prevent the occurrence of AF. Therefore, it is very important to determine whether 5-MTP can attenuate ibrutinib-induced atrial remodeling and AF occurrence while preserving the antitumor effect of ibrutinib. To support our conclusion regarding the translational application of 5-MTP, further studies examining the effect of 5-MTP on the antitumor effect of ibrutinib are necessary.
6. Conclusions
The present study demonstrates that 5-MTP treatment decreases the risk of ibrutinib-related AF. This effect can be primarily attributed to the amelioration of maladaptive left atrial remodeling and the restoration of calcium-handling protein homeostasis. Mechanistically, treatment with 5-MTP significantly enhances the activation of the cardiac PI3K-Akt signaling pathway.
Author contribution statement
Wei Shuai: Conceived and designed the experiments; Wrote the paper.
Bo Peng: Performed the experiments; Wrote the paper.
Jun Zhu: Performed the experiments.
Bin Kong: Analyzed and interpreted the data.
Hui Fu: Contributed reagents, materials, analysis tools or data.
He Huang: Conceived and designed the experiments.
Funding statement
Dr Wei Shuai was supported by The Nature Science Foundation of Hubei Province {2022CFB708}.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19501.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shanafelt T.D., Wang X.V., Kay N.E., Hanson C.A., O'Brien S., Barrientos J., Jelinek D.F., Braggio E., Leis J.F., Zhang C.C., Coutre S.E., Barr P.M., Cashen A.F., Mato A.R., Singh A.K., Mullane M.P., Little R.F., Erba H., Stone R.M., Litzow M., Tallman M. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2019;381(5):432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald W.J., Rabe K.G., Kabat B.F., Herrmann J., Ding W., Kay N.E., Kenderian S.S., Muchtar E., Leis J.F., Wang Y., Chanan-Khan A.A., Schwager S.M., Koehler A.B., Fonder A.L., Slager S.L., Shanafelt T.D., Call T.G., Parikh S.A. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes. Ann. Hematol. 2021;100(1):143–155. doi: 10.1007/s00277-020-04094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onitilo A.A., Piwuna T.O., Islam N., Furuya-Kanamori L., Kumar S., Doi S.A.R. Determinants of atrial fibrillation development among patients undergoing ibrutinib therapy. Clin. Med. Res. 2022;20(1):16–22. doi: 10.3121/cmr.2021.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mato A.R., Hill B.T., Lamanna N., Barr P.M., Ujjani C.S., Brander D.M., Howlett C., Skarbnik A.P., Cheson B.D., Zent C.S., Pu J.J., Kiselev P., Foon K., Lenhart J., Henick Bachow S., Winter A.M., Cruz A.L., Claxton D.F., Goy A., Daniel C., Isaac K., Kennard K.H., Timlin C., Fanning M., Gashonia L., Yacur M., Svoboda J., Schuster S.J., Nabhan C. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann. Oncol. 2017;28(5):1050–1056. doi: 10.1093/annonc/mdx031. [DOI] [PubMed] [Google Scholar]
- 5.Weeks K.L., Gao X., Du X.J., Boey E.J., Matsumoto A., Bernardo B.C., Kiriazis H., Cemerlang N., Tan J.W., Tham Y.K., Franke T.F., Qian H., Bogoyevitch M.A., Woodcock E.A., Febbraio M.A., Gregorevic P., McMullen J.R. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ. Heart. Fail. 2012;5(4):523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 6.McMullen J.R., Boey E.J., Ooi J.Y., Seymour J.F., Keating M.J., Tam C.S. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L., Li L., Ruan Y., Zuo S., Wu X., Zhao Q., Xing Y., Zhao X., Xia S., Bai R., Du X., Liu N., Ma C.S. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16(9):1374–1382. doi: 10.1016/j.hrthm.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Deng W.G., Saunders M., Gilroy D., He X.Z., Yeh H., Zhu Y., Shtivelband M.I., Ruan K.H., Wu K.K. Purification and characterization of a cyclooxygenase-2 and angiogenesis suppressing factor produced by human fibroblasts. Faseb. J. 2002;16(10):1286–1288. doi: 10.1096/fj.01-0844fje. [DOI] [PubMed] [Google Scholar]
- 9.Wu K.K., Kuo C.C., Yet S.F., Lee C.M., Liou J.Y. 5-methoxytryptophan: an arsenal against vascular injury and inflammation. J. Biomed. Sci. 2020;27(1):79. doi: 10.1186/s12929-020-00671-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu W.T., Tseng Y.H., Jui H.Y., Kuo C.C., Wu K.K., Lee C.M. 5-Methoxytryptophan attenuates postinfarct cardiac injury by controlling oxidative stress and immune activation. J. Mol. Cell. Cardiol. 2021;158:101–114. doi: 10.1016/j.yjmcc.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Fang L., Chen H., Kong R., Que J. Endogenous tryptophan metabolite 5-Methoxytryptophan inhibits pulmonary fibrosis by downregulating the TGF-beta/SMAD3 and PI3K/AKT signaling pathway. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118399. [DOI] [PubMed] [Google Scholar]
- 12.Ponader S., Chen S.S., Buggy J.J., Balakrishnan K., Gandhi V., Wierda W.G., Keating M.J., O'Brien S., Chiorazzi N., Burger J.A. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie Y., Fu C., Zhang H., Zhang M., Xie H., Tong X., Li Y., Hou Z., Fan X., Yan M. Celastrol slows the progression of early diabetic nephropathy in rats via the PI3K/AKT pathway. BMC. Complement. Med. Ther. 2020;20(1):321. doi: 10.1186/s12906-020-03050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F., Wang L., Jiao Y., Wang Z. Qishen Huanwu capsule reduces pirarubicin-induced cardiotoxicity in rats by activating the PI3K/Akt/mTOR pathway. Ann. Palliat. Med. 2020;9(5):3453–3461. doi: 10.21037/apm-20-1746. [DOI] [PubMed] [Google Scholar]
- 15.Coppola C., Riccio G., Barbieri A., Monti M.G., Piscopo G., Rea D., Arra C., Maurea C., De Lorenzo C., Maurea N. Antineoplastic-related cardiotoxicity, morphofunctional aspects in a murine model: contribution of the new tool 2D-speckle tracking. OncoTargets Ther. 2016;9:6785–6794. doi: 10.2147/OTT.S106528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuai W., Kong B., Fu H., Shen C., Jiang X., Huang H. MD1 deficiency promotes inflammatory atrial remodelling induced by high-fat diets. Can. J. Cardiol. 2019;35(2):208–216. doi: 10.1016/j.cjca.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z., Li R., Wang X., Li J., Yuan M., Liu E., Liu T., Li G. Attenuation of atrial remodeling by aliskiren via affecting oxidative stress, inflammation and PI3K/Akt signaling pathway. Cardiovasc. Drugs Ther. 2021;35(3):587–598. doi: 10.1007/s10557-020-07002-z. [DOI] [PubMed] [Google Scholar]
- 18.Tang C.P.S., McMullen J., Tam C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors, Leuk. Lymphoma. 2018;59(7):1554–1564. doi: 10.1080/10428194.2017.1375110. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y.F., Hsu Y.J., Wu H.F., Lee G.L., Yang Y.S., Wu J.Y., Yet S.F., Wu K.K., Kuo C.C. Endothelium-derived 5-methoxytryptophan is a circulating anti-inflammatory molecule that blocks systemic inflammation. Circ. Res. 2016;119(2):222–236. doi: 10.1161/CIRCRESAHA.116.308559. [DOI] [PubMed] [Google Scholar]
- 20.Wu K.K. Control of tissue fibrosis by 5-methoxytryptophan, an innate anti-inflammatory metabolite. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.759199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landstrom A.P., Dobrev D., Wehrens X.H.T. Calcium signaling and cardiac arrhythmias. Circ. Res. 2017;120(12):1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuomi J.M., Xenocostas A., Jones D.L. Increased susceptibility for atrial and ventricular cardiac arrhythmias in mice treated with a single high dose of ibrutinib. Can. J. Cardiol. 2018;34(3):337–341. doi: 10.1016/j.cjca.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 23.van Oort R.J., McCauley M.D., Dixit S.S., Pereira L., Yang Y., Respress J.L., Wang Q., De Almeida A.C., Skapura D.G., Anderson M.E., Bers D.M., Wehrens X.H. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122(25):2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 25.Dobrev D., Wehrens X.H. Role of RyR2 phosphorylation in heart failure and arrhythmias: controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 2014;114(8):1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. ; discussion 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMullen J.R., Amirahmadi F., Woodcock E.A., Schinke-Braun M., Bouwman R.D., Hewitt K.A., Mollica J.P., Zhang L., Zhang Y., Shioi T., Buerger A., Izumo S., Jay P.Y., Jennings G.L. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 2007;104(2):612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pretorius L., Du X.J., Woodcock E.A., Kiriazis H., Lin R.C., Marasco S., Medcalf R.L., Ming Z., Head G.A., Tan J.W., Cemerlang N., Sadoshima J., Shioi T., Izumo S., Lukoshkova E.V., Dart A.M., Jennings G.L., McMullen J.R. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am. J. Pathol. 2009;175:998–1009. doi: 10.2353/ajpath.2009.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballou L.M., Lin R.Z., Cohen I.S. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ. Res. 2015;116(1):127–137. doi: 10.1161/CIRCRESAHA.116.303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwell I.B., Ip A., Cohen J.B. PI3K inhibitors: understanding toxicity mechanisms and management, oncology. (Williston Park). 2017;31(11):821–828. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.